- 1State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China
- 2University of Chinese Academy of Sciences, Beijing, China
- 3Institute of Plant and Microbial Biology, Academia Sinica, Taipei, Taiwan
Acyl carrier proteins (ACPs) are a group of small acidic proteins functioning as important cofactors in the de novo synthesis of fatty acids. In Arabidopsis, ACPs are encoded by a small gene family comprising five plastid members, AtACP1 to AtACP5, and three mitochondrial members. The biological functions and the transcriptional responses to abiotic stresses of most AtACPs have yet to be elucidated. The present study extends previous findings and provides new knowledge on the function of ACPs by examining the responses of AtACP-encoding genes to several abiotic stresses and, in particular, the role of AtACP5 in the adaptation to salt stress. Phylogenetic analysis showed that AtACP1, AtACP2, AtACP3, and AtACP5 can be classified into one group and separated from a group comprising AtACP4 and ACP homologs from related species. Quantitative RT-PCR analysis revealed that the expression of AtACP1, AtACP2, and AtACP3 was induced by drought. Both iron deficiency and nitrogen starvation resulted in down-regulation of AtACP4. The most pronounced response was observed for AtACP5, the expression of which was dramatically decreased by salt stress. Knock-out of AtACP5 showed increased sensitivity to NaCl stress, whereas transgenic lines overexpressing AtACP5 displayed increased salt tolerance relative to the wild-type. Overexpression of AtACP5 further led to an altered composition of fatty acids, mainly a decrease of oleic acid (C18:1) and an increase of palmitic acid (C16:0), and to a lower Na+/K+ ratio when compared to the salt stressed wild-type. The comprehensive transcriptional information on the small plastid AtACP gene family in response to various abiotic stresses and the further investigation of the AtACP5 indicate that AtACP5 might be critical for salt tolerance through alterations of the composition of fatty acids and, subsequently, the Na+/K+ ratio.
Introduction
Due to their sessile life style, plants are unavoidably exposed to environmental stresses throughout their life cycle. To deal with such constraints, plants have evolved mechanisms that allow for efficient adaption to an ever-changing environment. Fatty acids (FAs) are pivotal constituents of cellular membranes, suberin, and cutin waxes that provide structural barriers to the environment (Beisson et al., 2007). Cellular membranes are a major target of environmental signals, and the maintenance of their integrity, permeability, fluidity and functionality of transport proteins by recalibrating lipid composition is critical for the resistance to different stresses (Rodriguez-Vargas et al., 2007; Huynh et al., 2012; Maejima et al., 2014).
Acyl carrier proteins (ACPs) play a central role in de novo FA synthesis. Fatty acid synthases (FASs) can be separated into two distinct classes. TypeI FASs consist of a single, large, multifunctional polypeptide and are common to mammals, fungi, and some bacteria. Type II FASs are found in archaea, bacteria and plants and are characterized by the involvement of discrete, mono-functional enzymes for FA synthesis. ACP exists as a separated domain (Jenke-Kodama et al., 2005). Plant ACPs are small (9 kD) separated polypeptides with 70–80 mostly acidic residues, modified by the covalent attachment of 4′-phosphopantetheine to a centrally localized serine (Wakil et al., 1983; Marrakchi et al., 2002). De novo biosynthesis of FAs proceeds via a conserved set of reactions, which are carried out during the elongation cycle (Smith and Sherman, 2008). ACPs are central components of FASs, which covalently bind all fatty acyl intermediates. In the first step, ACP synthase (ACPS) attaches a phosphopantetheine group from CoA on a serine residue of ACP in a conserved Asp-Ser-Leu motif to form holo-ACP (Mofid et al., 2002). The initial substrate of FASs, malonyl-CoA, is transferred to ACP and the acetyl-CoA unit (C2) is expanded to a butyryl group (C4). The synthetic cycle is repeated multiple times until saturated C16 or C18 acyl-ACPs are produced for utilization in membrane biosynthesis (Chan and Vogel, 2010).
Besides the production of saturated FAs (SFAs), ACPs are also involved in the biosynthesis of unsaturated FAs (UFAs), which proceeds via a slightly different reaction scheme. In Escherichia coli, most of the C10 acyl chain substrates are processed by β-hydroxyacyl dehydratase (FabZ) to form trans-2-decenoyl-ACP. Subsequently, enoyl reductase (FabI), β-Oxoacyl synthase I (FabB) or β-Oxoacyl synthase II (FabF) catalyzes the formation of SFAs. Alternatively, this fatty acyl intermediate can be converted through dehydration and isomerization to cis-3-decenoyl-ACP catalyzed by β-hydroxyacyl dehydratase (FabA) (Heath and Rock, 1996; Leesong et al., 1996), which can be further extended to UFAs by FabB (Feng and Cronan, 2009). Therefore, in E. coli FabBs and FabIs compete for cis-3- and trans-2-decenoyl-ACP, respectively, and the relative abundance of these two enzymes determines the ratio of saturated to UFAs in the membrane. The vast majority of UFAs in E. coli are cis-9-hexadecenoic acid (palmitoleic acid) and cis-11-octadecenoic acid (vaccenic acid) (Cronan, 1967; Morein et al., 1996).
The levels of UFAs can be changed to adjust membrane lipid fluidity to environmental conditions, mainly by regulating the activity of FA desaturases. For example, enhancement of polyunsaturated FA levels in chloroplast lipids was shown to be an effective means to resist chilling stress, while an opposite trend was observed during heat stress (Iba, 2002). Free linolenic acid itself is a stress signal and the precursor for phyto-oxylipin biosynthesis (Blee, 2002). Increasing evidence suggests that chloroplast oleic acid (18:1) levels are critical for normal pathogen defense responses in Arabidopsis, including programmed cell death and systemic acquired resistance (SAR) (Kachroo et al., 2001). Also, the Arabidopsis FAD2, an endoplasmic reticulum (ER)-localized ω-6 desaturase, which converts oleic acid (18:1) to linoleic acid (18:2) by inserting a double bond at the ω-6 position, is required for chilling and salt tolerance (Miquel et al., 1993; Zhang et al., 2012). All higher plants studied so far contain multiple isoforms of ACP, some of which are expressed ubiquitously while others appear to be expressed in a tissue-specific manner (Battey and Ohlrogge, 1990; Hlousekradojcic et al., 1992). The genome of the model plant Arabidopsis thaliana harbors three genes encoding mitochondrial ACPs, namely mtACP1 (At2g44620), mtACP2 (At1g65290), and mtACP3 (At5g47630), and five plastidial ACPs, AtACP1 (At3g05020), AtACP2 (At1g54580), AtACP3 (At1g54630), AtACP4 (At4g25050), and AtACP5 (At5g27200) (Meyer et al., 2007). Based on protein analysis, AtACP1, AtACP2, and AtACP3 are expressed in all tissues examined (Hlousekradojcic et al., 1992), whereas AtACP4 is expressed predominantly in leaves and the mRNA levels are increased by light (Shintani, 1996; Bonaventure and Ohlrogge, 2002). AtACP1 and AtACP3 are regulated by the transcription factor WRINKLED1 (WRI1) and are required for the accumulation of triacylglycerols in Arabidopsis seeds (Ruuska et al., 2002; Maeo et al., 2009). The expression of both genes followed a bell-shaped pattern that increased through the early seed development stages and peaked between 8 and 11 days after flowering (Ruuska et al., 2002). AtACP2 was associated with the cell cycle (Menges et al., 2002), and its expression was up-regulated in developing siliques (Moire et al., 2004) and in cytokinin receptor mutants (Rashotte et al., 2003). AtACP4 was required to perceive the mobile SAR signal in distal tissues of Arabidopsis (Xia et al., 2009). Except for AtACP5, all plastid AtACPs showed increased protein abundance upon phosphate deficiency (Lan et al., 2012).
Generally, the expression levels of ACPs relate to the FA composition. Overexpression (OE) of AtACP1 resulted in decreased 16:3 and increased 18:3 FAs (Branen et al., 2001). Compromised expression of AtACP4 by both antisense AtACP4 and T-DNA insertion led to reduced proportion of 16:3 FAs (Branen et al., 2003; Ajjawi et al., 2010) and caused a chlorotic phenotype and compromised photosynthetic competence (Ajjawi et al., 2010). Although all Arabidopsis ACP isoforms have been characterized except AtACP5, little is known regarding their putative involvement in the acclimation to abiotic stresses. Understanding the role of plastidial ACPs in environment stresses in general, and the molecular function of AtACP5, are the two main aims of the present study. We here described the transcriptional responses of five plastidial ACPs to various abiotic stresses such as salt, drought and deficiencies in nitrogen, phosphorus, potassium, and iron in Arabidopsis. It was found that the expression of AtACP1-3 was induced by drought, AtACP4 was down-regulated in nitrogen- and iron-deficient plants, and AtACP5 was dramatically inhibited by salt stress. Loss of AtACP5 function resulted in hypersensitivity to salt stress, while AtACP5 OE lines showed increased salt tolerance. The results showed that alterations in FA composition were causative for the different phenotypes observed under salt stress. Specifically, decreased oleic acid and increased palmitic acid levels in AtACP5 OE plants were shown to contribute to increased salt tolerance, possibly by maintaining the cellular homeostasis of Na+ and K+.
Materials and Methods
Plant Material and Treatments
Plants were grown in a growth chamber on agar medium as described by Estelle and Somerville (1987). Seeds of A. thaliana Col-0 accession and the T-DNA insertion mutant atacp5 (SALK_111501C) were ordered from NASC1. Seeds were surface sterilized with 70% ethanol and bleach (0.5% NaClO + 0.5% Tween) and sown on solid agar plates containing ES medium (Estelle and Somerville, 1987). The medium was composed of (mM): KNO3 (5), MgSO4 (2), Ca (NO3)2 (2), and KH2PO4 (2.5), (μM): H3BO3 (70), MnCl2 (14), ZnSO4 (1), CuSO4 (0.5), NaCl (10), Na2MoO4 (0.2), and Fe(III)-EDTA (40), solidified with 0.8% agar, 1% sucrose, and 4.7 mM MES. The pH was adjusted to 5.5. Plates were vernalized in darkness for 2 days at 4°C and then transferred to a growth chamber at 22°C and 70% humidity under a 16-h-light/8-h-dark photoperiod.
To observe the seedling phenotype under salt stress, 50 seeds of each genotype were germinated and grown for 20 days on ES media supplemented with 0, 125 or 150 mM NaCl. The growth status of the plants was divided into four categories: non-germination, normal, sub-healthy, and dead. The percentage of each category was determined. For each treatment, all 50 seedlings were collected and weighed together, and the average fresh weight (FW) per seedling was calculated. Similar experiments were carried out with 5-day-old seedlings transferred to ES media supplemented with 0 or 125 mM NaCl for another 9 days.
To analyze the salt sensitivity of mature plant, 10-day-old seedlings were transferred to soil to grow for additional 11 days and then treated with NaCl solution either by stepwise increase or added once to the maximum of 250 mM NaCl. The average FW of rosette leaves per plant was calculated.
Characterization of the T-DNA Insertion Mutant
Homozygosity of the mutants was determined by PCR from genomic DNA using gene-specific (LP: 5′-CTCAGAGATGAAGGATGCTGG-3′; RP: 5′-CCATCTCTCTCGATCAGATCG-3′) and T-DNA left border primers LBa1, and further analyzed by DNA sequencing to confirm the insertion site of the T-DNA in the gene.
DNA Constructs and Plant Transformation
To generate the AtACP5 OE construct (35S::AtACP5), the full-length ORF (420 bp) of the AtACP5 gene was amplified with AtACP5 specific primers (forward: 5′-CTAGGTACCATGGCGACAAGTTTCTGCT-3′, KpnI; reverse: 5′-CATCTGCAGCTAAGCAGTCTTCTCTTGGACG-3′, PstI), ligated into the same sites of the modified binary vector pCAMBIA2301 behind the cauliflower mosaic virus 35S promoter. The T-DNA construct was introduced into wild-type plants via Agrobacterium-mediated transformation as described previously (Clough and Bent, 1998).
Total RNA Isolation and Quantitative Real-Time RT-PCR Analyses
Plant samples were collected in liquid nitrogen and stored at -80°C for RNA extraction. Total RNA was isolated according to the manufactures’ instructions using Trizol reagent (Invitrogen). One microgram total RNA was used for reverse transcription (TaKaRa, with gDNA Eraser, Cat#RP047A). The cDNA was diluted 12 times, and 2 μl were used as a template in a 10 μl PCR reaction. Real-time RT-PCR analysis was performed using SYBR Green Perfect mix (TaKaRa, Cat# RR420A) on a Thermo PIKOREAL 96 Real-Time PCR System, with a program of 40 cycles and the following conditions: 95°C for 5 s, 60°C for 30 s with TUA3 (tubulin alpha-3, At5g19770) as endogenous control. The primers used in this study are listed in Supplementary Table 1.
FA Composition
The FA composition in seedling tissues was determined as previously described (Li et al., 2006; Wang et al., 2012) with slight modification. Ten-day-old seedlings (0.15 g) of each genotype were heated in 2 ml 10% methanol-KOH at 80°C for 2 h. After the mixture was cooled to room temperature, 1 ml of 6 N HCl was added and the mixture was extracted twice with 2 ml of hexane. The extracts were dried under nitrogen stream. To this end, 1 ml of 5% (v/v) sulfuric acid in MeOH (freshly prepared for each use), 25 μl BHT solution (0.2% butylated hydroxy toluene in MeOH), 50 μg triheptadecanoin (as a triacylglycerol internal standard to generate methyl heptadecanoate), and 300 μl toluene as co-solvent were added to the tube. The mixture was vortexed for 30 s and then heated to 90–95°C for 1.5 h. After cooling to room temperature, 1.5 ml 0.9% NaCl (w/v) were added and fatty acid methyl esters (FAMEs) were extracted with 3 ml × 2 ml hexane. Pooled extracts were evaporated under nitrogen and then dissolved in 400 μl hexane. The FAME extracts were analyzed by gas chromatography (GC). GC analysis was performed on a Thermo GC Ultra with a flame ionization detector (FID) on a DB23 column (30 m by 0.25 mm i.d., 0.25 μm film; J&W Scientific, Folsom, CA, United States). The commercial standard FAME mixture (Sigma, CRM47885) was estimated quantitatively. The GC conditions were: split mode injection (1:40), injector and FID temperature, 260°C; oven temperature program, 150°C for 3 min, then increasing at 10°C/min to 240°C and holding this temperature for 5 min.
Determination of the Na+ and K+ Concentrations
Ten-day-old seedlings grown on ES media supplemented with or without 125 mM NaCl were used for cation concentration determination as previously described (AOAC, 1990). Seedlings were harvested, rinsed with deionized water, and dried at 70°C for 2 days. One-hundred milligrams of ground dry matter was then extracted with 20 ml deionized water by boiling for 2 h. The extract was filtered and the volume was set to 25 ml. Na+ and K+ concentrations were determined by flame spectrophotometry using a standard curve (coefficients > 0.99) made by standard Na+ and K+ solutions, and the relative Na+ and K+ concentrations were calculated as mg/g DW.
Results
Arabidopsis Plastidial ACPs Are Phylogenetically Divided into Two Clades
To investigate the divergence between Arabidopsis ACPs and ACPs in other species, we performed a protein BLAST search with the amino acid sequences of AtACP1-AtACP5. Highest scores (similarities of more than 90%) were obtained for Brassicaceae species such as Arabidopsis lyrata subsp. lyrata and Camelina sativa (Supplementary Table 2). Extending the search for related proteins in the genomes of the monocotyledonous species rice (Oryza sativa) and maize (Zea mays) and the dicotyledons soybean (Glycine max) and tobacco (Nicotiana tabacum) yielded relatively low similarities of the amino acid sequences (less than 50%). We then analyzed the phylogenetic relationships of the orthologous ACPs using the MEGA6.06 software package (Figure 1). This analysis subdivided the proteins into two clades with AtACP1, AtACP2, AtACP3, AtACP5 and most ACPs from Brassicaceae species comprising one clade, and AtACP4 and ACPs from species unrelated to Brassicaceae forming a second clade, suggesting that the divergence between AtACP4 and the other four AtACPs appeared early in evolution. In the first clade, AtACP2, AtACP3, and some AtACP2/3-like protein from other Brassicaceae species form a subgroup. The high homology between AtACP2 and AtACP3 (similarity 92.6%) and clusters of arrangement in the Chr 1 suggested that these genes were created by a local gene duplication. AtACP1 and AtACP5 showed moderate similarity (67.9%) and fell into another group. Multiple alignment of these ACPs by DNAMAN revealed that the highly conserved amino acid sequence LGADSLDTVEIVM include the serine residue to which the cofactor 4′-phosphopantetheine is attached (Supplementary Figure 1). These results suggest that the five plastidial Arabidopsis ACPs contain a highly conserved motif that may play similar molecular roles in different processes.
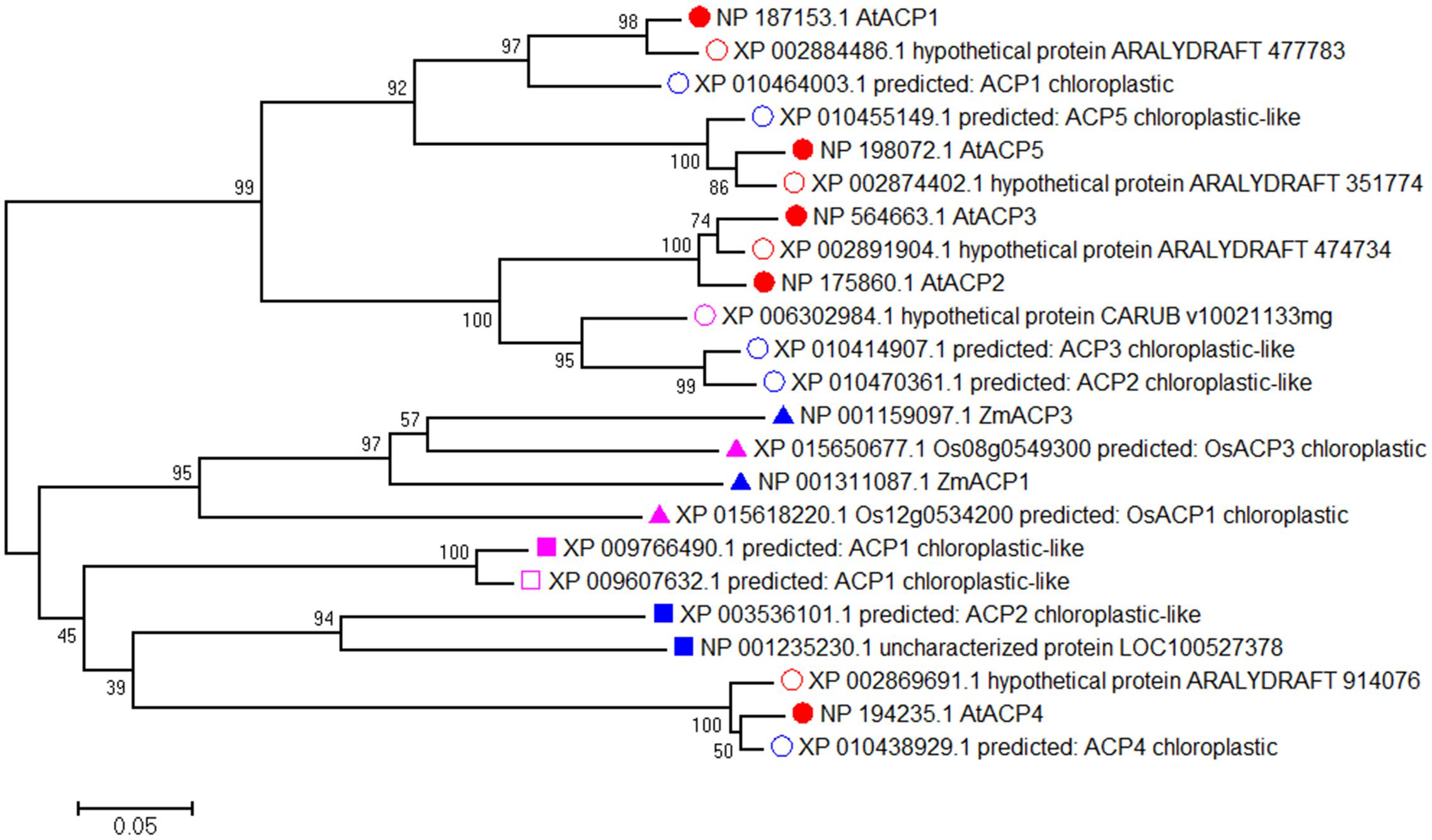
FIGURE 1. The phylogenetic tree of AtACP1-5 proteins. A phylogenetic tree was conducted using full-length maize (Zea mays, blue triangle), rice (Oryza sativa, purple triangle), soybean (Glycine max, blue quadrate) and tobacco (Nicotiana sylvestris, purple quadrate; Nicotiana tomentosiformis, purple unfilled quadrate) and other species (Arabidopsis lyrata subsp. lyrata, red unfilled circular; Camelina sativa, blue unfilled circular; Capsella rubella, purple unfilled circular) with the highest similarity amino acid sequences related to AtACP1-5 from Arabidopsis (red filled circular) by MEGA6.06 software using neighbor-joining method. The numbers beside each node represent bootstrap values based on 1,000 replications. The scale bar indicates the relative amount of change along branches.
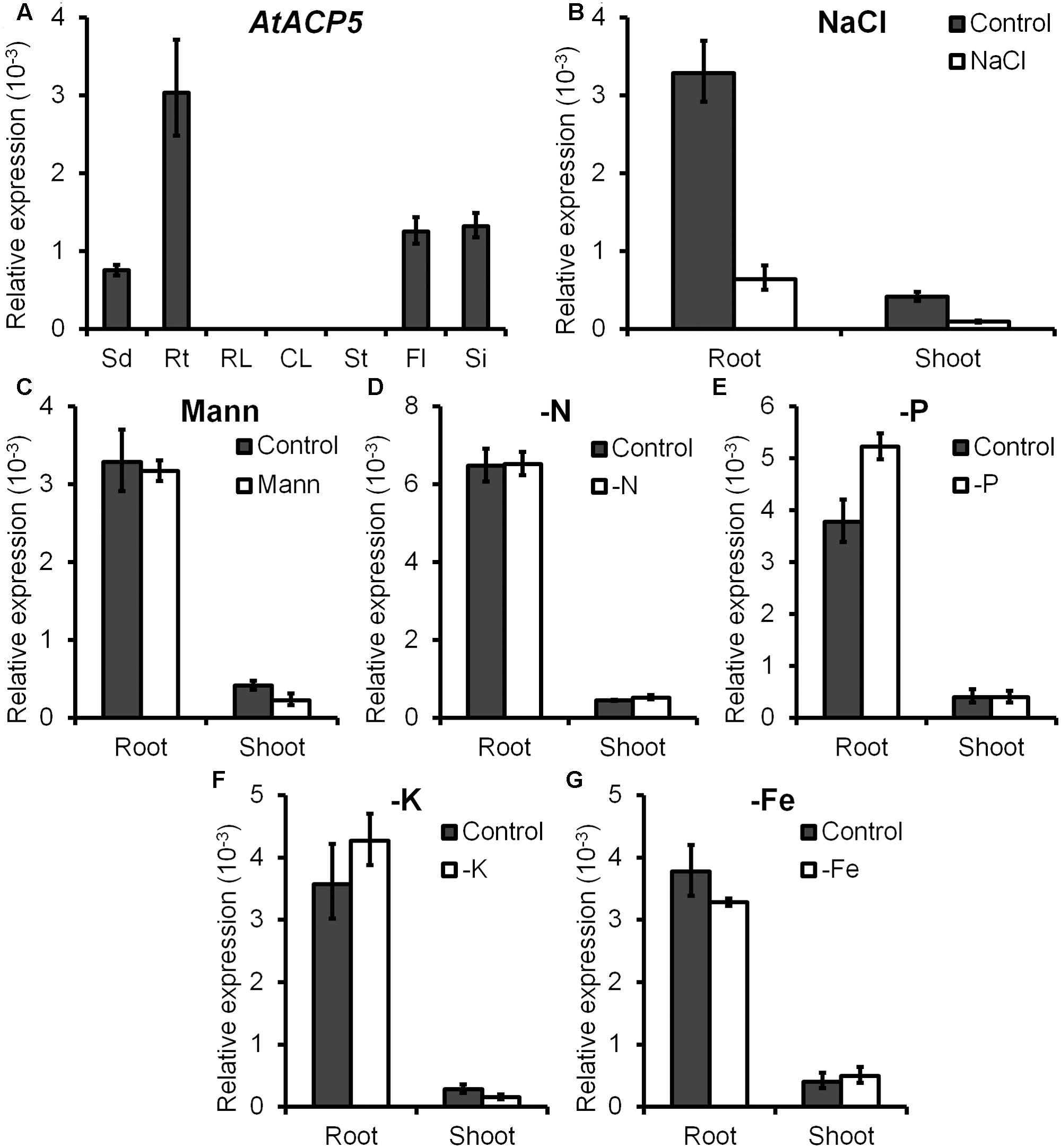
FIGURE 2. AtACP5 is preferentially expressed in root and chiefly repressed under NaCl treatment. Expression analyses of AtACP5 in the wild-type Col-0 plants. (A) Quantitative real-time RT-PCR (qRT-PCR) analyses of AtACP5 transcripts. Total RNA was extracted from 7-day-old seedlings (Sd), roots of 14-day-old seedlings grown on ES agar media (Rt), and rosette leaves (RL), cauline leaves (CL), stems (St), flowers (Fl), and young siliques (Si) of 5-week-old plants grown on nutrition soil. (B–G) qRT-PCR analysis of AtACP5 upon treatment with different stresses, including 150 mM NaCl, 300 mM mannitol, nitrogen-, phosphorus-, potassium-, and iron-deficiency. Ten-day-old seedling were grown on ES media and total RNA was extracted from shoots and roots after 3-day-treatment. Expression values were calculated using 2-ΔCT method with TUA3 (tubulin alpha-3, At5g19770) as endogenous control. Data represents the average of three independent experiments ±SD.
Overexpression of AtACP5 Enhances Salt Stress Tolerance
Since AtACP5 specifically responded to salt stress, we therefore further explored a potential function of AtACP5 in the salt stress response using a genetic approach. The mutant SALK_111501C carrying a T-DNA insertion in the second intron of AtACP5 was identified and confirmed to be dramatically reduced at mRNA level, herein named as atacp5 (Supplementary Figure 4). Overexpression lines harboring a 35S::AtACP5 construct were generated, and three homozygous OE lines designated as AtACP5-OE12, AtACP5-OE32, and AtACP5-OE41 with massively increased expression levels were selected for further experiments (Supplementary Figure 4). Twenty-day-old seedlings of the wild-type, atacp5 mutant plants and the three OE lines were grown on ES media supplemented with 0, 125 or 150 mM NaCl for growth analysis (Figure 3A). Growth was divided into four categories: non-germinating, normal (green leaves), sub-healthy (yellowish-green leaves), and dead (chlorophyll bleaching). Under control conditions (ES media), no differences in germination rate and growth status were observed between the three genotypes. However, growing plants on media containing 125 mM NaCl significantly increased the percentage of dead atacp5 mutant plants (21%) when compared with wild-type plants (3%) and OE lines (1–3%). The percentage of normal seedlings listed in descending order were 31–48% for OE lines, 30% for wild-type plants, and 12% for atacp5 mutants. Raising the NaCl concentration to 150 mM decreased growth of all genotypes under investigation. For seedlings carrying the 35S::AtACP5 construct, 13–22% were classified as normal, a rate that was significantly higher than that of the wild-type (Figure 3B). By contrast, among atacp5 mutant plants less than half normal or sub-healthy seedlings were counted relative to wild-type plants. A similar picture was observed with respect to the FW. While under control conditions no significant differences were observed between the genotypes, OE lines grew larger and gained more FW than wild-type plants on both high-salt media; atacp5 plants produced the lowest FW during the experimental period (Figure 3C). Five-day-old seedlings transferred to ES media supplemented with 0 or 125 mM NaCl and grown for 9 days showed similar results (Supplementary Figure 5).
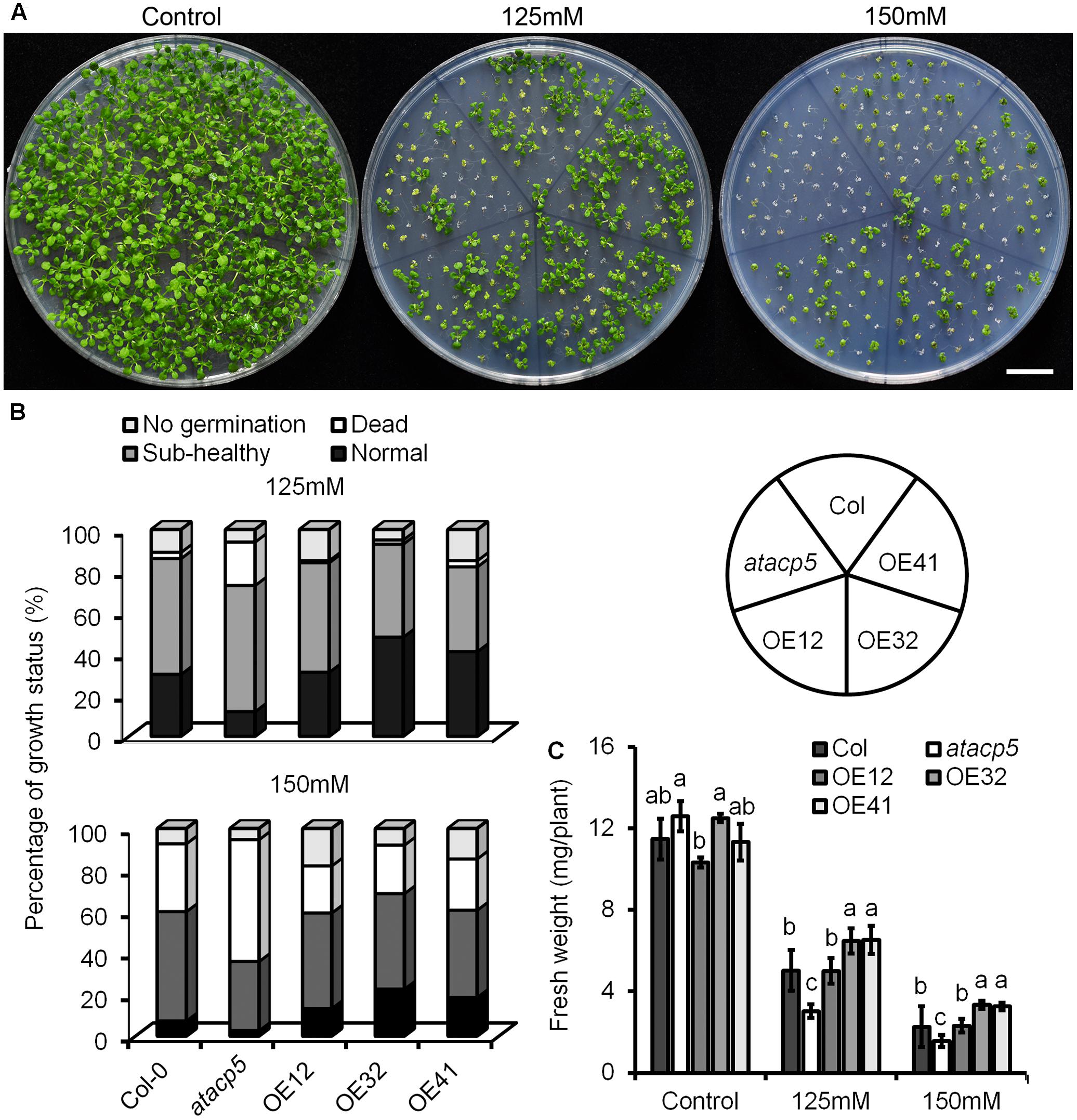
FIGURE 3. NaCl hypersensitive phenotypes of the atacp5 mutant. (A) Plates with 20-day-old seedlings of Col-0, atacp5, OE12, OE32, and OE41 grown in Petri dishes with ES media supplemented with 0 mM (control), 125 mM or 150 mM NaCl. Growth status and survival rate of the salt-stressed seedlings. (B) For each treatment, three plates each containing 50 seedlings for each genotype were scored. Black column represents percentage of normal seedlings, dark gray column represents percentage of sub-healthy ones with yellowish cotyledons, white column represents percentage of white and dead seedlings, and gray column represents ungerminated seeds. (C) Statistical analysis of the fresh weight of whole plant. Illustrated are the mean values ±SD (n = 3). Data from one of two experiments with similar results are shown. Different letters represent significantly different values at P < 0.05 (Duncan’s multiple range test). Scale bar = 2 cm (A).
To assess the sensitivity of mature plants to salt stress, we transferred 10-day-old seedlings from media to soil for additional 11 days with subsequent NaCl treatment (Figure 4A). While wild-type and mutant plants displayed progressive chlorosis, reduced leaf size and general growth inhibition when watered with a NaCl-containing solution, significantly higher FW of rosette leaves from OE lines relative to the wild-type indicated increased tolerance to NaCl (Figure 4B).
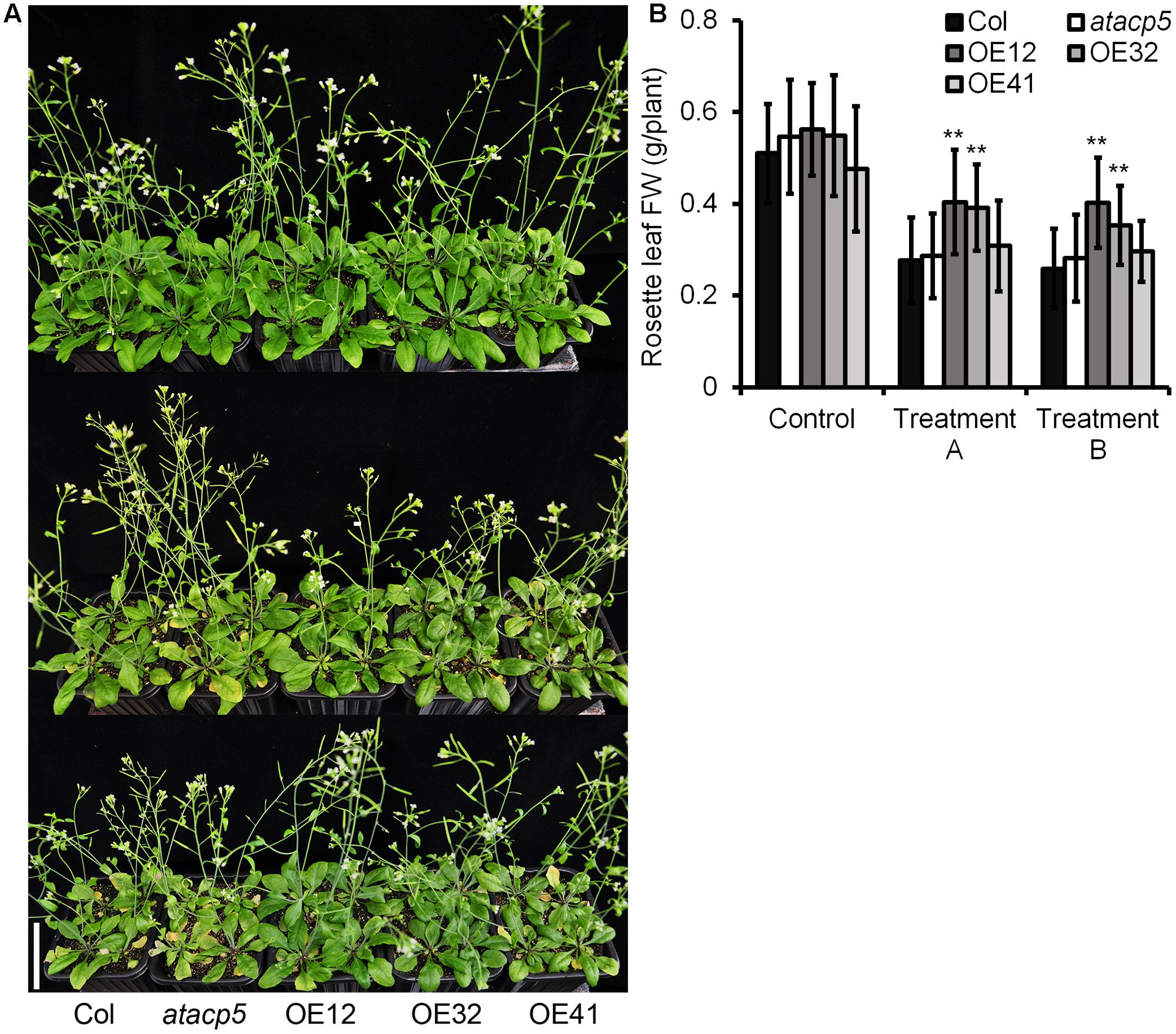
FIGURE 4. NaCl tolerance phenotypes of the overexpressing AtACP5. Wild-type Col-0, mutant atacp5, three overexpression transgenic lines OE12, OE32, and OE41 were used in assessing salt tolerance. Each plant line each set of 60 plants was divided into three groups (Control, treatment A, and treatment B) of 20 plants each. We applied 25 ml water every other day over the 16-day watering treatment. The control group received no NaCl supplementation. The remaining groups were watered with NaCl solution. Treatment A: the concentrations of NaCl supplementation were increased stepwise by 83.3 mM every other days for each line, to the indicated maximum 250 mM NaCl; Treatment B: salt solution was added once to the indicated maximum 250 mM NaCl. (A) Photographs after 16-day salt treatment (upper, control; middle, treatment A; down, treatment B). (B) Statistical analysis of the fresh weight of the rosette leaves. Illustrated are the mean values ± SD (n = 20). Data from one of two experiments with similar results are shown. Statistical analysis was performed by using an unpaired two-tailed Student’s t-test: ∗∗P < 0.01. Scale bar = 5 cm (A).
Overexpression of AtACP5 Results in Decreased Oleic and Increased Palmitic Acid Levels under Salt Stress
Acyl carrier protein is a critical cofactor for FA biosynthesis. In order to investigate the effect of AtACP5 on FA levels, we analyzed the major FA species in 10-day-old seedlings by GC. Analysis of FA composition revealed that in OE lines the levels of monounsaturated FAs (oleic acid, C18:1n9c) under both control condition and salt stress decreased by approximately 20 and 40%, respectively, whereas the levels of SFAs (palmitic acid, C16:0) increased under salt stress compared to wild-type plants (Figures 5A,B). No differences in patterns were observed for seedlings FAMEs in the atacp5 mutant lines compared to Col-0, probably due to the fact that this gene product is primarily limited in root and low abundance. Notably, the percentage of SFAs (C16:0 and C18:0) increased differentially between the two growth types, whereas the percentage of UFAs (C18:1, C18:2, and C18:3) decreased in all plants under salt stress relative to control conditions (Figure 5C). This suggests that increased levels of SFAs (C16:0) and reduced concentrations of UFAs (C18:1) improved the salt tolerance of OE lines.
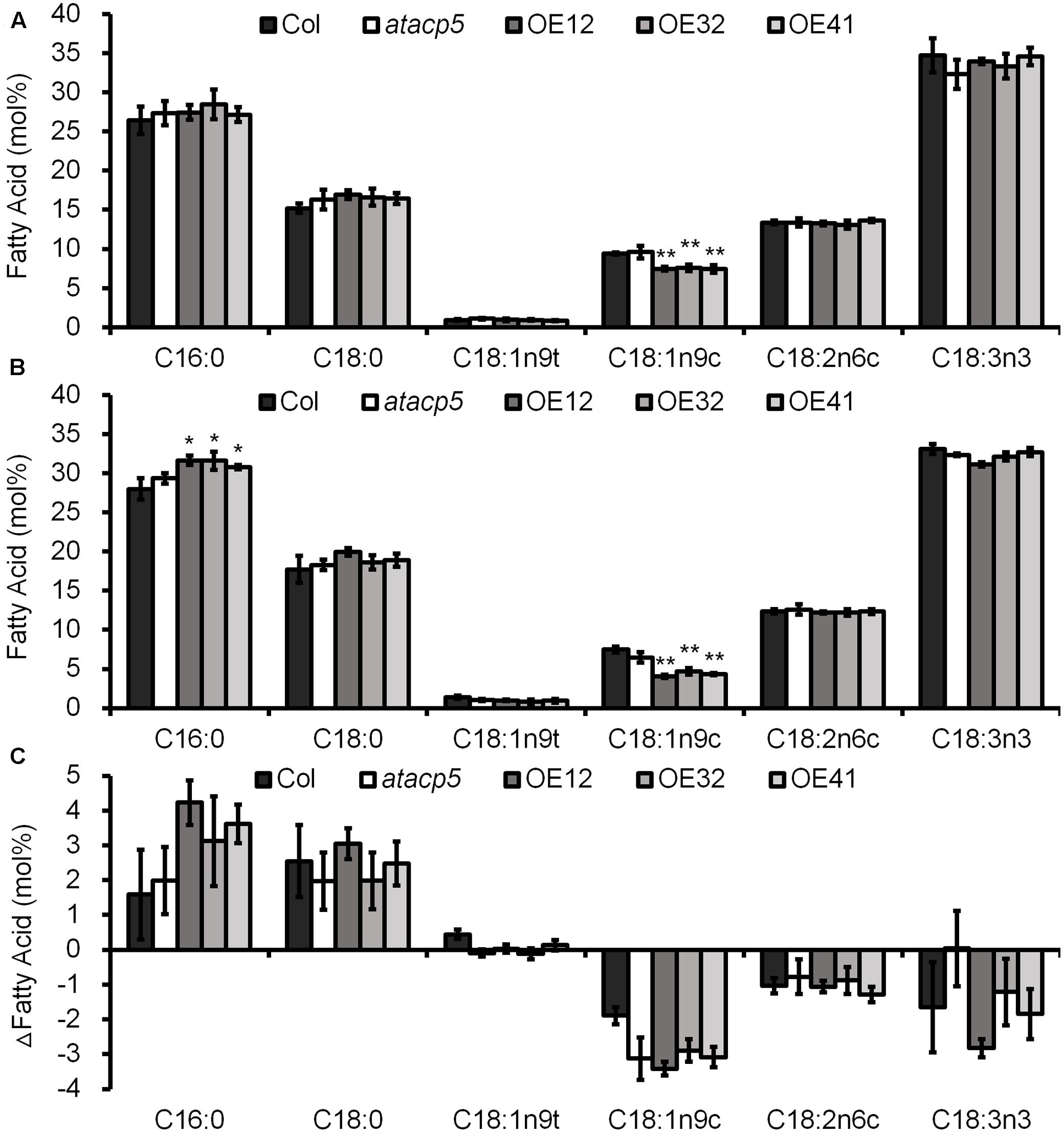
FIGURE 5. Overexpressing AtACP5 caused decreased seedlings C18:1n9c fatty acids. Fatty acid analyses of wild-type Col-0, mutant atacp5, and three overexpression transgenic lines OE12, OE32, and OE41. Ten-day-old seedlings germinated and grown in Petri dishes with ES media supplemented with 0 (control, A), 125 mM NaCl (B). The ΔFatty acid (difference value NaCl versus control treatment, C). Error bars represent SD values for three biological replicates (each replicate consisted of 50 seedlings). Statistical analysis was performed by using an unpaired two-tailed Student’s t-test: ∗P < 0.05, ∗∗P < 0.01.
AtACP5 Is Preferentially Expressed in Roots and Repressed under NaCl Treatment
To decipher the biological function of the five plastidial ACPs in Arabidopsis, we first examined the expression patterns of these genes in wild-type plants grown under normal and various abiotic stress conditions by quantitative real-time RT-PCR (qRT-PCR). Results showed that expression of AtACP5 was detected in all test tissues including seedlings, roots, flowers, and siliques but not in rosette leaves, cauline leaves and stems, with being highest in roots (Figure 2A). Consistent with previous protein analyses, transcripts of AtACP1, AtACP2, and AtACP3 were present in all tissues (Supplementary Figure 2) (Hlousekradojcic et al., 1992). Among them, AtACP4 was observed to be the most abundant gene, which was also expressed across all tissues. In line with previous reports (Shintani, 1996), AtACP4 mRNA was most abundant in cauline leaves, rosettes and seedlings (Supplementary Figure 2). To investigate the expression responses of AtACPs to various abiotic stresses, plants were subjected to various treatments followed by qRT-PCR analysis. Upon treatment with 150 mM NaCl, the transcript level of AtACP5 was reduced to 20% of the value observed under control conditions (Figure 2B). However, no significant differences in expression upon other various abiotic treatments were observed for AtACP5 (Figures 2C–G). These results suggested AtACP5 might be involved in salt stress process. Expression levels of AtACP1, AtACP2, and AtACP3 in shoots were up-regulated by drought (300 mM mannitol) (Supplementary Figure 3B), while transcript level of AtACP4 was reduced by nitrogen and iron deficiency (Supplementary Figure 3). These results confirmed that the five AtACPs respond differently to various abiotic stresses and could be associated with multiple processes.
OE Lines Accumulate Less Na+ under High-Salt Conditions
The cytosolic Na+/K+ ratio is a key determinant of plant salinity tolerance. To investigate whether the maintenance of cellular ion homeostasis contributes to improved tolerance of AtACP5 OE lines, we compared the Na+ and K+ concentrations of the wild-type, atacp5 mutant plants and three OE lines. No significant differences in the Na+ and K+ concentration were detected between the genotypes when seedlings were grown in the absence of NaCl (Figure 6). Upon treatment with 125 mM NaCl, OE lines accumulated less Na+ than the wild-type while K+ levels were maintained, leading to a significantly decreased Na+/K+ ratio in OE lines (Figure 6).
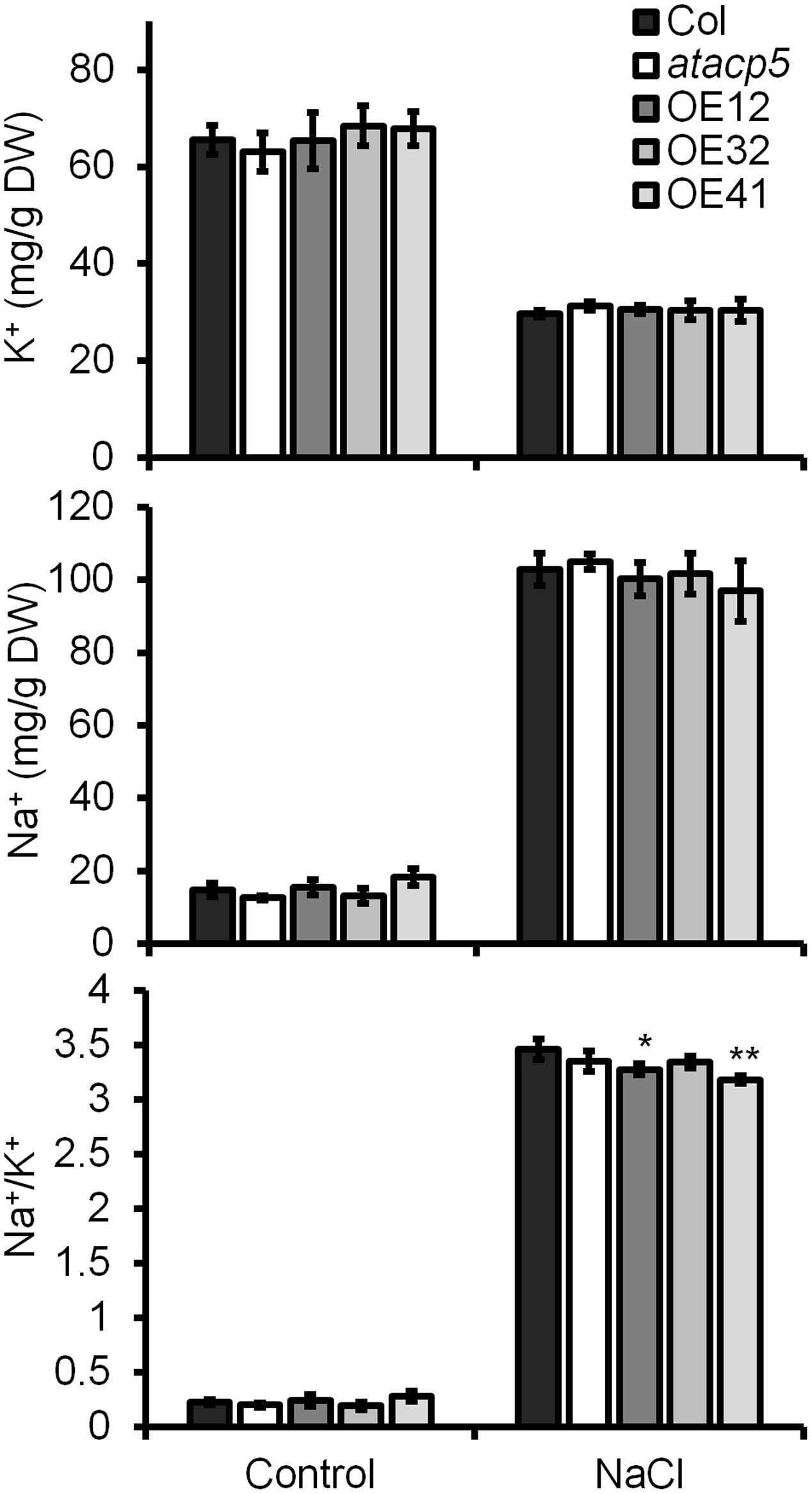
FIGURE 6. Overexpression (OE) lines accumulates less Na+ relative to Col-0 and atacp5 in media supplemented with NaCl. Ten-day-old seedlings grown on ES media supplemented without (control) or with 125 mM NaCl (NaCl) were used. Results are presented as means and standard errors from three independent experiments. Statistical analysis was performed by using an unpaired two-tailed Student’s t-test: ∗P < 0.05, ∗∗P < 0.01.
Discussion
Acyl carrier proteins are a family of universal, relatively abundant and highly conserved carriers of acyl, representing the core of the FAS system. Most species encode at least one ACP, but multiple isoforms and even dozens of homologous carrier proteins involved in FAS and secondary metabolism have been reported, suggesting functional diversity. For instance, more than 85 ACPs are encoded in the genome of Streptomyces avermitilis (Ikeda et al., 2003), the soybean (G. max, complex allotetraploid) genome harbors 25 ACPs (Wang et al., 2014). Arabidopsis, with its relatively simple genome, encodes five plastidial and three mitochondrial ACPs (Meyer et al., 2007). Phylogenetic analysis of 517 putative ACP proteins from 43 plant species identified a legume-specific subclade (Wang et al., 2014), supporting the assumption of functional diversification.
In the present investigation, phylogenetic analysis of Arabidopsis ACPs, regardless using neighbor-joining, maximum likelihood or minimum-evolution methods, revealed a subclade comprising all Arabidopsis plastidial ACP proteins (except AtACP4) and putative ACPs from other Brassicaceae (Figure 1), indicating divergence between different families during evolution. The formation of another subclade of AtACP4 suggests that AtACP4 and ACPs from other species unrelated to Brassicaceae might be derived from a different ancient gene. Apart from gene duplication and de novo gene generation, horizontal gene transfer (HGT) is an important way of acquiring new genes. Most HGT events have been occurred in prokaryotes (Gogarten et al., 2002) and unicellular eukaryotes (Keeling and Palmer, 2008), while the HGT is also involved in the evolution of high plant (Bock, 2010). The first example of nuclear HGT in seed plant is a Mu-like element (MULE) transposon between in Setaria species (millets) and in the rice (O. sativa) (Diao et al., 2006). Taken together, the exact mechanism of AtACP4 evolution awaits more evidence. Nevertheless, all Arabidopsis ACPs display the basic features of ACP proteins. Sequence alignment with homologous ACP proteins identified the characteristic and highly conserved LGADSLDTVEIVM motif comprising the DSL motif (Supplementary Figure 1), which represents the recognition site for ACPs (Mofid et al., 2002).
In plants, ACPs are expressed both ubiquitously and in a tissue-specific manner (Battey and Ohlrogge, 1990; Hlousekradojcic et al., 1992). The presence of multiple ACP isoforms and their differential responsiveness to external stimuli indicate that individual ACPs may play different roles in the adaptation to environmental conditions. For example, it was shown that the expression of AtACP4 was induced by light, whereas the abundance of AtACP2 or AtACP3 mRNA was not responsive to the light regime (Bonaventure and Ohlrogge, 2002). In the present study, we found that the expression of AtACP4, but not of the other ACPs under study was down-regulated by nitrogen and iron deficiency (Figure 2D). AtACP4 antisense plants (Branen et al., 2003) and T-DNA insertion mutants (acp4-1 and acp4-2) showed a different degree of leaf bleaching appearance, reduced photosynthetic efficiency, and a decreased concentrations of 16:3 FAs. The lower concentrations of 16:3 FAs are attributed to the lower concentration of 16:3 FAs on the monogalactosyldiacylglycerol (MGDG) moiety, and most of the 16:3 FAs in Arabidopsis leaf tissue are found on the sn-2 position of MGDG (Ajjawi et al., 2010). AtACP4 thus is a major player controlling the biosynthesis of FAs for chloroplast membrane lipids. Similarly, plant experiencing nitrogen or iron deficiency developed similar chlorotic phenotypes. Iron deficiency has been well documented to impair chlorophyll biosynthesis and chloroplast development (Mori, 1999), while N-starvation changes the chloroplast structure associated with alterations in thylakoid membrane structure and degradation of plastidial membrane lipids, leading to a damaged photosynthetic systems (Siaut et al., 2011; Wang et al., 2011). These results suggest that the expression level of AtACP4 positively correlated with the photosynthetic systems status, and probably functions as a hub among light, nitrogen and iron deficiency. The expression of AtACP5 was significantly repressed under salt stress (Figure 2B); while no significant differences in transcript levels were observed under other stresses, especially under drought stress (Figures 2C–G). These results thus indicated that the down-regulated expression of AtACP5 under salt stress might be due to ion toxicity rather than osmotic stress. Consistent with these results, a knock-out T-DNA insertion mutant exhibited increased salt sensitivity whereas AtACP5 OE lines showed increased tolerance to salt treatment (Figures 3, 4), indicating a possible role of AtACP5 in salt stress tolerance.
AtACP5 OE lines accumulated more palmitic acid 16:0 and less oleic acid 18:1 FAs compared with wild-type plants (Figures 5A, B). The major fate of 16:0 and 18:l acyl chains produced in the plastid is to form the hydrophobic portion of glycerolipid molecules, which are components of all cellular membranes (Ohlrogge and Browse, 1995). Surprisingly, atacp5 loss-of-function did not contain less FAs, which could be expected from the observed increase in the OE lines. One possible reason is genetic redundancy; the Arabidopsis genome harbors five plastidial ACPs carrying a highly conserved consensus motif. Overexpression of AtACP1 resulted in decreased concentration of 16:3 and increased levels of 18:3 FAs (Branen et al., 2001); inhibiting the expression of AtACP4 led to reduced 16:3 FA concentration (Branen et al., 2003; Ajjawi et al., 2010). These genes may function in concert with AtACP5 to regulate FA homeostasis. An alternative explanation is that the modification of FA composition is local rather than systemic. The detected FAs in the present study were from 10-day-old intact seedlings rather than from roots, which will dilute the contributions of the roots to the total FAs. Furthermore, AtACP5 is primary expressed in root and could be functional mainly in the roots. In summary, although no significant differences in FA profiles were found between atacp5 and the wild-type, the results from the AtACP5 OE lines suggest that a complex regulatory network of FA metabolism exists in plants and AtACP5 is a key player in FA homeostasis. Future study focusing on the root FA profiles among the three genotypes would expand our understandings toward the functions of AtACP5 in plants.
Fatty acids are major components of membranes and as such part of the mechanisms by which cellular processes are adapted to environmental constraints. Changes in plasma membrane lipids are an adaptation mechanism to salinity in broccoli roots (Lopez-Perez et al., 2009). Studies in cucumber, soybean, and borage (Borago officinalis L.) have reported that salinity caused a significant increase of SFAs and UFAs in thylakoid membranes, plasma membrane, and leaf, respectively (Surjus and Durand, 1996; Jaffel-Hamza et al., 2013; Shu et al., 2015). Similarly, increased salinity was shown to reduce the plasma membrane fluidity in the halophyte Spartina patens calli, which was associated with increased FA saturation (Wu et al., 2005). The amount of 18:3 FAs was found to be reduced under salt stress in salt-tolerant, but not salt-sensitive citrus cells (Gueta-Dahan et al., 1997), wheat (Triticum aestivum) (Mohamed et al., 1994), and soybean (G. max) (Surjus and Durand, 1996). Due to their role in membrane rigidity, SFAs in the membrane bilayer reduces its permeability and fluidity (Van Blitterswijk et al., 1981). Moller et al. (2007) reported that reduction of polyUFAs in membranes contribute to salt tolerance via amelioration of the oxidative effects of salt. On the contrary, some studies reported that increasing UFAs resulted in salt tolerance. In Synechococcus, increasing the expression of the desA gene, which encodes the Δ12 acyl-lipid desaturase involving in the synthesis of diunsaturated FAs, enhanced NaCl tolerance due to an increased ability to repair the photosynthetic and Na+/H+ antiport systems (Allakhverdiev et al., 2001). Application of exogenous linoleic acid exhibited protective effects on tonoplast function in barley seedlings under salt stress (Zhao and Qin, 2005). An increase of UFAs in membrane lipids contributed to an alleviated salt-induced photoinhibition of PSII in Thellungiella halophile (Sui and Han, 2014). However, Chalbi et al. (2013), also revealed that the ability to increase unsaturation in FAs was not a determinant factor for salt resistance in barley species. Nevertheless, in Arabidopsis, the FA desaturases FAD2 and FAD6, which enhance the degree of unsaturation of FAs, were found to be required for salt tolerance (Zhang et al., 2009, 2012). ER-localized FAD2 and plastid-localized FAD6 encode two ω-6 desaturases that convert oleic acid (18:1) to linoleic acid (18:2) by inserting a double bond at the ω-6 position, FAD3 (ER) and FAD7 and FAD8 (plastids) encode three ω-3 desaturases, which convert linoleic acid (18:2) to linolenic acid (18:3) by inserting a double bond at the ω-3 position. Both fad2 and fad6 mutants showed increased cytoplasmic Na+ accumulation and enhanced sensitivity to salt stress due to increased 18:1 and decreased 18:2 FA levels. In agreement with the decreased drought tolerance of transgenic FAD7 antisense tobacco plants (Im et al., 2002), OE of either FAD3 or FAD8 increased tolerance to drought in transgenic tobacco plants (Zhang et al., 2005). Since increased 18:1 FAs resulted in increased sensitivity to salt, it is reasonable to assume that decreased amounts of 18:1 FA levels contribute to salt tolerance. In agreement with previous studies in Arabidopsis mentioned above, in the present study, although the ratio between SFAs and UFAs was increased under salt stress in all genotypes (Figure 5C), OE of AtACP5 further led to an altered composition of FAs, mainly a decrease of oleic acid (C18:1) and an increase of palmitic acid (C16:0), which could explain why OE of AtACP5 can increase salt tolerance. ACPs have been documented to be involved in the biosynthesis of both SFAs and UFAs (Heath and Rock, 1996; Leesong et al., 1996; Feng and Cronan, 2009; Chan and Vogel, 2010). Thus, manipulating the expression of ACPs could be a strategy to increase the tolerance of plants to environmental stresses including salt.
Under saline conditions, rapid influx of Na+ from the soluble phase of the soil into the cytoplasm of cortical cells in plant roots occurs through non-selective cation channels (NSCCs) rather than through the high-affinity K+ transporter HKT1 (Essah et al., 2003). The relatively high permeability for Na+ of many depolarization-activated NSCCs (DA-NSCCs) suggests they are also important contributors to the uptake and translocation of Na+, and thus their function impacts on salt tolerance. Increased 3′,5′-cyclic guanosine monophosphate (cGMP) affected monovalent cation homeostasis during salt stress and reduced Na+ uptake through NSCCs such as CNGC3 (Gobert et al., 2006). Some NSCCs respond to physical stimuli, relaying external signals into electrical and/or Ca2+ signals through the action of mechanosensitive channels (MCs) whose gating depends on changes in tension forces on the membrane. Maintenance of appropriate tension forces depends on membrane integrity and fluidity, which are largely affected by the composition of lipids in general and FAs in particular (Mikami and Murata, 2003). To maintain ion homeostasis, plants could secrete Na+ out of the cell via plasma membrane-bound Na+/H+ antiporters (e.g., SOS1) and sequester Na+ into the vacuole via Na+/H+ antiporters (e.g., NHX1) on the tonoplast (Wu et al., 1996; Apse et al., 1999). In the present study, no significant changes in the transcripts of AtSOS1 and AtNHX1 were detected (Supplementary Figure 6), suggesting that both secreting and sequestering Na+ might not be predominated among genotypes. However, Na+ concentration was slightly reduced while K+ concentration was unchanged in OE lines, leading to a lower Na+/K+ ratio, which might confer OE lines more tolerant to salt stress. The exact mechanisms by which overexpressing AtACP5 increases salt tolerance awaits further study, although it could be possible that ectopic expression of AtACP5 alters the membrane integrity and fluidity through the alterations of FA composition (Figure 5).
Conclusion
We found that Arabidopsis plastidial ACPs are a highly conserved family of FAS system proteins, which respond disparately to different stresses. The atacp5 mutant plants showed increased salt sensitivity associated with a higher mortality rate, weaker growth and lower FW when compared with wild-type plants. By contrast, AtACP5 OE lines performed better than the wild-type in each aspect. The primary difference between OE lines and wild-type plants were cytosolic Na+ concentration and FA composition, which indicates an involvement of AtACP5 in the homeostasis of FAs. These results suggest that the modification of FAs contribute to the maintenance of membrane integrity, which might lead to improved salt tolerance. The findings presented here will facilitate the understanding of the relationship between cellular microenvironment and ever-changing environmental conditions.
Author Contributions
PL designed and supervised this study; RS supervised this study; JH, CX, HW, and LW performed the experiments; WS polished the writing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Professor Zhaoliang Zhu is most appreciated for offering the chance to JH to pursue her doctor’s degree at ISSAS. We thank Professor Wenhua Zhang and Doctor Yang Bo from Nanjing Agriculture University for the fatty acid analysis. This work was supported by the National Key R&D Program (2016YFD0200308), the National Key Basic Research Program of China (No. 2015CB150501), the National Science Foundation in Jiangsu Provinces (BK20141511), and the Project of Priority and Key Areas, ISSCAS (ISSASIP1605). We are grateful to two reviewers for their invaluable comments and suggestions to substantially improve the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00987/full#supplementary-material
Footnotes
References
Ajjawi, I., Lu, Y., Savage, L. J., Bell, S. M., and Last, R. L. (2010). Large-scale reverse genetics in Arabidopsis: case studies from the chloroplast 2010 project. Plant Physiol. 152, 529–540. doi: 10.1104/pp.109.148494
Allakhverdiev, S. I., Kinoshita, M., Inaba, M., Suzuki, I., and Murata, N. (2001). Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. Plant Physiol. 125, 1842–1853. doi: 10.1104/pp.125.4.1842
AOAC (1990). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th Edn. Washington, DC: Association of Official Analytical Chemists.
Apse, M. P., Aharon, G. S., Snedden, W. A., and Blumwald, E. (1999). Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285, 1256–1258. doi: 10.1126/science.285.5431.1256
Battey, J. F., and Ohlrogge, J. B. (1990). Evolutionary and tissue-specific control of expression of multiple Acyl-carrier protein isoforms in plants and bacteria. Planta 180, 352–360. doi: 10.1007/BF00198786
Beisson, F., Li, Y. H., Bonaventure, G., Pollard, M., and Ohlrogge, J. B. (2007). The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19, 351–368. doi: 10.1105/tpc.106.048033
Blee, E. (2002). Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 7, 315–322. doi: 10.1016/S1360-1385(02)02290-2
Bock, R. (2010). The give-and-take of DNA: horizontal gene transfer in plants. Trends Plant Sci. 15, 11–22. doi: 10.1016/j.tplants.2009.10.001
Bonaventure, G., and Ohlrogge, J. B. (2002). Differential regulation of mRNA levels of acyl carrier protein isoforms in Arabidopsis. Plant Physiol. 128, 223–235. doi: 10.1104/pp.010397
Branen, J. K., Chiou, T. J., and Engeseth, N. J. (2001). Overexpression of acyl carrier protein-1 alters fatty acid composition of leaf tissue, in Arabidopsis. Plant Physiol. 127, 222–229. doi: 10.1104/pp.127.1.222
Branen, J. K., Shintani, D. K., and Engeseth, N. J. (2003). Expression of antisense acyl carrier protein-4 reduces leaf lipid content in Arabidopsis tissue. Plant Physiol. 132, 748–756. doi: 10.1104/pp.102.018622
Chalbi, N., Hessini, K., Gandour, M., Mohamed, S. N., Smaoui, A., Abdelly, C., et al. (2013). Are changes in membrane lipids and fatty acid composition related to salt-stress resistance in wild and cultivated barley? J. Plant Nutr. Soil Sci. 176, 138–147. doi: 10.1002/jpln.201100413
Chan, D. I., and Vogel, H. J. (2010). Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem. J. 430, 1–19. doi: 10.1042/BJ20100462
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
Cronan, J. E. Jr. (1967). The unsaturated fatty acids of Escherichia coli. Biochim. Biophys. Acta 144, 695–697. doi: 10.1016/0005-2760(67)90063-X
Diao, X., Freeling, M., and Lisch, D. (2006). Horizontal transfer of a plant transposon. PLoS Biol. 4:e5. doi: 10.1371/journal.pbio.0040005
Essah, P. A., Davenport, R., and Tester, M. (2003). Sodium influx and accumulation in Arabidopsis. Plant Physiol. 133, 307–318. doi: 10.1104/pp.103.022178
Estelle, M. A., and Somerville, C. R. (1987). auxin resistant mutants of arabidopsis with an altered morphology. J. Cell. Biochem. 206, 200–206. doi: 10.1007/bf00333575
Feng, Y., and Cronan, J. E. (2009). Escherichia coli unsaturated fatty acid synthesis: complex transcription of the fabA gene and in vivo identification of the essential reaction catalyzed by FabB. J. Biol. Chem. 284, 29526–29535. doi: 10.1074/jbc.M109.023440
Gobert, A., Park, G., Amtmann, A., Sanders, D., and Maathuis, F. J. (2006). Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. J. Exp. Bot. 57, 791–800. doi: 10.1093/jxb/erj064
Gogarten, J. P., Doolittle, W. F., and Lawrence, J. G. (2002). Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 19, 2226–2238. doi: 10.1093/oxfordjournals.molbev.a004046
Gueta-Dahan, Y., Yaniv, Z., Zilinskas, B. A., and Ben-Hayyim, G. (1997). Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in citrus. Planta 203, 460–469. doi: 10.1007/s004250050215
Heath, R. J., and Rock, C. O. (1996). Roles of the FabA and FabZ beta-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J. Biol. Chem. 271, 27795–27801. doi: 10.1074/jbc.271.44.27795
Hlousekradojcic, A., Postbeittenmiller, D., and Ohlrogge, J. B. (1992). Expression of constitutive and tissue-specific acyl carrier protein isoforms in Arabidopsis. Plant Physiol. 98, 206–214. doi: 10.1104/pp.98.1.206
Huynh, V. B., Repellin, A., Zuily-Fodil, Y., and Pham-Thi, A. T. (2012). Aluminum stress response in rice: effects on membrane lipid composition and expression of lipid biosynthesis genes. Physiol. Plant. 146, 272–284. doi: 10.1111/j.1399-3054.2012.01622.x
Iba, K. (2002). Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol. 53, 225–245. doi: 10.1146/annurev.arplant.53.100201.160729
Ikeda, H., Ishikawa, J., Hanamoto, A., Shinose, M., Kikuchi, H., Shiba, T., et al. (2003). Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21, 526–531. doi: 10.1038/nbt820
Im, Y. J., Han, O., Chung, G. C., and Cho, B. H. (2002). Antisense expression of an Arabidopsis omega-3 fatty acid desaturase gene reduces salt/drought tolerance in transgenic tobacco plants. Mol. Cells 13, 264–271.
Jaffel-Hamza, K., Sai-Kachout, S., Harrathi, J., Lachaal, M., and Marzouk, B. (2013). Growth and fatty acid composition of borage (Borago officinalis L.) leaves and seeds cultivated in saline medium. J. Plant Growth Regul. 32, 200–207. doi: 10.1007/s00344-012-9290-8
Jenke-Kodama, H., Sandmann, A., Muller, R., and Dittmann, E. (2005). Evolutionary implications of bacterial polyketide synthases. Mol. Biol. Evol. 22, 2027–2039. doi: 10.1093/molbev/msi193
Kachroo, P., Shanklin, J., Shah, J., Whittle, E. J., and Klessig, D. F. (2001). A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc. Natl. Acad. Sci. U.S.A. 98, 9448–9453. doi: 10.1073/pnas.151258398
Keeling, P. J., and Palmer, J. D. (2008). Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 9, 605–618. doi: 10.1038/nrg2386
Lan, P., Li, W., and Schmidt, W. (2012). Complementary proteome and transcriptome profiling in phosphate-deficient Arabidopsis roots reveals multiple levels of gene regulation. Mol. Cell. Proteom. 11, 1156–1166. doi: 10.1074/mcp.M112.020461
Leesong, M., Henderson, B. S., Gillig, J. R., Schwab, J. M., and Smith, J. L. (1996). Structure of a dehydratase-isomerase from the bacterial pathway for biosynthesis of unsaturated fatty acids: two catalytic activities in one active site. Structure. 4, 253–264. doi: 10.1016/S0969-2126(96)00030-5
Li, Y., Beisson, F., Pollard, M., and Ohlrogge, J. (2006). Oil content of Arabidopsis seeds: the influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 67, 904–915. doi: 10.1016/j.phytochem.2006.02.015
Lopez-Perez, L., Martinez-Ballesta, M. D., Maurel, C., and Carvajal, M. (2009). Changes in plasma membrane lipids, aquaporins and proton pump of broccoli roots, as an adaptation mechanism to salinity. Phytochemistry 70, 492–500. doi: 10.1016/j.phytochem.2009.01.014
Maejima, E., Watanabe, T., Osaki, M., and Wagatsuma, T. (2014). Phosphorus deficiency enhances aluminum tolerance of rice (Oryza sativa) by changing the physicochemical characteristics of root plasma membranes and cell walls. J. Plant Physiol. 171, 9–15. doi: 10.1016/j.jplph.2013.09.012
Maeo, K., Tokuda, T., Ayame, A., Mitsui, N., Kawai, T., Tsukagoshi, H., et al. (2009). An AP2-type transcription factor, WRINKLED1, of Arabidopsis thaliana binds to the AW-box sequence conserved among proximal upstream regions of genes involved in fatty acid synthesis. Plant J. 60, 476–487. doi: 10.1111/j.1365-313X.2009.03967.x
Marrakchi, H., Zhang, Y. M., and Rock, C. O. (2002). Mechanistic diversity and regulation of type II fatty acid synthesis. Biochem. Soc. Trans. 30, 1050–1055. doi: 10.1042/bst0301050
Menges, M., Hennig, L., Gruissem, W., and Murray, J. A. (2002). Cell cycle-regulated gene expression in Arabidopsis. J. Biol. Chem. 277, 41987–42002. doi: 10.1074/jbc.M207570200
Meyer, E. H., Heazlewood, J. L., and Millar, A. H. (2007). Mitochondrial acyl carrier proteins in Arabidopsis thaliana are predominantly soluble matrix proteins and none can be confirmed as subunits of respiratory Complex I. Plant Mol. Biol. 64, 319–327. doi: 10.1007/s11103-007-9156-9
Mikami, K., and Murata, N. (2003). Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog. Lipid Res. 42, 527–543. doi: 10.1016/S0163-7827(03)00036-5
Miquel, M., James, D. Jr., Dooner, H., and Browse, J. (1993). Arabidopsis requires polyunsaturated lipids for low-temperature survival. Proc. Natl. Acad. Sci. U.S.A. 90, 6208–6212. doi: 10.1073/pnas.90.13.6208
Mofid, M. R., Finking, R., and Marahiel, M. A. (2002). Recognition of hybrid peptidyl carrier proteins/acyl carrier proteins in nonribosomal peptide synthetase modules by the 4’-phosphopantetheinyl transferases AcpS and Sfp. J. Biol. Chem. 277, 17023–17031. doi: 10.1074/jbc.M200120200
Mohamed, M., Mansour, F., van Hasselt, P. R., and Kuiper, P. J. C. (1994). Plasma membrane lipid alterations induced by NaCl in winter wheat roots. Physiol. Plant. 92, 473–478. doi: 10.1111/j.1399-3054.1994.tb08838.x
Moire, L., Rezzonico, E., Goepfert, S., and Poirier, Y. (2004). Impact of unusual fatty acid synthesis on futile cycling through beta-oxidation and on gene expression in transgenic plants(1[w]). Plant Physiol. 134, 432–442. doi: 10.1104/pp.103.032938
Moller, I. M., Jensen, P. E., and Hansson, A. (2007). Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 58, 459–481. doi: 10.1146/annurev.arplant.58.032806.103946
Morein, S., Andersson, A., Rilfors, L., and Lindblom, G. (1996). Wild-type Escherichia coli cells regulate the membrane lipid composition in a “window” between gel and non-lamellar structures. J. Biol. Chem. 271, 6801–6809. doi: 10.1074/jbc.271.12.6801
Mori, S. (1999). Iron acquisition by plants. Curr. Opin. Plant Biol. 2, 250–253. doi: 10.1016/S1369-5266(99)80043-0
Ohlrogge, J., and Browse, J. (1995). Lipid biosynthesis. Plant Cell 7, 957–970. doi: 10.1105/tpc.7.7.957
Rashotte, A. M., Carson, S. D. B., To, J. P. C., and Kieber, J. J. (2003). Expression profiling of cytokinin action in Arabidopsis. Plant Physiol. 132, 1998–2011. doi: 10.1104/pp.103.021436
Rodriguez-Vargas, S., Sanchez-Garcia, A., Martinez-Rivas, J. M., Prieto, J. A., and Randez-Gil, F. (2007). Fluidization of membrane lipids enhances the tolerance of Saccharomyces cerevisiae to freezing and salt stress. Appl. Environ. Microbiol. 73, 110–116. doi: 10.1128/AEM.01360-06
Ruuska, S. A., Girke, T., Benning, C., and Ohlrogge, J. B. (2002). Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell 14, 1191–1206. doi: 10.1105/tpc.000877
Shintani, D. (1996). How Plants Manage Their Fatty Assets: A Study into the Organization and Regulation of the Plant Fatty Acid Biosynthetic Pathway. Ph.D. thesis, Michigan State University, Lansing, MI.
Shu, S., Yuan, Y. H., Chen, J., Sun, J., Zhang, W. H., Tang, Y. Y., et al. (2015). The role of putrescine in the regulation of proteins and fatty acids of thylakoid membranes under salt stress. Sci. Rep. 5:14390. doi: 10.1038/srep14390
Siaut, M., Cuine, S., Cagnon, C., Fessler, B., Nguyen, M., Carrier, P., et al. (2011). Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 11:7. doi: 10.1186/1472-6750-11-7
Smith, J. L., and Sherman, D. H. (2008). Biochemistry, An enzyme assembly line. Science 321, 1304–1305. doi: 10.1126/science.1163785
Sui, N., and Han, G. L. (2014). Salt-induced photoinhibition of PSII is alleviated in halophyte Thellungiella halophila by increases of unsaturated fatty acids in membrane lipids. Acta Physiol. Plant. 36, 983–992. doi: 10.1007/s11738-013-1477-5
Surjus, A., and Durand, M. (1996). Lipid changes in soybean root membranes in response to salt treatment. J. Exp. Bot. 47, 17–23. doi: 10.1093/jxb/47.1.17
Van Blitterswijk, W. J., Van Hoeven, R. P., and Van der Meer, B. W. (1981). Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim. Biophys. Acta 644, 323–332. doi: 10.1016/0005-2736(81)90390-4
Wakil, S. J., Stoops, J. K., and Joshi, V. C. (1983). Fatty-acid synthesis and its regulation. Annu. Rev. Biochem. 52, 537–579. doi: 10.1146/annurev.bi.52.070183.002541
Wang, J., Toth, K., Tanaka, K., Nguyen, C. T., Yan, Z., Brechenmacher, L., et al. (2014). A soybean acyl carrier protein, GmACP, is important for root nodule symbiosis. Mol. Plant Microbe. Interact. 27, 415–423. doi: 10.1094/MPMI-09-13-0269-R
Wang, L. P., Shen, W. Y., Kazachkov, M., Chen, G. Q., Chen, Q. L., Carlsson, A. S., et al. (2012). Metabolic interactions between the lands cycle and the kennedy pathway of glycerolipid synthesis in Arabidopsis developing seeds. Plant Cell 24, 4652–4669. doi: 10.1105/tpc.112.104604
Wang, S., Pan, Y., Liu, C., Chuang, L., and Chen, C. (2011). Characterization of a green microalga UTEX 2219-4: effects of photosynthesis and osmotic stress on oil body formation. Bot. Stud. 52, 305–312.
Wu, J., Seliskar, D. M., and Gallagher, J. L. (2005). The response of plasma membrane lipid composition in callus of the halophyte Spartina patens (Poaceae) to salinity stress. Am. J. Bot. 92, 852–858. doi: 10.3732/ajb.92.5.852
Wu, S. J., Ding, L., and Zhu, J. K. (1996). SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8, 617–627. doi: 10.1105/tpc.8.4.617
Xia, Y., Gao, Q. M., Yu, K. S., Lapchyk, L., Navarre, D., Hildebrand, D., et al. (2009). An intact cuticle in distal tissues is essential for the induction of systemic acquired resistance in plants. Cell Host Microbe 5, 151–165. doi: 10.1016/j.chom.2009.01.001
Zhang, J. T., Liu, H., Sun, J., Li, B., Zhu, Q., Chen, S. L., et al. (2012). Arabidopsis fatty acid desaturase FAD2 is required for salt tolerance during seed germination and early seedling growth. PLoS ONE 7:e30355. doi: 10.1371/journal.pone.0030355
Zhang, J. T., Zhu, J. Q., Zhu, Q., Liu, H., Gao, X. S., and Zhang, H. X. (2009). Fatty acid desaturase-6 (Fad6) is required for salt tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 390, 469–474. doi: 10.1016/j.bbrc.2009.09.095
Zhang, M., Barg, R., Yin, M. G., Gueta-Dahan, Y., Leikin-Frenkel, A., Salts, Y., et al. (2005). Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 44, 361–371. doi: 10.1111/j.1365-313X.2005.02536.x
Keywords: acyl carrier proteins, salt stress, fatty acids, Arabidopsis, abiotic stress
Citation: Huang J, Xue C, Wang H, Wang L, Schmidt W, Shen R and Lan P (2017) Genes of ACYL CARRIER PROTEIN Family Show Different Expression Profiles and Overexpression of ACYL CARRIER PROTEIN 5 Modulates Fatty Acid Composition and Enhances Salt Stress Tolerance in Arabidopsis. Front. Plant Sci. 8:987. doi: 10.3389/fpls.2017.00987
Received: 20 March 2017; Accepted: 24 May 2017;
Published: 08 June 2017.
Edited by:
Shucai Wang, Northeast Normal University, ChinaReviewed by:
Klaas J. Jan Hellingwerf, University of Amsterdam, NetherlandsShiyou Lu, Wuhan Botanical Garden (CAS), China
Copyright © 2017 Huang, Xue, Wang, Wang, Schmidt, Shen and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Lan, plan@issas.ac.cn
 Jiexue Huang
Jiexue Huang Caiwen Xue1,2
Caiwen Xue1,2 Han Wang
Han Wang Wolfgang Schmidt
Wolfgang Schmidt Renfang Shen
Renfang Shen Ping Lan
Ping Lan