- State Key Laboratory for Biocontrol, School of Life Science, Sun Yat-sen University, Guangzhou, China
Seed setting rate is one of the most important components of rice grain yield. To date, only several genes regulating setting rate have been identified in plant. In this study, we showed that laccase-13 (OsLAC13), a member of laccase family genes which are known for their roles in modulating phenylpropanoid pathway and secondary lignification in cell wall, exerts a regulatory function in rice seed setting rate. OsLAC13 expressed in anthers and promotes hydrogen peroxide production both in vitro and in the filaments and anther connectives. Knock-out of OsLAC13 showed significantly increased seed setting rate, while overexpression of this gene exhibited induced mitochondrial damage and suppressed sugar transportation in anthers, which in turn affected seed setting rate. OsLAC13 also induced H2O2 production and mitochondrial damage in the root tip cells which caused the lethal phenotype. We also showed that high abundant of OsmiR397, the suppressor of OsLAC13 mRNA, increased the seed setting rate of rice plants, and restrains H2O2 accumulation in roots during oxidative stress. Our results suggested a novel regulatory role of OsLAC13 gene in regulating seed setting rate by affecting H2O2 dynamics and mitochondrial integrity in rice.
Introduction
Rice is one of the most important food crops. Grain size, grain number, and panicle number are the determinants of rice grain yield. Seed setting rate determines grain number and is susceptive to environmental conditions, which often lead to decrease of rice yield. Several genes related to seed setting rate have been reported in rice, such as PTB1 positively regulate seed setting rate by controlling pollen tube growth (Li et al., 2013), GSD1 affects seed setting rate through regulating plasmodesmatal conductance (Gui et al., 2014), and THIS1 regulates both seed setting and plant architecture (Liu et al., 2013). In higher plants, male reproductive organogenesis requires the establishment of anthers and filaments. Abnormal reproductive organogenesis also reduces seed setting rate. For example, OsSPX1, a rice SPX domain gene, is involved in anther and pollen development. Down-regulation of OsSPX1 leads to semi-male sterility and ultimately resulted in low seed-setting rate and grain yield (Zhang et al., 2016). Knock-out of GSL5 which encodes the callose synthase disrupts normal microspore development during late meiosis and exhibits a severe reduction of seed setting rate (Shi et al., 2015). At the late stage of pollen maturation, starch accumulates in the pollen as an energy reserve for germination. Thus, starch accumulation serves as a marker of pollen maturity (Datta et al., 2002). As a non-photosynthetic organ, the anther obtains sugars mainly from source organs such as leaves and sink organs such as lemma and palea (Goetz et al., 2001). The connective attaches the anther to the filament, which acts as the conduit and provides a link for vascular transport of photosynthetic sugars and other essential nutrients to the anther and the sugars deposited as starch in the pollen provide energy for development following pollination (Cardarelli and Cecchetti, 2014). However, the importance of filaments in anther development and male fertility has not been studied in detail.
Plants generate reactive oxygen species (ROS) by using molecular oxygen as a terminal electron acceptor, creating molecules such as superoxide anion (O2-), hydroxyl radicals (OH-), and hydrogen peroxide (H2O2) (Hu et al., 2011). ROS are highly reactive and toxic in damaging proteins, lipids, DNA, and carbohydrates (Gill and Tuteja, 2010). Moreover, recent work has identified ROS, particularly H2O2, as important second messengers in signal transduction networks that regulate plant developmental processes such as cell expansion, polar growth, flower development, and stress responses (Alvarez et al., 1998; Skopelitis et al., 2006). Notably, several recent studies showed that ROS affect pollen maturation and male fertility by accumulating in the tapetum and pollen tube (Wu et al., 2010; Hu et al., 2011; Xie et al., 2014), suggesting that ROS serve as important regulatory molecules for male reproductive development.
As one of the evolutionarily oldest enzymes in both fungi and plants, laccases (LACs) have been studied for years. Most studies on plant laccases have mainly focused on secondary lignification in cell walls (Berthet et al., 2011; Lu et al., 2013; Zhao et al., 2013; Wang et al., 2014; Bryan et al., 2016), via the phenylpropanoid pathway (Vogt, 2010). However, LACs have a wide range of substrates and thus might have diverse and complicated functions. Indeed, recent studies showed that LACs in higher plants have more varied functions than expected. For example, our recent study showed that LACs regulate grain yield in both rice and Arabidopsis thaliana (Zhang et al., 2013; Wang et al., 2014). Other LAC genes also affect seed coat color and nutrient transportation in Arabidopsis (Turlapati et al., 2011), suggesting that LACs affect important plant traits.
In this study, we reported a novel function of OsLAC13 in regulating seed setting rate and H2O2 dynamics in rice. Knock-out of OsLAC13 increases seed setting rate. By contrast, higher expression level of OsLAC13 reduces seed setting rate dramatically by inducing H2O2 accumulation in filaments and anther connectives and suppressing the maturation of pollen grains. The integrity of mitochondria was damaged both in the phloem cells of vascular tissue and in the root tip cells when elevating the expression level of OsLAC13. We also showed that OsLAC13 is under the regulation of OsmiR397 during anther development and stress response of rice plants. Our data therefore report a novel regulatory role of OsLAC13 gene in regulating H2O2 dynamics and seed setting rate in rice.
Materials and Methods
Plant Growth Conditions and Generation of Transgenic Rice Plants
The growth conditions and generation of transgenic plants were conducted as described by Zhang et al. (2013). Briefly, the Zhonghua 11 (Oryza sativa japonica) rice cultivar was used in the experiments. Rice plants were grown in the field in Guangzhou, China (23°08′ N, 113°18′ E), where the growing season extends from late April to late September. The average low temperature range is ∼22.9–25.5°C, and the average high temperature range is ∼29.7–32.9°C. The day length ranged from 12 to 13.5 h. Plants were maintained with routine management practices. OsLAC13, pre-OsmiR397a, pre-OsmiR397b, and pre-mmiR397 (pre-mmiR397 contains several mismatches to the OsLAC binding site but can also produce a 21-nt small RNA) were overexpressed under the control of the CaMV35S promoter. The OsLAC13-RNAi transgenic plants were generated using pRNAi-35S vectors, which cloned the OsLAC13 gene fragments in the sense and antisense orientations, and the construct expressing the RNA hairpin was driven by the CaMV35S promoter. T3 seeds that were homozygous for the transgene were harvested and several lines with high expression levels were used for further analysis. The OsLAC13 knock-out muntants were constract by CRISPR-Cas9 based genome editing technology as discribed (Ma et al., 2015). The primers are as follows. Target site 1: 5′-ggcAgcagcaacgaagaacagagg-3′ and 3′-cgtcgttgcttcttgtctcccaaa-5′. Target site 2: 5′-gccGtacgtgtgcgtgcaggcac-3′ and 3′-atgcacacgcacgtccgtgcaaa-5′.
DAPI Staining
The 4′,6-diamidino-2-phenylindole (DAPI) staining was performed essentially as described previously, with minor modifications (Ross et al., 1996). The fixed tissue was washed twice with water and twice with 10 mM citrate buffer, pH 4.5. Four to six anthers were placed in a small drop of 60% acetic acid on a slide and pressed with another slide to release microspore mother cells. The slides were then separated and the samples dried at room temperature for 5 min. A total of 5 μL DAPI solution (1 μg/mL DAPI in a buffer with 50% glycerol and 10 mM citrate, pH 4.5) was placed onto the slide, which was then covered with a cover glass and sealed with nail polish. Slides were examined under a fluorescence microscope (Leica DM5000B).
Transmission Electron Microscopy
Samples were fixed in 5% (w/v) glutaraldehyde, 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.0, and were then post-fixed in 2% OsO4 in PBS, pH 7.2. Following ethanol dehydration, samples were embedded in acrylic resin. Ultrathin sections (50–70 nm) were double-stained with 2% (w/v) uranyl acetate and 2.6% (w/v) lead citrate aqueous solution and examined with a JEOL JEM – 100CX II transmission electron microscope.
In Vitro Pollen Germination Assay
Pollen germination tests were performed as described previously (Zhou et al., 2011). Briefly, pollen grains were placed on a clean cover glass, and 20 μL of Brewbaker and Kwach medium (10% sucrose, 100 mg/L boric acid, 300 mg/L calcium nitrate, 200 mg/L magnesium sulfate, and 100 mg/L potassium nitrate) were added. The cover glass was placed in a humid dish, and incubated for 60 min at 25°C in the dark. The pollen grains were then observed under a microscope. Pollen grains with a pollen tube elongated longer than the diameter of the pollen grain were scored as successful germination.
Soluble Sugar Assays by GC-MS and Measurement of Starch
Metabolites were analyzed essentially as described, with modifications (Lisec et al., 2006). Briefly, about 50 mg (fresh weight) of anther, lemma, palea, or flag leaf was harvested and ground into a fine powder in liquid nitrogen. To stop enzymatic activity, 700 μL methanol was immediately added to the powder and 120 μL of 0.2 mg/mL rabitol (Sigma–Aldrich) was then added. The mixture was shaken at 950 RPM at 70°C for 10 min. After centrifugation at 11,000 g for 10 min, the supernatant was transferred to a new tube and dried for sugar assays; the remaining pellet was used to assay starch contents using a starch assay kit (product number SA20-1KT; Sigma–Aldrich). For sugar assays, 40 μL of methoxyamination reagent was used at 37°C for 2 h. Afterward, 40 μL of N-methyl-N-(trimethylsilyl) trifluoroacetamide was added, and the mixture was incubated at 37°C for 30 min. GC–MS analysis was performed using an Agilent 6890 series gas chromatograph fitted with a capillary column (0.25 mm × 30 m, 0.25 mm film thickness [HP–5MS]). The gas chromatograph was combined with a quadrupole mass selective detector (Agilent).
Histochemical Assays of Superoxide Anion and Hydrogen Peroxide
In vivo hydrogen peroxide staining was performed as described by Barcelo (1998) using TMB. Freshly collected spikelets were put in the staining solution (0.1 mg/mL TMB in Tris-acetate, pH 5.0) under vacuum conditions for 15 min and then incubated at 25°C until the blue color appeared. Production of superoxide anion was visualized by incubating intact anthers in 10 mM K-citrate buffer, pH 6.0, containing 0.5 mM NBT (Liszkay et al., 2004).
In Vitro Induction of H2O2 by OsLAC13
For mammalian expression system, full-length OsLAC13 gene was contrasted into pCDH-CMV-MCS-EF1-Puro vectors that inserted eGFP. Then, 15ug OsLAC13-pCDH vector were transfected with Lipofectamine LTX (Invitrogen Corporation, Carlsbad, CA, United States) into the 3 × 106 293T cells plated in 10 cm dish before 24 h. Add 60 μl of PremoTM Cellular H2O2 Sensor (Moleqular probes) per 75,000 cells in growth medium. Finally, 48 h after transfection, cells were analyzed using a confocal laser scanning microscope (Zeiss 7 DUO NLO) at 400- and 488-nm excitation, and emission at 515-nm.
For rice protoplast transient transfection, OsLAC13 was overexpressed under the control of the CaMV35S promoter. Two-week-old rice shoots were used to isolate protoplast. A bundle of rice plants (approximately 30 seedlings) was cut together into approximately 0.5 mm strips with propulsive force using sharp razors. The strips were incubated in an enzyme solution (1.5% cellulose RS, 0.75% macerozyme R-10, 0.6 M mannitol, 10 mM MES at pH 5.7, 10 mM CaCl2 and 0.1% BSA) for 4–5 h in the dark with gentle shaking (40–50 rpm). After the enzymatic digestion, an equal volume of W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, and 2 mM MES at pH 5.7) was added, followed by shaking (60–80 rpm) for 30 min. Protoplasts were released by filtering through 40-μm nylon mesh into round bottom tubes, washing the strips with W5 solution 3–5 times. The pellets were collected by centrifugation at 800 rpm for 3 min in a swinging bucket. After washing once with W5 solution, the pellets were then resuspended in MMG solution (0.4 M mannitol, 15 mM MgCl2 and 4 mM MES at pH 5.7) at a concentration of 2 × 106 cells mL-1. PEG-mediated transfections were carried out as previously described (Zhang et al., 2011). Protoplasts were observed 24 h after transfection using a confocal laser scanning microscope (Zeiss 7 DUO NLO) at 400- and 488-nm excitation, and emission at 515-nm. Each experiment was repeated at least three times.
Results
OsLAC13 Regulates Seed Setting Rate and Male Reproductive Organogenesis in Rice
To identified the functions of OsLAC13, we first constructed the transgenic plants that knockout of OsLAC13 (lac13) by CRISPR-Cas9 based genome editing technology (Supplementary Figure 1A) and analyzed their phenotypes. We found that the seed setting rate (the ratio of number of filled grains to total number of spikelets) of the lac13 plants increased significantly compared with that of the wild-type (WT) plants. Consistently, the seed setting rate was decreased dramatically in the OsLAC13 over-expression plants (OXLAC13), and was increased slightly in the OsLAC13 RNA interference lines (LAC13 RNAi) (Figure 1A and Supplementary Figure 1B). These results indicated OsLAC13 plays a role in regulating seed setting rate in rice.
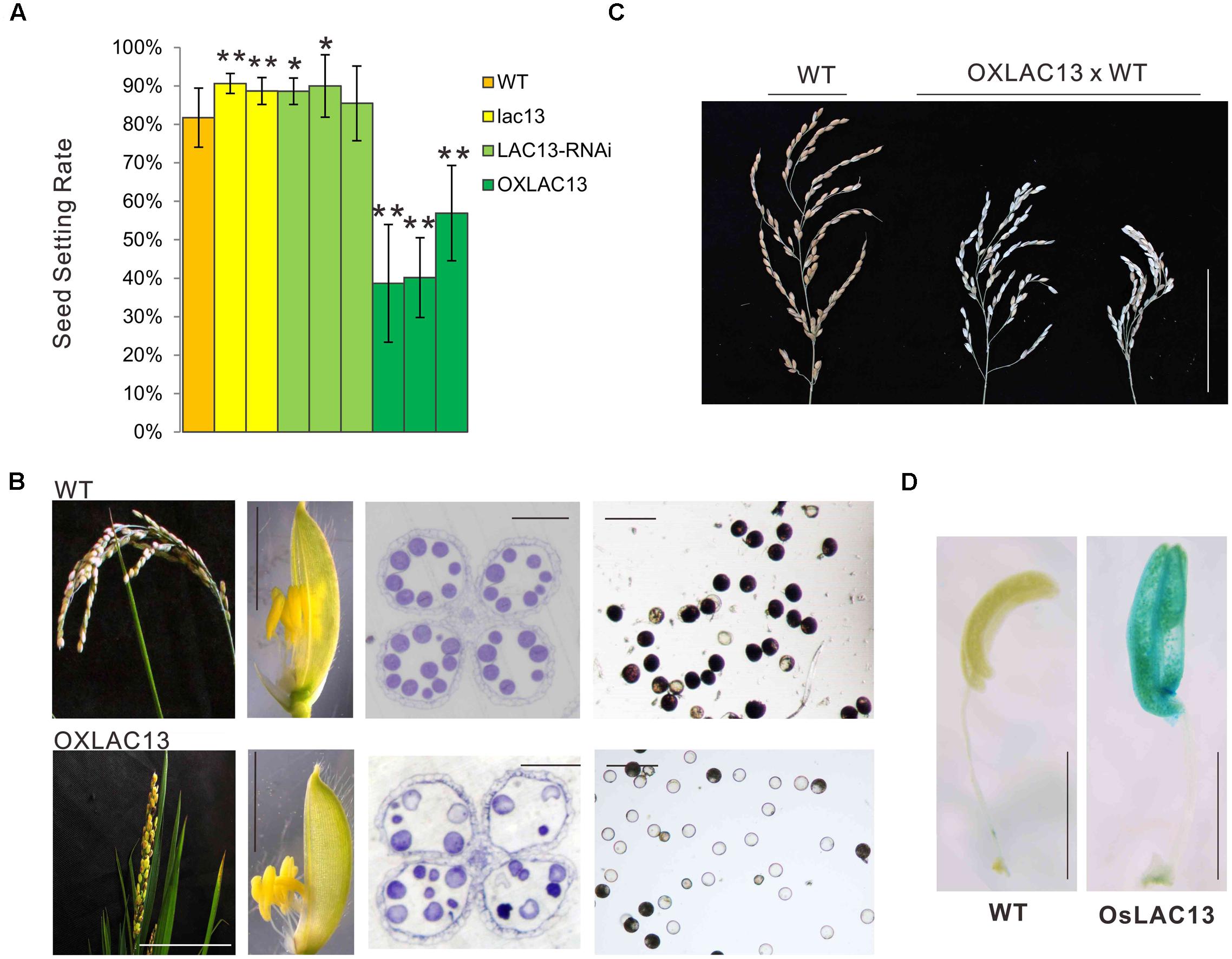
FIGURE 1. OsLAC13 regulates seed setting rate and male reproductive organogenesis. (A) The seed setting rates of WT, OsLAC13 knock out (lac13), OsLAC13-RNAi, and OXLAC13 plants. Values are the means ± s. d. (n = 40 plants). Significant differences were identified at the 5% (∗) and 1% (∗∗) probability levels using Student’s t-test. (B) The panicles of OXLAC13 plants crossed with WT plants. Scale bars, 10 cm. (C) The panicles (scale bars, 2.5 cm), the spikelet (scale bars, 4 mm), the transverse semithin sections of mature anthers (scale bars, 100 μm) and the mature pollen grains stained for starch with I2-KI (scale bars, 100 μm) of WT and OXLAC13 plants from the left panels to the right panels. (D) Expression patterns of OsLAC13 by GUS staining (Blue). Scale bars, 2 mm.
We then analyzed the floral developmental process of the transgenic plants to identify how OsLAC13 control seed setting rate. The phenotypical analysis showed that, compared with wild-type and the lac13 plants, the OXLAC13 plants showed unfavorable characteristics including small panicles, lethal phenotype and semi-sterility (Figure 1B). The OXLAC13 pistils are developed normally (Supplementary Figure 1D). The OXLAC13 plants produced apparently normally developed but slightly smaller spikelets and anthers, although less than 1% of the anthers appeared twisted (Figure 1B and Supplementary Figure 1E). The decreased seed setting rate in the OXLAC13 plants was induced by abnormal male reproductive organogenesis, that most of the mature pollen grains lacked starch, as revealed by iodine-potassium iodide staining of semi-thin sections (Figure 1B). The hybrids of the OXLAC13 plants and the WT plants also showed semi-sterile phenotype (Figure 1C). These results suggested that OsLAC13 restrains male reproductive organogenesis and negatively regulates rice setting rate.
OsLAC13 Restrains Carbohydrate Transportation to Anthers and Filament Elongation
To further characterize the role of OsLAC13 in anther development, we analyzed the spatial expression patterns of OsLAC13 in anthers byβ-glucuronidase (GUS) activity analysis. OsLAC13 is highly expressed in anthers, especially in anther connectives (Figure 1D). We then performed a detailed analysis of anther morphology. The OXLAC13 plants undergoes normal meiosis, as revealed by DAPI staining (Supplementary Figure 2A), indicating that high levels of OsLAC13 transcripts does not affect meiosis. We also investigated the development of the microspores of the OXLAC13 plants after meiosis, and found no obvious phenotypic alterations. The pollen grains developed normally to the trinucleate stage, but had slow starch deposition in the spores, as shown by toluidine blue staining of semi-thin sections (Supplementary Figures 2B,C). These results indicated that the OXLAC13 anthers have no defects during microspore development except for suppressed nutrient accumulation in anthers and pollen grains.
In the mature anthers, the WT pollen grains were deeply stained, indicating that they contained stored starch, but the OXLAC13 pollen grains were almost unstained, showing the failure of starch deposition (Figure 1B). We also monitored the in vitro germination rate of pollen grains. The results showed that the WT pollens had a germination rate of ∼79.8%, but the OXLAC13 pollens only had a germination rate of ∼12.6% (Figure 2A). This result was generally consistent with the result of iodine-potassium iodide staining. We further analyzed the sugar contents of the carbohydrate source tissues (flag leaf, lemma, and palea) during anther development (Zhang et al., 2010) to examine whether the reduction of starch accumulation in the OXLAC13 pollen grains was caused by failure of carbohydrate synthesis in the OXLAC13 plants. However, the results showed that the source tissues of the OXLAC13 plants had similar or only slightly higher levels of sugars (sucrose, glucose, and fructose) and starch as that of the WT plants at both the stages that before and after starch deposition in pollen grains, implying that the OXLAC13 plants have no defect in carbohydrate synthesis (Supplementary Figures 3A–C). The anther vascular tissues from filaments to anther connectives is responsible for transporting nutrients to anthers, thus the roles of anther vascular tissues might be blocked in the OXLAC13 plants.
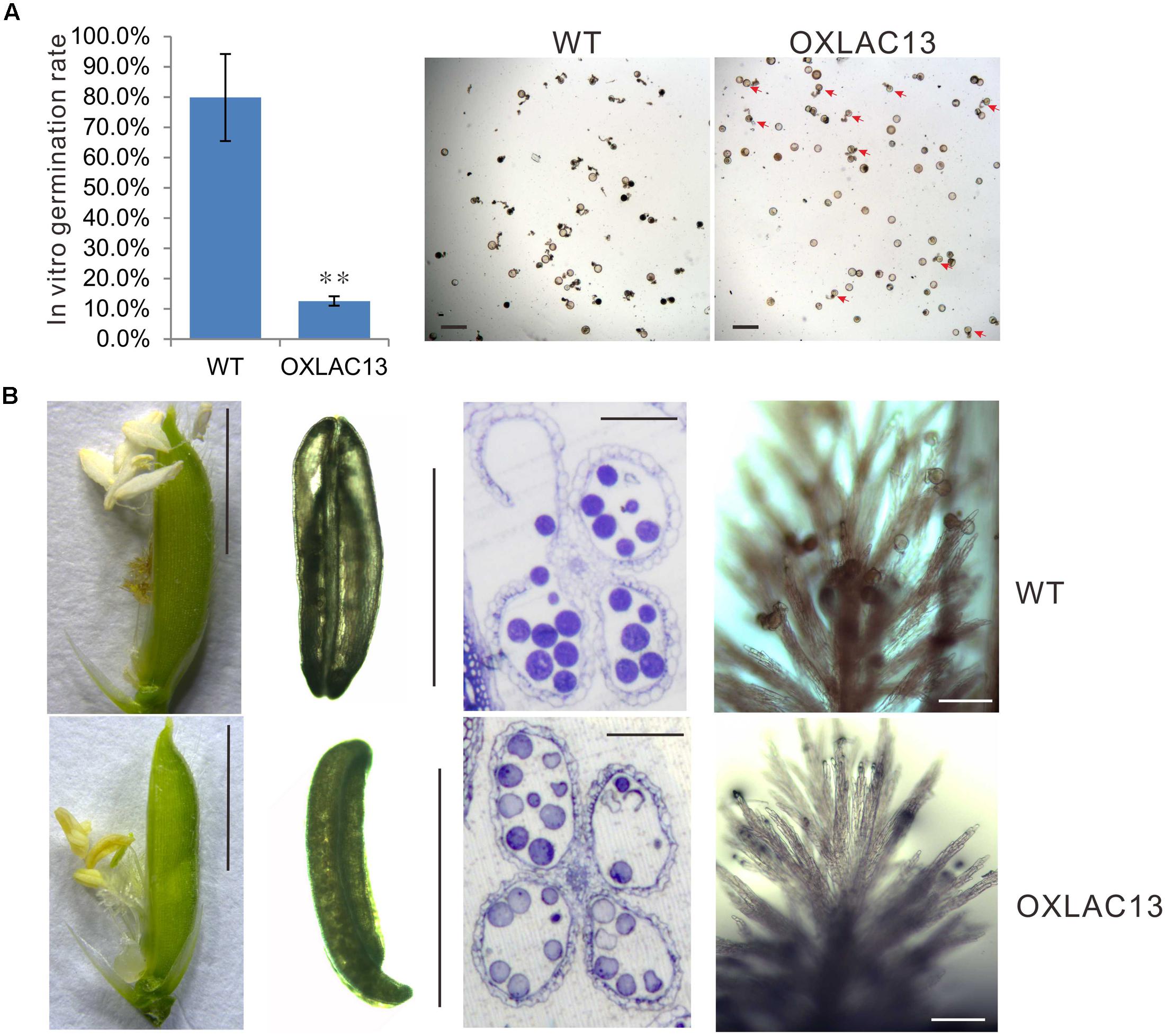
FIGURE 2. Defects of OXLAC13 plants in late anther development. (A) In vitro germination rates of WT and OXLAC13 pollen. Scale bars, 100 μm. Values are means ± s. d. (n = 200 pollen grains). Red arrows indicate the succesfully germinated OXLAC13 pollen grains. Significant differences were identified at the 5% (∗) and 1% (∗∗) probability levels using Student’s t-test. (B) Spikelets 1 day after flowering (Scale bars, 4 mm), anthers after flowering (Scale bars, 2 mm), transverse sections of anthers after flowering (Scale bars, 100 μm) and the stigmas 2 h after flowering (Scale bars, 100 μm) of WT and OXLAC13 plants from the left panels to the right panels.
Noticeably, we also observed a failure of filament elongation in the OXLAC13 spikelets (Figure 2B). At the flowering stage, the WT filaments elongated and the anthers reach the top of the spikelet, and then dehisce, releasing pollen grains over the stigma of the pistil for pollination in the WT plants. After anthesis, the spikelet remains closed and the empty anthers are outside the spikelet (Figure 2B). However, the filaments of about 43.9% of the OXLAC13 spikelets failed to elongate and about 40.2% of the anthers did not dehisce (Figure 2B). Consistent with this, only 37.3% of the OXLAC13 stigmas had more than 20 pollen grains when over 93% of the WT stigmas had more than 20 pollen grains at 2 h after anthesis, and 42.4% of the OXLAC13 stigmas did not have any pollen grains at all (Figure 2B), showing that the semi-sterility of the OXLAC13 plants is caused by a series of defects in late anther development and pollination, including filament elongation. Consider the expression patterns of OsLAC13, together with these results, it could be speculated that OsLAC13 possibly affects the roles of anther vascular tissue including sugar transportation from source tissues to anthers, and filaments elongation.
OsLAC13 Induces Hydrogen Peroxide Production and Affects the Number and Integrity of the Mitochondria in Stamen Vascular Cells
The observations above showed that the OXLAC13 plants might have defects in carbohydrate transport to anthers, and anther vascular tissues is responsible for transporting nutrients to anthers. We also found that the filaments in about 43.9% of the OXLAC13 anthers also failed to elongate (Figure 2B). Thus we speculated that the OXLAC13 plants might have abnormal anther vascular tissue and filaments, which restrained carbohydrate transport to anthers and failed to lift anthers to the top of the OXLAC13 spikelets. Laccases regulate lignin synthesis and the lignification of xylem in vascular bundles (Berthet et al., 2011; Lu et al., 2013; Zhao et al., 2013; Wang et al., 2014); moreover, vascular tissues transport carbohydrates. Therefore, we first used transmission electron microscopy to examine the vascular bundles in filaments and connectives of anthers. We observed only a slight increase of lignification in the secondary wall of vessels in the OXLAC13 plants compared with that of the WT plants (Figures 3A,B and Supplementary Figure 3E), and the vascular bundle showed no apparently abnormalities in morphology (Supplementary Figure 3E), which suggested that the sterility in the OXLAC13 plants might not be caused by the OsLAC13 functions that associated with lignification. This phenomenon is similar to that observed in Arabidopsis, in which loss of function of only one LAC gene fails to induce an apparent phenotype in vascular bundles (Berthet et al., 2011).
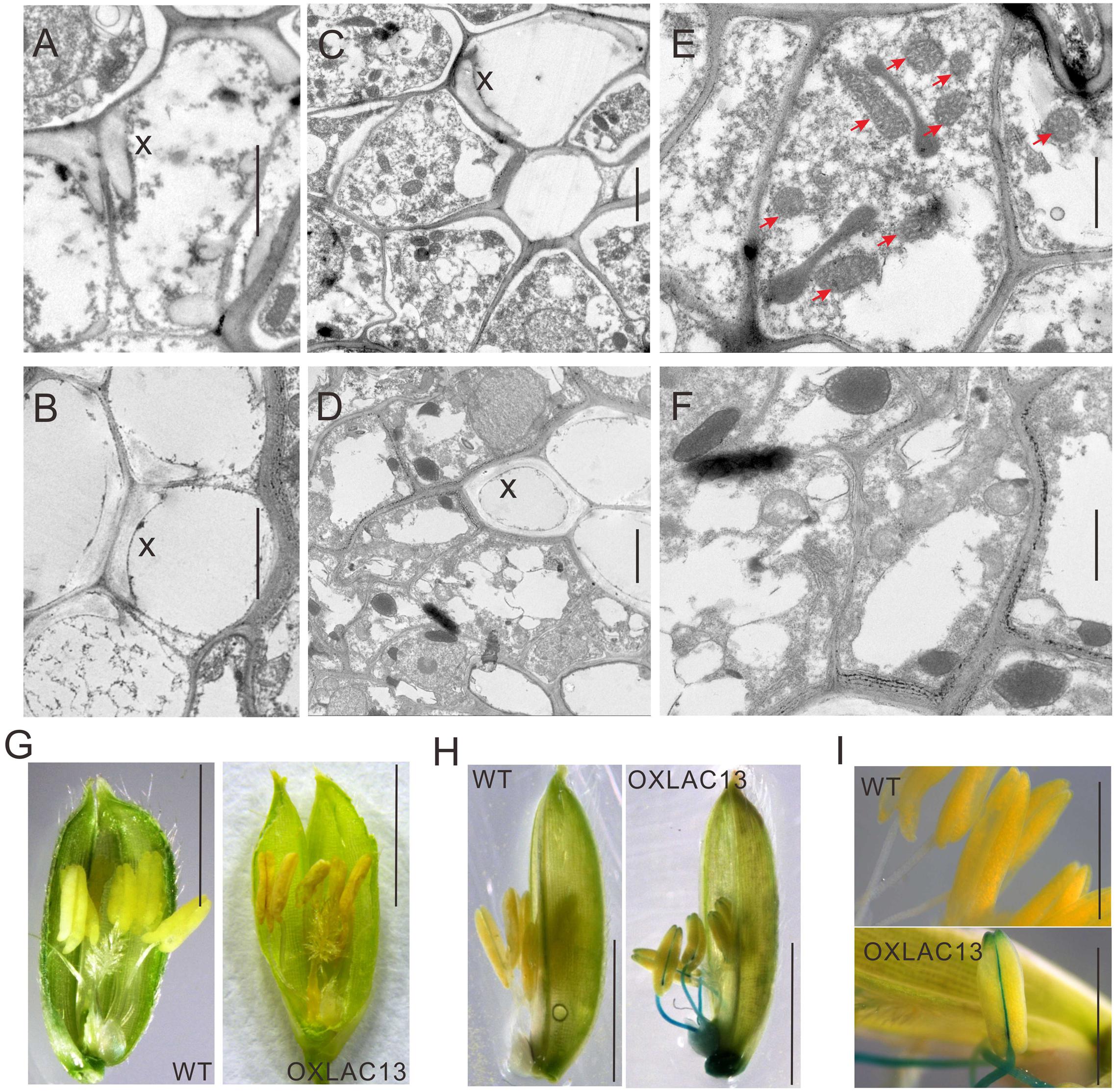
FIGURE 3. Transmission electron micrographs of vascular and ROS accumulation in WT and OXLAC13 anthers. (A,B) The secondary xylem of WT (A) and OXLAC13 (B) anther vascular. Scale bars, 2 μm. (C,D) The phloem of WT (C) and OXLAC13 (D) anther vascular. Scale bars, 2 μm. (E,F) Magnified image of the phloem of WT (E) and OXLAC13 (F). The red arrows show the normal mitochondria in WT phloem (E). Scale bars, 1 μm. X, secondary xylem. (G) Flowers of WT and OXLAC13 plants at the mature stage, showing that the OXLAC13 flowers turn brown at this stage. Scale bars, 4 mm. (H) TMB staining of H2O2 production at the late anther development stage showing blue color. Scale bars, 4 mm. (I) A higher-magnification image of the anthers at the late anther development stage after TMB staining. Scale bars, 2 mm.
As sugars are transported by phloem, we then observed the ultrastructure of the phloem cells in the WT and the OXLAC13 plants. Interestingly, we found that the WT phloem cells have lots of mitochondria with clear mitochondrial cristae (Figures 3C,E), but in the OXLAC13 phloem, the mitochondria are anomalous and seem to undergo degradation. We observed very few organelle with mitochondrial cristae in the OXLAC13 phloem cells during starch deposition in pollen during late anther development (Figures 3D,F), suggesting that OsLAC13 could affect the number and integrity of mitochondria in the stamen vascular cells.
We also noticed that 48% of the OXLAC13 anthers started to turn brown before anthesis (Figure 3G). Anomalous organelles and brown anthers might indicate oxidative stress caused by higher levels of ROS, which cause oxidative damage to cellular structures and molecules (Gechev et al., 2006). Superoxide anion, hydrogen peroxide, and hydroxyl radicals are major ROS in plants (Hu et al., 2011). Given that hydroxyl radicals are unstable and difficult to detect directly in biological samples (Hu et al., 2011), we detected superoxide anion and hydrogen peroxide in the WT and the OXLAC13 anthers during development. Measurement of superoxide anion with nitroblue tetrazolium (NBT) showed no obvious differences in superoxide anion between the WT and the OXLAC13 stamens (Supplementary Figure 4A). We then used 3, 5, 3, 5′-tetramethylbenzidine (TMB) to analyze the cellular localization of hydrogen peroxide in anthers. Intriguingly, although we observed no obvious difference between the anthers of the WT and the OXLAC13 plants during early anther development (Supplementary Figure 4A), we did observe apparent differences during late anther development. Hydrogen peroxide started to accumulate in the filaments and the connectives of the OXLAC13 anthers in late anther development, when no hydrogen peroxide was detected in the WT anthers (Figures 3H,I).
To verify whether OsLAC13 induces H2O2 accumulation in cells, we performed in vitro experiments in both rice cells and mammalian cell, respectively. OsLAC13 was transfected into the mammalian expression system HEK-293T or rice protoplast cells. As expected, OsLAC13 could induce apparent H2O2 accumulation in both the rice protoplast cells and the HEK-293T cells after 24 h or 48 h transfection, respectively (Supplementary Figure 5). These results showed that H2O2 accumulation was directly or indirectly driven by OsLAC13 but not by biotic and abiotic stresses during development in the OXLAC13 plants.
H2O2 has been reported to affect mitochondrial functions that lipid peroxidation mediated by the interaction between ROS and membrane lipids can affect mitochondrial membrane integrity (Keunen et al., 2011). Mitochondria are required for plant development and they supply cellular energy by respiration for various biological processes, including sugar transport in phloem, and the respiratory rate in the phloem cells is much higher than in most of other tissues (Kuhn and Grof, 2010; Eom et al., 2012). In anthers, mitochondria also supply essential energy for elongation of filaments during flowering. These results suggest that OsLAC13 is likely to promote hydrogen peroxide production in filaments and the anther connectives, and in turn affects the integrity of the mitochondria in the stamen vascular cells and might be account for pollen development. We further analyzed three OXLAC13 lines with different OsLAC13 expression level, and found that a higher OsLAC13 expression level induced a slightly higher H2O2 production, and a lower seed setting rate (Supplementary Figures 4C–E). This result suggested that the induction of H2O2 by OsLAC13 in anthers might subsequently affected seed setting rate.
Hydrogen Peroxide Dynamics and Oxidative Stress Responses Are Affected in the Transgenic Plants that Over-Expressing of OsmiR397, the Mediator of OsLAC13
OsLAC13 negatively regulates pollen development by promoting H2O2 production, that it might be suppressed by other molecule to ensure normal anther development. OsLAC13 has been reported to be cleaved by OsmiR397 in rice. We first analyzed the expression patterns of OsmiR397 in anthers, and found it was highly expressed in filaments, anther connectives and pollen sacs (Figure 4A). The biological role of OsmiR397 in anthers might be suppressing OsLAC13 expression and maintaining modest H2O2 level and seed setting rate. Consistent with the OsLAC13 knockout plants, the seed setting rate increased in the OsmiR397 over-expressing plants (OXmiR397), but not in the transgenic plants that over-expressing mutated OsmiR397 gene (OXmmR397) (Figure 4B and Supplementary Figures 1B,C), which contains several mismatches to the OsLAC13 binding site and couldn’t cleave the mRNA of OsLAC13. The hybrids of the OXLAC13 plants and the OXmiR397 plants have similar seed setting rate with that of the WT plants, whereas the hybrids of the OXLAC13 plants and the OxmmiR397 plants have low seed setting rate (Figure 4C), implying that the cleavage of OsLAC13 by OsmiR397 is essential for seed setting rate maintenance in rice plants.
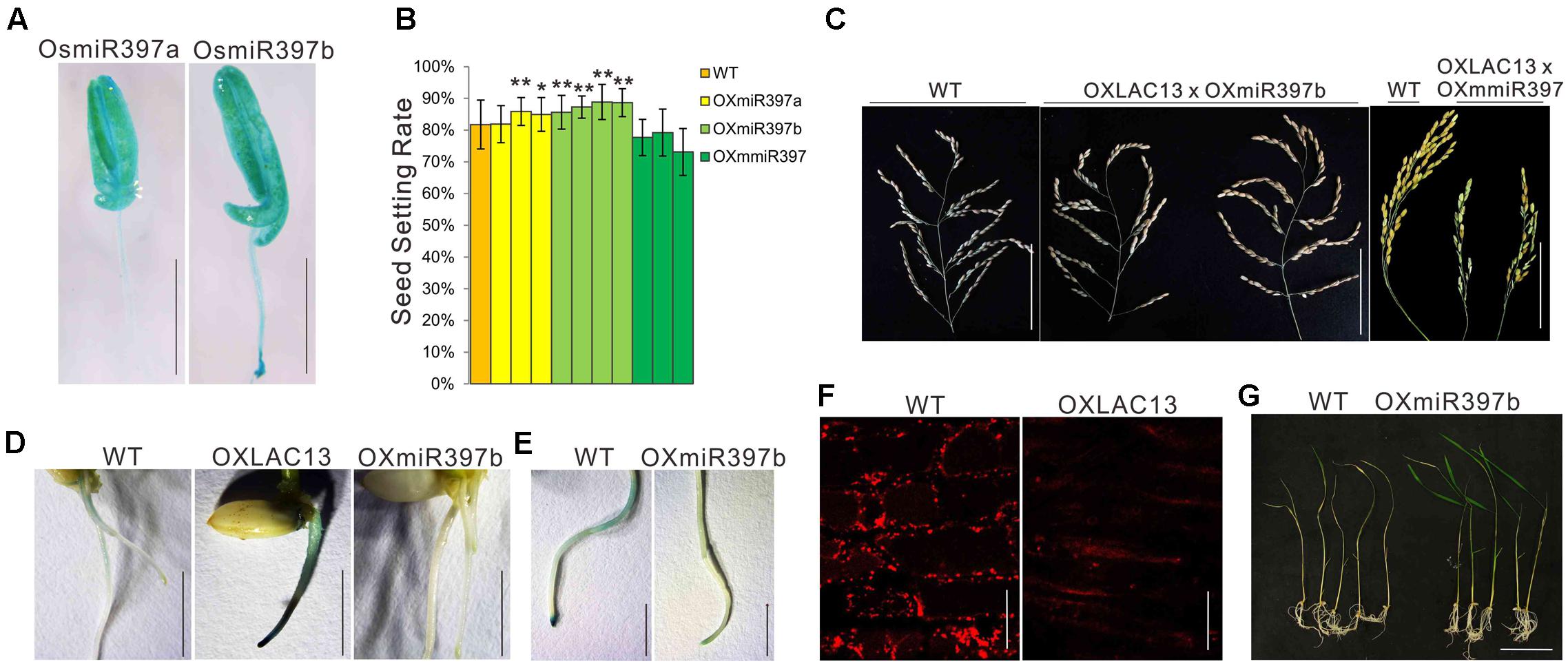
FIGURE 4. OsLAC13 and its mediator OsmiR397 regulate hydrogen peroxide dynamics during root growth and stress response. (A) Expression patterns of OsmiR397a and OsmiR397b by GUS staining (Blue). Scale bars, 2 mm. (B) The seed setting rates of WT, OXmiR397a, OXmiR397b, and OxmmiR397 plants. Values are the means ± s. d. (n = 40 plants). Significant differences were identified at the 5% (∗) and 1% (∗∗) probability levels using Student’s t-test. (C) The panicles of OXLAC13 plants crossed with OXmiR397b and OxmmiR397 plants, respectively. Scale bars, 10 cm. (D) TMB staining showed abnormal H2O2 deposited at the root tips of OXLAC13 seedling, whereas slight H2O2 deposited in the WT roots and no H2O2 was observed in the OXmiR397 roots. Scale bars, 5 mm. (E) TMB staining of the WT and the OXmiR397 roots after 24 h MV treatment. Scale bars, 2 mm. (F) The mitochondria in WT and OXLAC13 root tips were stained by Mito Tracker red. No evident mitochondrion was observed in the OXLAC13 root tip. Scale bars, 20 μm. (G) The WT and OXmiR397b seedlings after 1 days MV treatment and 3 days recovering. Scale bars, 5 cm.
We next ask if OsmiR397 mediated OsLAC13 also regulates H2O2 producing and mitochondrial function in other tissues. We found that, compared with the wild-type plants, the rice plants with ectopic expression of OsLAC13 showed lethal phenotypes, and the lethal plants had very few or no root (Supplementary Figure 4B). It has been evidenced that excess H2O2 is harmful to organisms, and we then analyze the H2O2 contents and mitochondrial functions in the OXLAC13 roots. We detected the cellular localization of H2O2 by TMB staining after seed germination. The OXLAC13 roots were strongly stained 5 days after germination especially at the root tips, and the WT roots were slightly stained at the zone of maturation and no staining could be observed at the root tips, whereas the OXmiR397 plants showed no staining throughout the roots (Figure 4D), indicating that OsLAC13 started to promote H2O2 production from the beginning of the plant development, and OsmiR397 reduced H2O2 storage. To monitor the effect of the elevated H2O2 contents in the OXLAC13 root tips on mitochondrial functions, we then detected active mitochondria in the root tip cells in which active mitochondria can be effectively stained using Mito Tracker red (Molecular Probes) as a marker. Consistently, the active mitochondria were not evident in the root tip of the poorly grown OXLAC13 seedling, while the active mitochondria distributed homogeneously in the WT root tip (Figure 4F).
It is known that oxidative stresses could induce H2O2, thus to further verify the effects of OsmiR397 on H2O2 dynamic, we then used methyl viologen (MV) to induce oxidative stress to the roots of the WT and the OXmiR397 plants, and detected the contents of H2O2. After 24 h 20 μM MV treatment, the root tips of the WT plants showed strong TMB staining, but the OXmiR397 root tips only showed slightly staining (Figure 4E). Consistently, after 3 days recovering, most of the OXmiR397 seedlings survived while most of the WT seedlings died (Figure 4G). It has been reported that miR397 is induced by different stresses in various species (Sunkar and Zhu, 2004; Wang et al., 2013; Maeda et al., 2016). Thus OsmiR397 regulated OsLAC13 abundance might play a role in stress response by suppressing H2O2 production in roots. These data showed that OsmiR397 directs OsLAC13 down-regulation is involved in affecting H2O2 dynamics during development and stress responses.
In conclusion, our results showed that OsLAC13 regulates seed setting rate and plant growth in rice through promoting the production of hydrogen peroxide, and induce mitochondrial disruption. This process is under the regulation of OsmiR397. In stamen, OsLAC13 might suppress sugar transport to the pollen grains and prevent the elongation of the filaments, which then lead to decreased seed setting rate.
Discussion
A Novel Function of Laccase in Plant Seed Setting Rate Regulation
Laccases belong to a large group of enzymes termed the blue copper proteins, including ascorbic acid oxidase and plastocyanin. Plant laccases are well-known to be involved in lignin synthesis (Turlapati et al., 2011; Cesarino et al., 2013; Lu et al., 2013; Zhao et al., 2013; Awasthi et al., 2015). Recent studies also showed laccase genes regulate seed yield in rice and in Arabidopsis (Zhang et al., 2013; Wang et al., 2014). In this study, we found a new function of OsLAC13 in regulating H2O2 dynamics and rice seed setting rate. Knock-out or knock-down of OsLAC13 increased seed setting rate, while overexpression of OsLAC13 lead to abnormal male reproductive organogenesis by promoting H2O2 accumulation in filaments and connectives of anthers and affecting the integrity of mitochondria in the vascular tissues. We also showed the regulatory role of OsLAC13 is under the control of OsmiR397 (Zhang et al., 2013). High expression level of OsmiR397 increases seed setting rate and restores the semi-sterility of the rice plants caused by high abundance of OsLAC13 mRNA.
We have reported that OsLAC13 suppressed brassinosteroid (BR) signaling and in turn affected grain yield in rice (Zhang et al., 2013). BRs are also essential for male fertility in plants. Some studies showed that BRs are involved in pollen tube and filament elongation by regulating related genes (Szekeres et al., 1996; Bouquin et al., 2001; Li et al., 2001; Kim et al., 2005; Ye et al., 2010), although the detailed molecular mechanisms have not been identified. In this study, we identified that OsLAC13 regulates seed setting rate and male reproductive organogenesis through H2O2 pathway. Whether BR signaling is also involved in the OsLAC13-mediated process has not been demonstrated and more efforts are necessary to further investigate the functions of this gene. These findings indicated that laccases in higher plants are much more complicated than previously estimated.
ROS in Male Reproductive Organogenesis
Most ROS molecules form as toxic by-products of aerobic metabolism in plants subjected to abiotic stresses (Bailey-Serres and Mittler, 2006). In recent years, studies have discovered the important roles of ROS in regulating plant development (Miller et al., 2008). ROS, particularly H2O2, serve as signaling molecules in diverse processes (Quan et al., 2008), such as cell expansion (Suzuki et al., 1999), apical dominance (Semighini and Harris, 2008), senescence (Cui et al., 2013; Allu et al., 2014), and flower development (Zafra et al., 2010; Hu et al., 2011; Kaya et al., 2014; Schippers et al., 2016), as well as stress responses (Xia et al., 2010; Baxter et al., 2014; Considine et al., 2015).
Several recent studies have reported the close connection between ROS and male reproductive organogenesis in plants. For example, Hu et al. (2011) studied the roles of the transcriptional regulator MADS3 in male sterility. Mutation of rice MADS3 affects its regulation of MT-1-4b, which has superoxide anion and hydroxyl radical-scavenging activity; the resulting increased level of superoxide anion causes decreased pollen fertility (Hu et al., 2011). Wu et al. (2010) reported the roles of H2O2 in pollen tube growth, showing that spermidine-derived H2O2 signals Ca2++ influx and thereby regulates pollen tube growth. ROS are also critical for tapetal programmed cell death and pollen development, and excessive accumulation of ROS also occurs in the anthers of cytoplasmic male sterile rice (Jiang et al., 2007; Luo et al., 2013; Xie et al., 2014; Li et al., 2015). In general, the appropriate amount of ROS in the tapetum is crucial for generating fertile anthers in plants, but abnormal production of ROS can harm the male reproductive organ (Hu et al., 2011; Xie et al., 2014). In this study, we reported that overexpression of OsLAC13 could induce the accumulation of H2O2 in the filaments and connectives of anthers, which then affected mitochondrial integrity in the vascular tissue cells. Our findings showed that ROS accumulation in filaments also affects male reproductive organogenesis in plants, and in turn affects grain setting rate.
The Possible Mechanism of OsLAC13 in Hydrogen Peroxide Production and Deposition in Plant
In plant laccases are proposed to function in the formation of lignin by promoting the oxidative coupling of monolignols, a family of naturally occurring phenols (Berthet et al., 2011; Lu et al., 2013; Zhao et al., 2013; Wang et al., 2014). The range of substrates which various laccases can attack is very wide, and substrates similar to a p-diphenol will be oxidized by laccases (Mayer and Staples, 2002). This characteristic makes the actions of laccases much complicated that up to now most functions of laccases in plants remain largely unknown.
Fungi laccases have been reported to catalyze reduction of molecular oxygen to H2O, but not H2O2 (Morozova et al., 2007). However, the catalytic activity of plant laccases is not well identified. It has been reported that laccases in different species have a fairly broad but distinctive substrate spectrum amongst the enzymes (Reiss et al., 2013). Thus, whether plant laccases could produce H2O2, and which substrate might be responsible for H2O2 production have not been reported yet.
In the study, we showed that H2O2 could be a product of a rice laccase catalysis, showing the differences between plant and fungi laccases. In vitro experiments showed that expressing OsLAC13 in mammalian cells could induce H2O2 production independently. This result indicated that OsLAC13 regulates H2O2 with no need of other plant specific protein. It has also been reported that, in plant, peroxidase uses ascorbate as the reductant to remove H2O2 (Noctor et al., 2000). Importantly, OsLAC13 and L-ascorbate oxidase belong to the blue oxidase family. Moreover, OsLAC13 has over 85% similarity with the L-ascorbate oxidase in Zea mays, implying that plant laccase, at least OsLAC13 could oxdize the reductant of peroxidase to restrain H2O2 removal. We have compared the ascorbate contents in the OXLAC13, the OXmiR397a/b and the WT plants. Interestingly, the ascorbate content was lower in the OXLAC13 plants and was higher in the OxmiR397a/b plants compared with that of the WT plants, negatively associated with the content of H2O2 (Supplementary Figure 3D). These data suggested that plant laccase might induce hydrogen peroxide deposition although further studies are necessary before the conclusion was made especially the enzyme substrates need to be declared.
Importance of Filaments and Connectives of Anthers in Regulating Crop Seed Setting Rate
Crop domestication is essential for food supply, and increasing seed setting rate is critical for crop domestication. Except environmental factors, reproductive organogenesis and pollination are determinants of seed setting rate. Male reproductive organogenesis include both the early phase of stamen formation and morphogenesis and the late phase of pollen grain maturation, stamen filament elongation, and anther dehiscence (Cardarelli and Cecchetti, 2014). Most studies of the male reproductive organogenesis focused on meiosis during pollen formation, the roles of the tapetum in pollen maturation, and the process of pollen tube elongation, but studies on filaments and connectives of anthers remain limited. Filaments and connectives function as conduits for water and nutrients and as a support that elongates to allow pollen deposition on the receptive stigma. The importance of filaments and connectives of anthers in male reproductive organogenesis and seed setting rate regulation has not been specifically studied yet.
Pollen development depends on energy supply. Mitochondria produce ATP via respiration and are essential for cellular energy production (Chen and Liu, 2014; Horn et al., 2014). The biogenesis of the sporophytic and gametophytic cells of plant anthers is thought to demand more cellular energy than other organs (Chen and Liu, 2014) and thus anthers have a lot of mitochondria. Some cytoplasmic male sterility genes cause mitochondrial dysfunction where the mitochondria fail to provide enough ATP for male development; this dysfunction then induces sterility (Ling et al., 1979). Carbohydrate transport in the phloem also needs energy, which is generated by mitochondrial respiration (Kuhn and Grof, 2010; Eom et al., 2012), and phloem tissues usually have a large number of mitochondria to support nutrient transport. The filaments and connectives of anthers have clear importance for supplying nutrients during anther development. In this study, we showed that the abnormal accumulation of ROS in the filaments and connections of anthers could injure the integrity of mitochondria, and this might decrease the energy supply available for carbohydrate transport and filament elongation. We indeed observed a blockage of carbohydrate transport from shells to anthers and a failure of filament elongation in some OXLAC13 plants. Thus it could be proposed that proper energy supply by mitochondria in filaments and connectives of anthers aids male reproductive organogenesis and improves seed setting rate in plants.
Author Contributions
YY, Q-FL, and J-PZ carried out mutant screening and validation experiments. FZ performed ultrathin section experiments and Y-FZ and Y-ZF carried out histochemical assays. Y-QC conceived of the study, and participated in its design and coordination and helped to draft the manuscript. Y-CZ designed and carried out the functional analysis and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 91335104 and 31401352), the Science and Technology Transgenic project (2014ZX0800934B), and grants from Guangdong Province (No. 2014T70833 and 2016A030308015) and Guangzhou (201606080912429, 201707020018, and 201710010029) and from the Foundation of China Post-doctoral Science (No. 2014T70833).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01324/full#supplementary-material
References
Allu, A. D., Soja, A. M., Wu, A., Szymanski, J., and Balazadeh, S. (2014). Salt stress and senescence: identification of cross-talk regulatory components. J. Exp. Bot. 65, 3993–4008. doi: 10.1093/jxb/eru173
Alvarez, M. E., Pennell, R. I., Meijer, P. J., Ishikawa, A., Dixon, R. A., and Lamb, C. (1998). Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92, 773–784. doi: 10.1016/S0092-8674(00)81405-1
Awasthi, M., Jaiswal, N., Singh, S., Pandey, V. P., and Dwivedi, U. N. (2015). Molecular docking and dynamics simulation analyses unraveling the differential enzymatic catalysis by plant and fungal laccases with respect to lignin biosynthesis and degradation. J. Biomol. Struct. Dyn. 33, 1835–1849. doi: 10.1080/07391102.2014.975282
Bailey-Serres, J., and Mittler, R. (2006). The roles of reactive oxygen species in plant cells. Plant Physiol. 141:311. doi: 10.1104/pp.104.900191
Barcelo, A. R. (1998). Hydrogen peroxide production is a general property of the lignifying xylem from vascular plants. Ann. Bot. 82, 97–103. doi: 10.1006/anbo.1998.0655
Baxter, A., Mittler, R., and Suzuki, N. (2014). ROS as key players in plant stress signalling. J. Exp. Bot. 65, 1229–1240. doi: 10.1093/jxb/ert375
Berthet, S., Demont-Caulet, N., Pollet, B., Bidzinski, P., Cezard, L., Le Bris, P., et al. (2011). Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 23, 1124–1137. doi: 10.1105/tpc.110.082792
Bouquin, T., Meier, C., Foster, R., Nielsen, M. E., and Mundy, J. (2001). Control of specific gene expression by gibberellin and brassinosteroid. Plant Physiol. 127, 450–458. doi: 10.1104/pp.010173
Bryan, A. C., Jawdy, S., Gunter, L., Gjersing, E., Sykes, R., Hinchee, M. A., et al. (2016). Knockdown of a laccase in Populus deltoides confers altered cell wall chemistry and increased sugar release. Plant Biotechnol. J. 14, 2010–2020. doi: 10.1111/pbi.12560
Cardarelli, M., and Cecchetti, V. (2014). Auxin polar transport in stamen formation and development: how many actors? Front. Plant Sci. 5:333. doi: 10.3389/fpls.2014.00333
Cesarino, I., Araujo, P., Sampaio Mayer, J. L., Vicentini, R., Berthet, S., Demedts, B., et al. (2013). Expression of SofLAC, a new laccase in sugarcane, restores lignin content but not S:G ratio of Arabidopsis lac17 mutant. J. Exp. Bot. 64, 1769–1781. doi: 10.1093/jxb/ert045
Chen, L., and Liu, Y. G. (2014). Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 65, 579–606. doi: 10.1146/annurev-arplant-050213-040119
Considine, M. J., Sandalio, L. M., and Foyer, C. H. (2015). Unravelling how plants benefit from ROS and NO reactions, while resisting oxidative stress. Ann. Bot. 116, 469–473. doi: 10.1093/aob/mcv153
Cui, M. H., Ok, S. H., Yoo, K. S., Jung, K. W., Yoo, S. D., and Shin, J. S. (2013). An Arabidopsis cell growth defect factor-related protein, CRS, promotes plant senescence by increasing the production of hydrogen peroxide. Plant Cell Physiol. 54, 155–167. doi: 10.1093/pcp/pcs161
Datta, R., Chamusco, K. C., and Chourey, P. S. (2002). Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol. 130, 1645–1656. doi: 10.1104/pp.006908
Eom, J. S., Choi, S. B., Ward, J. M., and Jeon, J. S. (2012). The mechanism of phloem loading in rice (Oryza sativa). Mol. Cells 33, 431–438. doi: 10.1007/s10059-012-0071-9
Gechev, T. S., Van Breusegem, F., Stone, J. M., Denev, I., and Laloi, C. (2006). Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28, 1091–1101. doi: 10.1002/bies.20493
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Goetz, M., Godt, D. E., Guivarc’h, A., Kahmann, U., Chriqui, D., and Roitsch, T. (2001). Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc. Natl. Acad. Sci. U.S.A. 98, 6522–6527. doi: 10.1073/pnas.091097998
Gui, J., Liu, C., Shen, J., and Li, L. (2014). Grain setting defect1, encoding a remorin protein, affects the grain setting in rice through regulating plasmodesmatal conductance. Plant Physiol. 166, 1463–1478. doi: 10.1104/pp.114.246769
Horn, R., Gupta, K. J., and Colombo, N. (2014). Mitochondrion role in molecular basis of cytoplasmic male sterility. Mitochondrion 19(Pt B), 198–205. doi: 10.1016/j.mito.2014.04.004
Hu, L., Liang, W., Yin, C., Cui, X., Zong, J., Wang, X., et al. (2011). Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell 23, 515–533. doi: 10.1105/tpc.110.074369
Jiang, P., Zhang, X., Zhu, Y., Zhu, W., Xie, H., and Wang, X. (2007). Metabolism of reactive oxygen species in cotton cytoplasmic male sterility and its restoration. Plant Cell Rep. 26, 1627–1634. doi: 10.1007/s00299-007-0351-6
Kaya, H., Nakajima, R., Iwano, M., Kanaoka, M. M., Kimura, S., Takeda, S., et al. (2014). Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26, 1069–1080. doi: 10.1105/tpc.113.120642
Keunen, E., Remans, T., Bohler, S., Vangronsveld, J., and Cuypers, A. (2011). Metal-induced oxidative stress and plant mitochondria. Int. J. Mol. Sci. 12, 6894–6918. doi: 10.3390/ijms12106894
Kim, T. W., Hwang, J. Y., Kim, Y. S., Joo, S. H., Chang, S. C., Lee, J. S., et al. (2005). Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 17, 2397–2412. doi: 10.1105/tpc.105.033738
Kuhn, C., and Grof, C. P. (2010). Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 13, 288–298. doi: 10.1016/j.pbi.2010.02.001
Li, J., Dai, X., Li, L., Jiao, Z., and Huang, Q. (2015). Metabolism of reactive oxygen species in cytoplasmic male sterility of rice by marking upmost pulvinus interval. Appl. Biochem. Biotechnol. 175, 1263–1269. doi: 10.1007/s12010-014-1346-8
Li, J., Nam, K. H., Vafeados, D., and Chory, J. (2001). BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 127, 14–22. doi: 10.1104/pp.127.1.14
Li, S., Li, W., Huang, B., Cao, X., Zhou, X., Ye, S., et al. (2013). Natural variation in PTB1 regulates rice seed setting rate by controlling pollen tube growth. Nat. Commun. 4:2793. doi: 10.1038/ncomms3793
Ling, S., Lee, J., and Warmke, H. E. (1979). Organelle size and number in fertile and T-cytoplasmic male-sterile corn. Am. J. Bot. 66, 141–148. doi: 10.2307/2442516
Lisec, J., Schauer, N., Kopka, J., Willmitzer, L., and Fernie, A. R. (2006). Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 1, 387–396. doi: 10.1038/nprot.2006.59
Liszkay, A., Van Der Zalm, E., and Schopfer, P. (2004). Production of reactive oxygen intermediates (O(2)(.-), H(2)O(2), and (.)OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 136, 3114–3123. doi: 10.1104/pp.104.044784
Liu, W., Zhang, D., Tang, M., Li, D., Zhu, Y., Zhu, L., et al. (2013). THIS1 is a putative lipase that regulates tillering, plant height, and spikelet fertility in rice. J. Exp. Bot. 64, 4389–4402. doi: 10.1093/jxb/ert256
Lu, S., Li, Q., Wei, H., Chang, M. J., Tunlaya-Anukit, S., Kim, H., et al. (2013). Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proc. Natl. Acad. Sci. U.S.A. 110, 10848–10853. doi: 10.1073/pnas.1308936110
Luo, D., Xu, H., Liu, Z., Guo, J., Li, H., Chen, L., et al. (2013). A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 45, 573–577. doi: 10.1038/ng.2570
Ma, X., Zhang, Q., Zhu, Q., Liu, W., Chen, Y., Qiu, R., et al. (2015). A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284. doi: 10.1016/j.molp.2015.04.007
Maeda, S., Sakazono, S., Masuko-Suzuki, H., Taguchi, M., Yamamura, K., Nagano, K., et al. (2016). Comparative analysis of microRNA profiles of rice anthers between cool-sensitive and cool-tolerant cultivars under cool-temperature stress. Genes Genet. Syst. 91, 97–109. doi: 10.1266/ggs.15-00056
Mayer, A. M., and Staples, R. C. (2002). Laccase: new functions for an old enzyme. Phytochemistry 60, 551–565. doi: 10.1016/S0031-9422(02)00171-1
Miller, G., Shulaev, V., and Mittler, R. (2008). Reactive oxygen signaling and abiotic stress. Physiol. Plant. 133, 481–489. doi: 10.1111/j.1399-3054.2008.01090.x
Morozova, O. V., Shumakovich, G. P., Gorbacheva, M. A., Shleev, S. V., and Yaropolov, A. I. (2007). “Blue” laccases. Biochemistry 72, 1136–1150. doi: 10.1134/s0006297907100112
Noctor, G., Veljovic-Jovanovic, S., and Foyer, C. H. (2000). Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1465–1475. doi: 10.1098/rstb.2000.0707
Quan, L. J., Zhang, B., Shi, W. W., and Li, H. Y. (2008). Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 50, 2–18. doi: 10.1111/j.1744-7909.2007.00599.x
Reiss, R., Ihssen, J., Richter, M., Eichhorn, E., Schilling, B., and Thony-Meyer, L. (2013). Laccase versus laccase-like multi-copper oxidase: a comparative study of similar enzymes with diverse substrate spectra. PLoS ONE 8:e65633. doi: 10.1371/journal.pone.0065633
Ross, K. J., Fransz, P., and Jones, G. H. (1996). A light microscopic atlas of meiosis in Arabidopsis thaliana. Chromosome Res. 4, 507–516. doi: 10.1007/BF02261778
Schippers, J. H., Foyer, C. H., and Van Dongen, J. T. (2016). Redox regulation in shoot growth, SAM maintenance and flowering. Curr. Opin. Plant Biol. 29, 121–128. doi: 10.1016/j.pbi.2015.11.009
Semighini, C. P., and Harris, S. D. (2008). Regulation of apical dominance in Aspergillus nidulans hyphae by reactive oxygen species. Genetics 179, 1919–1932. doi: 10.1534/genetics.108.089318
Shi, X., Sun, X., Zhang, Z., Feng, D., Zhang, Q., Han, L., et al. (2015). GLUCAN SYNTHASE-LIKE 5 (GSL5) plays an essential role in male fertility by regulating callose metabolism during microsporogenesis in rice. Plant Cell Physiol. 56, 497–509. doi: 10.1093/pcp/pcu193
Skopelitis, D. S., Paranychianakis, N. V., Paschalidis, K. A., Pliakonis, E. D., Delis, I. D., Yakoumakis, D. I., et al. (2006). Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18, 2767–2781. doi: 10.1105/tpc.105.038323
Sunkar, R., and Zhu, J. K. (2004). Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16, 2001–2019. doi: 10.1105/tpc.104.022830
Suzuki, K., Yano, A., and Shinshi, H. (1999). Slow and prolonged activation of the p47 protein kinase during hypersensitive cell death in a culture of tobacco cells. Plant Physiol. 119, 1465–1472. doi: 10.1104/pp.119.4.1465
Szekeres, M., Nemeth, K., Koncz-Kalman, Z., Mathur, J., Kauschmann, A., Altmann, T., et al. (1996). Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85, 171–182. doi: 10.1016/S0092-8674(00)81094-6
Turlapati, P. V., Kim, K. W., Davin, L. B., and Lewis, N. G. (2011). The laccase multigene family in Arabidopsis thaliana: towards addressing the mystery of their gene function(s). Planta 233, 439–470. doi: 10.1007/s00425-010-1298-3
Wang, C. Y., Zhang, S., Yu, Y., Luo, Y. C., Liu, Q., Ju, C., et al. (2014). MiR397b regulates both lignin content and seed number in Arabidopsis via modulating a laccase involved in lignin biosynthesis. Plant Biotechnol. J. 12, 1132–1142. doi: 10.1111/pbi.12222
Wang, M., Wang, Q., and Zhang, B. (2013). Response of miRNAs and their targets to salt and drought stresses in cotton (Gossypium hirsutum L.). Gene 530, 26–32. doi: 10.1016/j.gene.2013.08.009
Wu, J., Shang, Z., Jiang, X., Moschou, P. N., Sun, W., Roubelakis-Angelakis, K. A., et al. (2010). Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+ -permeable channels and pollen tube growth. Plant J. 63, 1042–1053. doi: 10.1111/j.1365-313X.2010.04301.x
Xia, X. J., Chen, Z., and Yu, J. Q. (2010). ROS mediate brassinosteroids-induced plant stress responses. Plant Signal. Behav. 5, 532–534. doi: 10.4161/psb.10989
Xie, H. T., Wan, Z. Y., Li, S., and Zhang, Y. (2014). Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 26, 2007–2023. doi: 10.1105/tpc.114.125427
Ye, Q., Zhu, W., Li, L., Zhang, S., Yin, Y., Ma, H., et al. (2010). Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. U.S.A. 107, 6100–6105. doi: 10.1073/pnas.0912333107
Zafra, A., Rodriguez-Garcia, M. I., and Alche Jde, D. (2010). Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biol. 10:36. doi: 10.1186/1471-2229-10-36
Zhang, H., Liang, W., Yang, X., Luo, X., Jiang, N., Ma, H., et al. (2010). Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 22, 672–689. doi: 10.1105/tpc.109.073668
Zhang, K., Song, Q., Wei, Q., Wang, C., Zhang, L., Xu, W., et al. (2016). Down-regulation of OsSPX1 caused semi-male sterility, resulting in reduction of grain yield in rice. Plant Biotechnol. J. 14, 1661–1672. doi: 10.1111/pbi.12527
Zhang, Y., Su, J., Duan, S., Ao, Y., Dai, J., Liu, J., et al. (2011). A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7:30. doi: 10.1186/1746-4811-7-30
Zhang, Y. C., Yu, Y., Wang, C. Y., Li, Z. Y., Liu, Q., Xu, J., et al. (2013). Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching. Nat. Biotechnol. 31, 848–852. doi: 10.1038/nbt.2646
Zhao, Q., Nakashima, J., Chen, F., Yin, Y., Fu, C., Yun, J., et al. (2013). LACCASE is necessary and nonredundant with PEROXIDASE for lignin polymerization during vascular development in Arabidopsis. Plant Cell 25, 3976–3987. doi: 10.1105/tpc.113.117770
Keywords: rice, seed setting rate, laccase, hydrogen peroxide, mitochondria
Citation: Yu Y, Li Q-F, Zhang J-P, Zhang F, Zhou Y-F, Feng Y-Z, Chen Y-Q and Zhang Y-C (2017) Laccase-13 Regulates Seed Setting Rate by Affecting Hydrogen Peroxide Dynamics and Mitochondrial Integrity in Rice. Front. Plant Sci. 8:1324. doi: 10.3389/fpls.2017.01324
Received: 16 May 2017; Accepted: 14 July 2017;
Published: 26 July 2017.
Edited by:
Liwen Jiang, The Chinese University of Hong Kong, Hong KongReviewed by:
Hao Wang, South China Agricultural University, ChinaZhaojun Ding, Shandong University, China
Copyright © 2017 Yu, Li, Zhang, Zhang, Zhou, Feng, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Chan Zhang, zhyuchan@mail.sysu.edu.cn
 Yang Yu
Yang Yu Quan-Feng Li
Quan-Feng Li Yan-Zhao Feng
Yan-Zhao Feng Yu-Chan Zhang
Yu-Chan Zhang