- 1Laboratory of Natural Products Pesticides, College of Plant Protection, Southwest University, Chongqing, China
- 2Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, National Engineering Research Center for Organic-Based Fertilizers, Nanjing Agricultural University, Nanjing, China
- 3State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China
- 4Key Laboratory of Horticultural Plant Biology, Ministry of Education, College of Horticulture and Forestry Sciences, Huazhong Agricultural University, Wuhan, China
- 5Key Laboratory of Potato Biology and Biotechnology, Ministry of Agriculture, College of Horticulture and Forestry Sciences, Huazhong Agricultural University, Wuhan, China
- 6Research Center of Bioenergy and Bioremediation, College of Resources and Environment, Southwest University, Chongqing, China
- 7Plant Protection Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou, China
- 8Shanghai Center for Plant Stress Biology, CAS Center for Excellence in Molecular Plant Sciences, Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences, Shanghai, China
- 9Oil Crops Research Institute, Chinese Academy of Agricultural Sciences, Wuhan, China
Bacterial wilt caused by plant pathogenic Ralstonia spp. is one of the most important diseases affecting the production of many important crops worldwide. In China, a large scientific community has been dedicated to studying bacterial wilt and its causative agent, Ralstonia pseudosolanacearum and R. solanacearum. Most of their work was published in Chinese, which has hindered international communication and collaboration in this field. In this review, we summarize the status of knowledge on geographical distribution, diversity, and host range of Ralstonia spp., as well as, the impact of bacterial wilt on important crops and disease control approaches, in China. We present areas of research and publications by Chinese scientists and propose the promotion of collaborative research within China and with the international community.
Introduction
Bacterial wilt disease caused by Ralstonia solanacearum is a serious threat to crop production worldwide (Hayward, 1991). R. solanacearum forms a highly diverse species complex encompassing four phylotypes, five races and six biovars that have geographically distinct distribution (Fegan and Prior, 2005). Recent reports propose to separate Ralstonia solanacearum species complex into three species: R. solanacearum (phylotype II), R. pseudosolanacearum (phylotype I and II), and R. syzygii (phylotype IV) (Safni et al., 2014; Prior et al., 2016). This species complex infects 100s of plants, including many economically important crops, such as tobacco, tomato, and potato (Hayward, 1991). Even though different approaches have been developed to control this disease, we still lack an efficient and environmentally friendly control measure for most of the host crops.
To exchange knowledge and control strategies of bacterial wilt disease, several International Bacterial Wilt Symposia (IBWS) have been organized in different locations across the world including Taiwan (1992), Guadeloupe (1997), White River (2002), York (2006), Wuhan (2011), and Toulouse (2016). The recent 6th IBWS held in July 2016 in Toulouse, France successfully brought together a community of researchers worldwide including agronomists, farmers, and private companies involved in the study and control of bacterial wilt. A total of 14 Chinese researchers from 6 institutions attended this symposium and presented their work on bacterial wilt. Even though bacterial wilt is becoming increasingly important in China, information exchange and coordinated research among different groups are relatively limited. Therefore, the First Chinese Bacterial Wilt Symposium was organized in Chongqing in December 2016 to bring together researchers to discuss the long-term strategies to understand and control bacterial wilt. This meeting was a landmark of bacterial wilt research in China and brought together both Chinese and international researchers.
Bacterial wilt is notoriously known in China as “Green wilt disease ( , Qing Ku Bing)” by farmers and scientists because the leaves of the infected plant remain green when the plant starts to shows wilt symptoms. Before the 1960s, limited research in China was conducted on bacterial wilt disease, even though the disease was first recorded on peanut in the 1930s (Ma and Gao, 1956). Guangdong Academy of Agricultural Sciences conducted the isolation of Ralstonia spp. from many plants in Guangdong Province in the middle of the 1960s. With the intensification of agricultural production, the prevalence of bacterial wilt has increased on Solanaceous crops as well as other hosts. In recent four decades, extensive research on pathogenic aspects and disease management strategies has been carried out throughout the country. Here we summarized the history and current status of bacterial wilt, disease control approaches, and the research community of China. This article will provide basic information and some suggestions for further research on this aggressive disease.
, Qing Ku Bing)” by farmers and scientists because the leaves of the infected plant remain green when the plant starts to shows wilt symptoms. Before the 1960s, limited research in China was conducted on bacterial wilt disease, even though the disease was first recorded on peanut in the 1930s (Ma and Gao, 1956). Guangdong Academy of Agricultural Sciences conducted the isolation of Ralstonia spp. from many plants in Guangdong Province in the middle of the 1960s. With the intensification of agricultural production, the prevalence of bacterial wilt has increased on Solanaceous crops as well as other hosts. In recent four decades, extensive research on pathogenic aspects and disease management strategies has been carried out throughout the country. Here we summarized the history and current status of bacterial wilt, disease control approaches, and the research community of China. This article will provide basic information and some suggestions for further research on this aggressive disease.
Distribution and Species Complexity
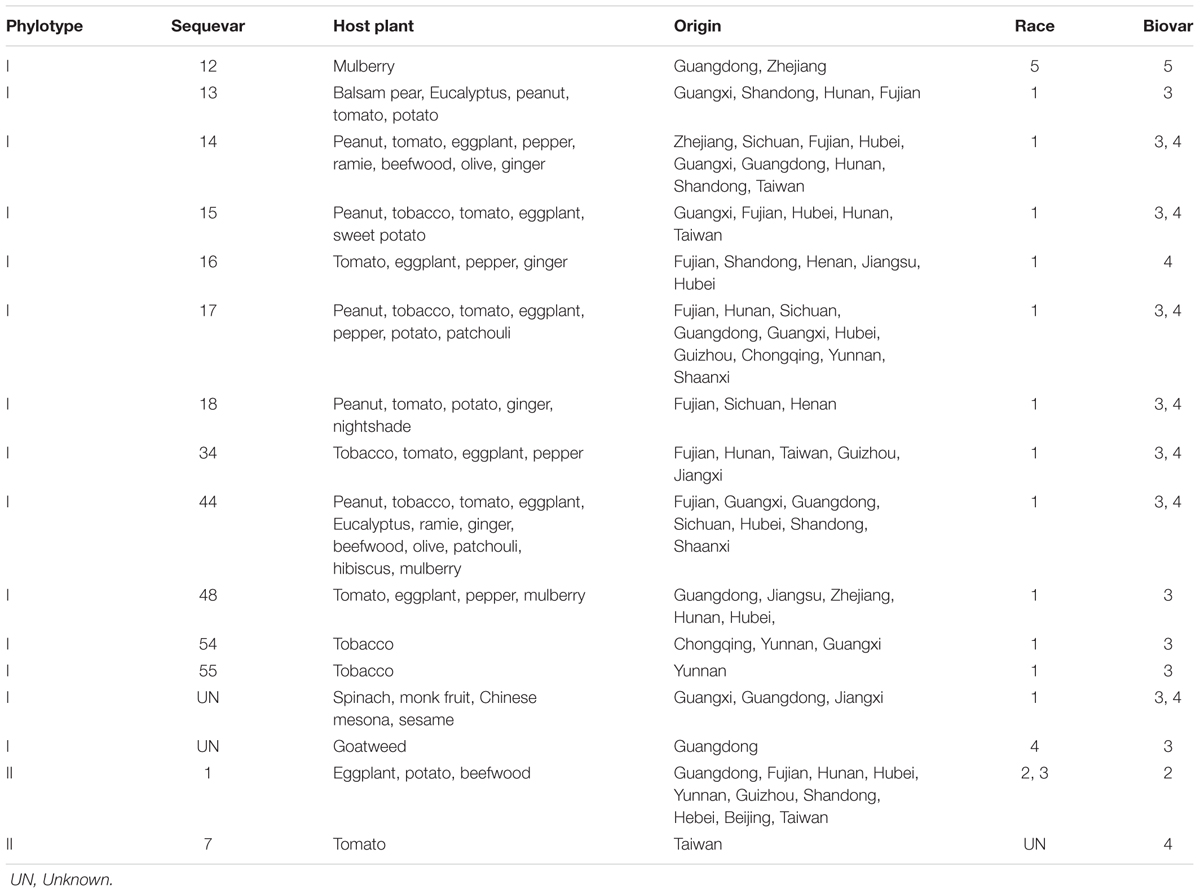
In China, bacterial wilt disease has been reported in 30 provinces, with more in southern and eastern areas than northern and western areas (Figure 1). We could not find any published report of bacterial wilt occurrence in Tibet (Xizang) and Macau (Figure 1). There were sporadic reports from Shangai, Hongkong, Jilin, Xinjiang and Liaoning before 2012 (Fan, 1987; Huang, 1991; Liu W.Y. et al., 2012), but not recently (Figure 1). Fujian, Guangxi, Guangdong, Sichuan, Taiwan, Chongqing, and Hunan are the provinces where Ralstonia spp. strains have been most frequently isolated and described based on the available reports (Figure 1). Although Ralstonia spp. is prevalent in tropical and subtropical regions of the world, it has recently become a recognized problem in temperate regions as well (Elphinstone, 2005). The similar trend was observed in China. During the last decade, bacterial wilt disease has been reported more frequently in temperate and cool areas. The epidemics of bacterial wilt from warm lowlands (southern and eastern parts of China) to cool highlands of low and high latitudes of North China’s provinces is possibly due to the global climate warming, and changes in the cropping systems in China (Kong, 2003; Zhou et al., 2012; Liu et al., 2017).
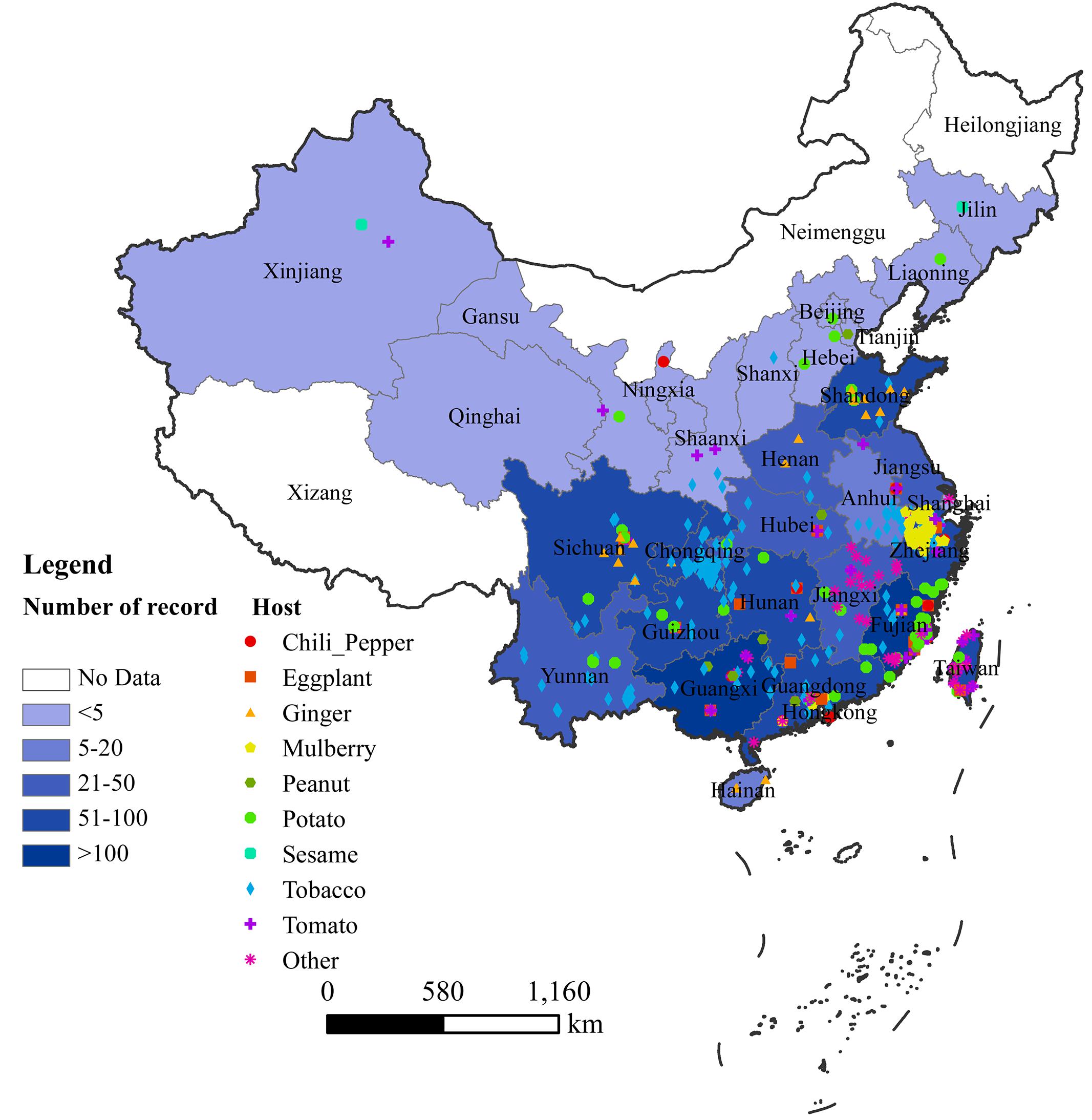
FIGURE 1. Geographical distribution of bacterial wilt in China. Records of each province were extracted from both CNKI and WoS, as well as information from website reports. Color depth indicates the number of records in each province.
Numerous studies have focused on the characterization of genetic diversity of Ralstonia spp. in China (Xu et al., 2009; Xue et al., 2011; Li et al., 2016; Liu et al., 2017). For instance, Ralstonia spp. infects more than 90 plant species in China (see Host Range). Some host plants such as peanut, potato, tobacco are commonly grown in more than 10 provinces and the topography and climate vary greatly between these agroecosystems (Chen and Zhang, 2000). The Ralstonia spp. strains from China belong to Race 1, 3, 4, and 5 and biovar 2, 3, 4, and 5 according to the race and biovar classification schemes (He et al., 1983; Hayward, 1991) (Table 1). Among those, Race 1 is most predominant being able to infect the largest number of hosts and being widely distributed across 17 provinces in China (Table 1). Overall, the evolutionary patterns of R. solanacearum in China are more divergent and complex than other parts of the world. This may be due to few countries have such a variety of environmental conditions for Ralstonia spp. as in China.
Based on the phylotyping scheme (Fegan and Prior, 2005), global Ralstonia spp. strains comprise four phylotypes indicating their geographical origins. Each phylotype is further subgrouped into sequevars based on endoglucanase gene similarity (Fegan and Prior, 2005). The phylotype I strains reported in China comprise 15 sequevars and phylotype II strains are consisting of 2 sequevars, see Table 1. R. pseudosolanacearum strains possess high level of phylogenetic diversity, comprising a total of 10 sequevars (12 – 18, 34, 44, and 48) with the largest host range (Xu et al., 2009; Xue et al., 2011; Wang L. et al., 2017). Recently, new sequevars were identified including sequevar 14M (Wang L. et al., 2017) isolated from potato and peanut in 2015 and sequevars 54 (Li et al., 2016) and 55 (Liu et al., 2017) isolated from tobacco in 2016. Ralstonia spp. isolated from the same hosts, for instance tobacco (Zheng et al., 2007; Li et al., 2016; Liu et al., 2017), peanut (Xie et al., 2009) and mulberry (Huang et al., 2017), show high genetic diversity. For example, potato and tobacco are infected by 9 sequevars (1, 13 – 18, 34, and 14 M) and 10 sequevars (1, 13 – 18, 34, 44, 54, 55) (Xu et al., 2009; Xue et al., 2011; Li et al., 2016; Liu et al., 2017; Wang X. et al., 2017). Interestingly, the diversity of tobacco isolate phylotype I is negatively affected by the elevation, i.e., less divergent or more geographically distinct in the highland areas (Liu et al., 2017) possibly due to recent adaptation to cool temperatures. Further research is required to understand the molecular bases determining environmental adaption of this pathogen. Importantly, 91% of 123 potato Ralstonia spp. isolates from 13 provinces belong to phylotype II/sequevar 1, race 3 biovar 2, R3B2 (Wang X. et al., 2017). R3B2, known as cold-tolerant group, significantly constraints Solanaceous crops at higher land or in temperate zones of Africa, Asia, and Latin America (Champoiseau et al., 2009; Milling et al., 2009).
Host Range
The history of bacterial wilt in China started with the first report of an outbreak on peanut in the 1930s. Ralstonia spp. were then isolated from a sweet potato in 1946 (Hwang et al., 1956) as well as from ginger, sesame, potato, tobacco, and tomato in the 1950s (Wang, 1959; He et al., 1983). Since 1960s, diseased area has expanded greatly and many new host plants have been observed indicative of host range expansion. In addition to herbaceous plants, bacterial wilt also attacks several woody plants, including olive, mulberry, Casuarina spp. and Eucalyptus spp. For example, the olive bacterial wilt became an emerging bacterial disease in China when the olive was introduced from Albania to China in 1964 (Sichuan Academy of Forestry, 1977). Bacterial wilt of C. equisetifolia was discovered at Yangjiang and Baixian of Guangdong in 1964 (Liang and Wang, 1982), and later extended to southeast coast including Guangdong, Guangxi, Fujian, and Hainan after a typhoon in 1969 (Sun et al., 2013). Until now, the host range of Ralstonia spp. in China encompasses more than 90 plant species belonging to 39 botanical families with the largest number of hosts in Solanaceae (Figure 2). New hosts are still being reported including roselle and chard in Taiwan (Wu et al., 2013; Lin et al., 2015) and fig in Fujian (Jiang et al., 2016). Around 20 of these host plants have not been reported to become infected by Ralstonia spp. in other countries and are thus specific to China (Italic and bold highlight in Figure 2).
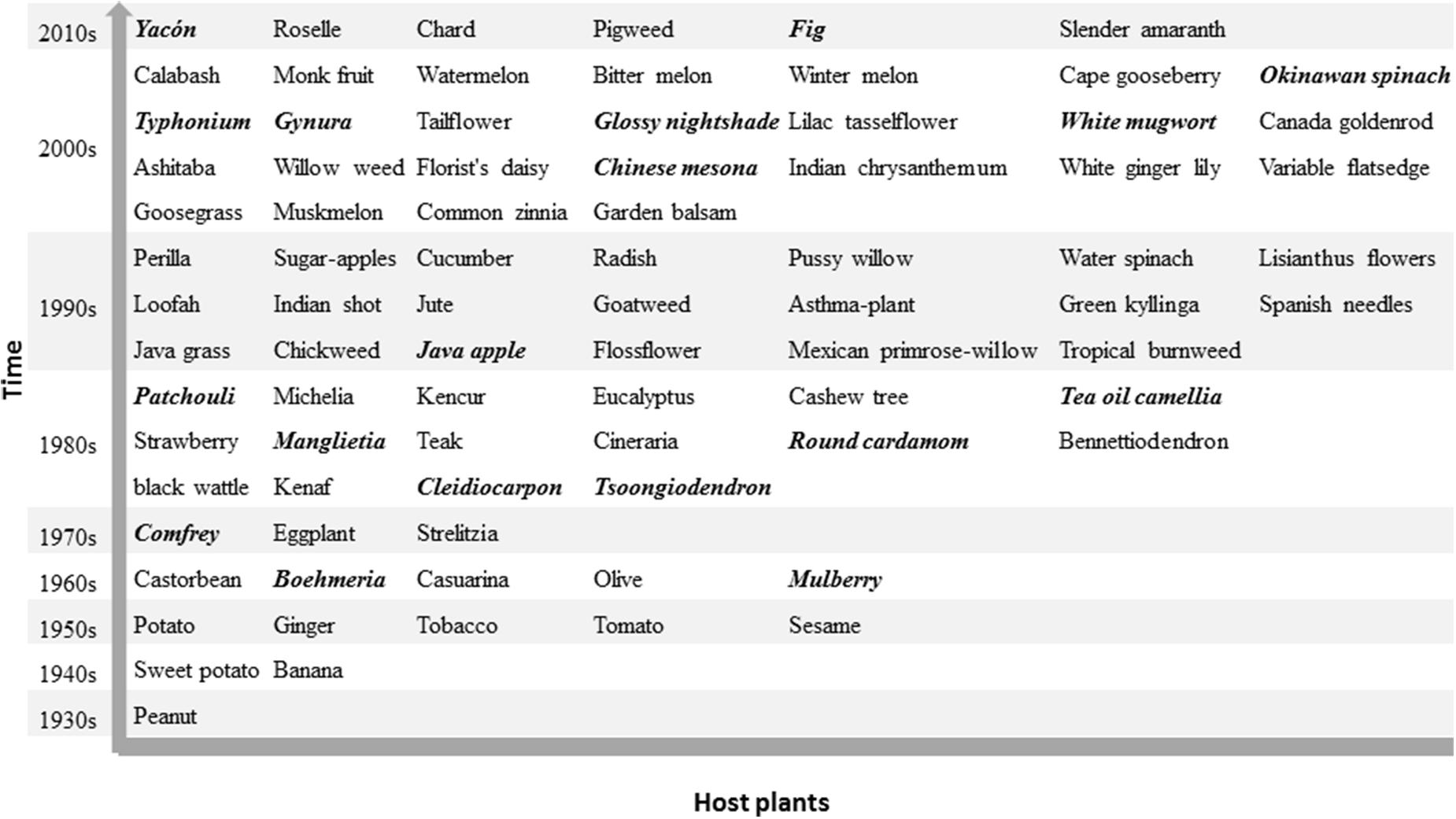
FIGURE 2. Host plants of the pathogenic Ralstonia spp. in China. A total of 84 plant species present here based on discovered decade since the first record on peanut in 1930s. Some of them are composed by more than one closed species like Casuarina spp., Eucalyptus spp. The common names in italic and bold indicate the host plants of Ralstonia spp. reported only in China. There is no ranked discovered time in this figure. Detail information of host plant and discovered year in Supplementary File 1.
Importance of Bacterial Wilt
Estimating economic losses caused by the bacterial wilt disease in China is difficult since direct yield losses vary widely according to host, cultivar, climate, soil type, cropping practice and pathogen strain. Therefore, the level of damage is commonly expressed on a crop-by-crop basis and can range from minimal crop loss to a very high economic damage. For instance, bacterial wilt of tomato is a severe problem in the southern provinces of the Yangtze River, ranging from 10% to 80% disease incidence depending on the crop seasons (Wei et al., 2011, 2015, 2017). In the case of potato, more than 10 provinces are infested by bacterial wilt disease with estimated yield losses ranging from 10–15% to even 80 or 100% in some hot-spot fields (Hua et al., 1985; Chen et al., 2005). The tobacco bacterial wilt broadly occurs in 14 out of the 22 main tobacco growing regions and has caused great economic losses in the recent years (Chen et al., 1997; Liu et al., 2017). Disease incidence is typically around 15–35%, but can reach up to 75% and even higher when associated with other root diseases such as Black shank caused by Phytophthora nicotianae var. nicotianae. In the wet and mono cropping tobacco areas, yield reduction ranges from 50 to 60% and up to even 100% during extreme outbreaks. With chili, disease incidence varies between 20 and 50% (Tan et al., 2014). Bacterial wilt is also prevalent in most ginger-growing areas, where it reduces the yield by 20–30% (Liu et al., 2005). Bacterial wilt of peanut has been observed in most of the 13 main peanut producing provinces. It is estimated to affect 800,000 hectares of agricultural land, which is nearly 16% of the total planting area in China. Yield losses in peanut varies between 10–20% and can reach up to 50–100% in extreme cases (Yu et al., 2011).
Bacterial wilt is also very serious in some woody and shrub plants, being especially significant in Eucalyptus spp. Since first reported on E. saligna and E. grandis in Guangxi in 1982 (Cao, 1982), this disease has been found in Guangdong, Yunnan, Hainan, Fujian, etc. (Wu and Liang, 1988). The bacterial wilt incidence of Eucalyptus spp. ranged from 20 to 40% in Guangdong and Hainan to 90% in Fujian (Wu et al., 2007). In beefwood (Casuarina spp.), bacterial wilt was first observed in Guangdong in 1964, and then was reported in Fujian, Guangxi, Hainan (Deng and Nan, 1979; Liang and Wang, 1982; Zheng et al., 1992). The bacterial wilt incidence of Beefwood varies around 50 and 90% (Sun et al., 2013). The bacterial wilt of mulberry was first found in 1973 at an orchard in Shunde of Guangdong (Lai et al., 1982), and expanded to Jiangxi in 1988 (Liu, 1996) and Zhejiang in 1992 (Chai and Dai, 1994). It was estimated that about 10% of the mulberry plantation was affected by bacterial wilt in China. The current outbreak in Zhejiang province has forced some growers to abandon mulberry cultivation, thus posing a serious threat to the local sericulture industry (Zhu et al., 2005; Pan et al., 2013). Therefore, bacterial wilt disease can be ranked as the most important disease in China due to its wide distribution and cumulative losses on many crops, trees and ornamental and medicinal plants.
Disease Control
Cultural practices are traditional and popular approaches to control the bacterial wilt disease in China. Effect of crop rotation and grifting on bacterial wilt have been widely evaluated in greenhouse and field conditions (Huang et al., 1997, 2009; Zhang et al., 2007; Huang and Lei, 2013; Ouyang et al., 2015). Weeding and soil disinfection might relieve or extenuate bacterial wilt because contaminated weeds and soil are a major sources of Ralstonia spp. infections in the field (Fang et al., 2013). Soil amendment and fumigation are widespread means used in soil management in China. For instance, the formulated product S-H mixture (4.4% bagasse, rice 8.4%, 4.25% oyster shell powder, urea, 8.25%, 1.04% potassium nitrate, 13.16 and 60.5% silicate slag SSP) is effective to control several soil-borne diseases including fusarium wilt and bacterial wilt of many crops by enhancing the fertility and microbial abundance in soil (Sun and Huang, 1985; Yao et al., 1994). Calcium amendments like CaO and CaCO3 are effective in controlling bacterial wilt by inhibiting pathogen survival through changes in the pH and nitrite accumulation in the field (Gong et al., 2013; He et al., 2014). Bacterial wilt of tobacco can also be suppressed by supplementation of mineral nutrients like calcium and molybdenum (Zheng S. et al., 2014). Organic fertilizer and biochar amendments are promising alternatives to suppress bacterial wilt by increasing the soil pH, electric conductivity, organic carbon and nitrogen availability and microbial activities (Cai et al., 2003; Xue et al., 2010; Wei et al., 2011; Zhang et al., 2013; Liu et al., 2015; Gu et al., 2016). Soil fumigants like Chloropicrin, Dazomet, and Bromomethane (phased out in 2015 in China) can be applied to control bacterial wilt and other soil-borne diseases (Wang et al., 2010).
Breeding of crop cultivars with suitable resistance is regarded as a key approach for integrated management of bacterial wilt. Remarkable progress has been achieved in developing resistant cultivars for some economically important crops in China including peanut (Sun et al., 1981; Chen et al., 2007; Liao, 2014), tobacco (Liu Y. et al., 2012), potato (Deng et al., 2014), tomato (Yin et al., 2005), pepper (Dang et al., 2013), and eggplant (Li et al., 2014). The resistance levels and the diversity of germplasms are very important for the genetic breeding of bacterial wilt resistance. In addition, identification of quantitative trait loci (QTL) associated with the resistance has enabled breeders to develop resistant cultivars through marked assisted selection (MAS), such as qBW-1 and qBW-2 in peanut (Zhao et al., 2016) and qBWR-3a/-3b and qBWR-5a/-5b in tobacco (Qian et al., 2013). Many factors have limited the breeding of plant resistance against bacterial wilt including the difficulty to conduct large-scale screening of resistant materials, lack of elite resistant parents, undesirable genetic linkages between resistance and other agronomic traits as well as the high diversity of Ralstonia spp. virulence factors (Liao, 2005).
Biological control is another promising way to reduce bacterial wilt severity. Biological agents have been used to control bacterial wilt in China for a long time (Meng, 1964). In principle, any microbe which is able to inhibit Ralstonia spp. population density, or to reduce its pathogenicity, has the potential for biological control of bacterial wilt. The most frequently applied microbial agents are Streptomyces spp. (Lu et al., 2013; Xiong et al., 2014), Bacillus spp. (Ran et al., 2005; Lei et al., 2010; Wei et al., 2011; Wang et al., 2015), Pseudomonas spp. (Yang et al., 2008; Qiao et al., 2015; Hu et al., 2016), avirulent Ralstonia spp. mutants (Chen et al., 2004; Yang et al., 2008), phage (Wang X. et al., 2017) and other microbes (Guo et al., 2004; Xue et al., 2009; Yang et al., 2012; Huang et al., 2013). Due to the unstable performance of application of single biocontrol agent under field conditions (Wei et al., 2011, 2015, 2017), beneficial microbial consortia that could better utilize available resources and produce antibiotics may help to improve the consistency and efficacy of bacterial wilt biocontrol (Wei et al., 2015; Hu et al., 2016; Yang et al., 2017). Novel strategies are also being developed to enhance disease suppression. A recent study demonstrated that combination of antibiotic-producing Bacillus strains and Ralstonia spp. specific phages had a better control effect on bacterial invasion of tomato due to additive synergistic effects (Wang X. et al., 2017).
Current Status of Bacterial Wilt Research in China
There have been three stages of the research on bacterial wilt in China. The first period (field practice) of research on bacterial wilt started with disease survey, pathogen identification in plant hosts, then extended to epidemiology and disease controls from the 1930s to the late 1970s. In second period, many researches had been moving from the field to laboratory, mainly on ecology, genetics, phytopathology, etc. This great increase was due to the improvement of education and the development of life science disciplines until the end of the 1990s. After that, the economic and scientific importance of bacterial wilt quickly gained attention across the international research community, prompting more scientists to dedicate to both fundamental and applied research on this topic. Meanwhile, the Chinese bacterial wilt research entered into a new era, i.e., the third period. It’s no longer simple field practice or laboratory research but from the field to the laboratory and back again. Researchers deploy practical strategies to cure the disease in the field, as it is exemplified by the extensive utilization of resistant cultivars in peanut and several Solanaceae crops, which has played an important role in reducing yield losses due to the disease.
Most publications related to bacterial wilt and Ralstonia spp. are written in Chinese, with 597 records from Web of Science (WoS) and 5441 records from China National Knowledge Infrastructure (CNKI) by the end of 2016. Only 50 publications were recorded in WoS but 1098 records in CNKI before the new century. Both records from WoS and CNKI have increased every year by following the trend of the world publication rate since the 1990s. The percentage of WoS publications from China increased from 1.64% (2001) to 14.42% (2016) and is now ranked now the second after the United States (Figure 3).
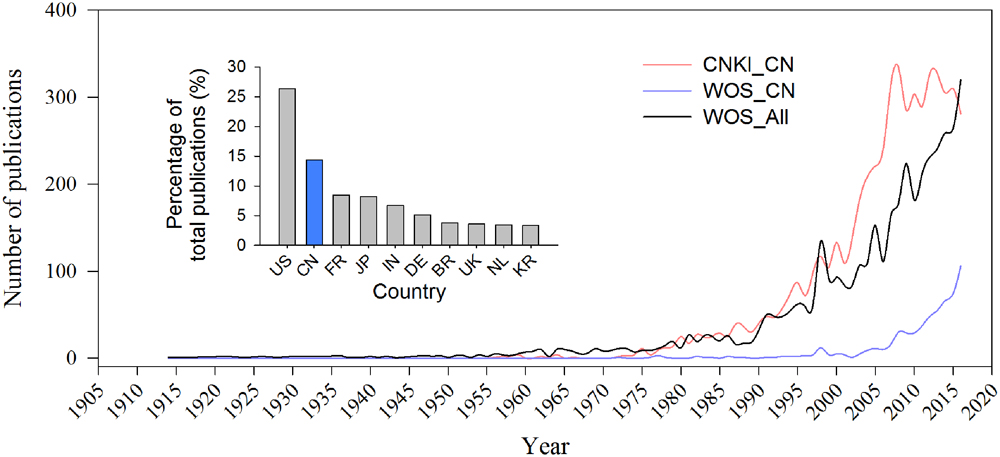
FIGURE 3. Publication records of the pathogenic Ralstonia spp. and bacterial wilt. Blue line indicates number of publications written in Chinese, i.e., records from CNKI. Red and black line show the records from Chinese organizations and world community on WoS. Small panel in Figure 3 shows the Top 10 numbers of reports on Ralstonia spp. from WoS published by researchers from United States (US), China (CN), France (FR), Japan (JP), India (IN), Germany (DE), Brazil (BR), United Kingdom (UK), the Netherlands (NL), and Korea (KR).
There are many organizations in China working on various research fields related to bacterial wilt and Ralstonia spp. biology. These areas include the ecology of the disease, pathogen diversity and evolutionary dynamics, microbiology and disease management and breeding of host-plant resistance. The main aims are described below:
(i) Ecology and diagnostics of bacterial wilt: assess the extent of problems caused by Ralstonia spp. to crops and understand the epidemiology of the disease in order to predict bacterial wilt outbreaks.
(ii) Genetic diversity and evolutionary dynamics of the pathogen: understand how host type and the environmental and geographic factors affect the evolution, diversification and population structure of Ralstonia spp.
(iii) Virulence mechanisms and host–plant responses: study pathogenicity determinants (e.g., type III/VI effectors) and complex regulation networks as well as the host responses upon Ralstonia spp. infections.
(iv) Control of bacterial wilt disease: delay or constrain bacterial wilt outbreaks and reduce yield losses by changing agricultural practices, developing chemicals and biocontrol agents, and breeding for resistant cultivars.
Perspectives
In recognition of the importance of bacterial wilt in China, Southwest University, Nanjing Agricultural University, Institute of Plant Protection of CAAS, Institute of Tobacco Research of CAAS, Oil Crops Research Institute of CAAS and Municipal Agriculture Committee of Chongqing jointly organized the 1st China Plant Bacterial Wilt Symposium (CPBW) in Chongqing from 11th to 14th December 2016. Around 200 participants attended the meeting, including researchers and scientists from 67 organizations and 15 provinces. In addition, the Chairman of 2016 IBWS, Dr. Stéphane Genin, attended this meeting and highlighted the contribution of Chinese researchers to the global bacterial wilt community. In this congress, 40 speakers presented their works involving genetic diversity, evolution and virulence effectors of Ralstonia spp., mechanisms of interaction with host plants, significance of plant resistance and various approaches to biocontrol bacterial wilt.
The emerging problems of Ralstonia spp. in China are common to most regions in the world. These challenges include unusually broad host range, high and complex genetic diversity, strong and rapid pathogen adaption to new environments such as cold regions of high latitude and altitude. The main task of bacterial wilt research is coordinated on the basic knowledge of Ralstonia spp. and innovative control treatments in the fields. Seeking green, efficient, environmentally friendly and feasible strategies for the management of bacterial wilt is a great challenge to the international scientific community. Through in-depth knowledge exchange and discussion, the attendees at the 1st CPBW reached a consensus and made a declaration toward the core research aims of the bacterial wilt research:
(i) Initiate a national network/platform for bacterial wilt research: develop collaborative innovations for cooperation and development, enhance communication and material exchanges, enable a concerted effort to jointly improve bacterial wilt research and favor the sustainable disease management.
(ii) Reinforcement of international exchanges and collaboration: strengthen cooperation in multidisciplinary fields and to maintain contributions to both theoretical and practical approaches.
(iii) Integrate production, education, and research: promote interactions between companies (application), universities (education) and research institutes (research) to find interdisciplinary solutions for the control of bacterial wilt.
(iv) Calling for policy guidance: gain attention from relevant governmental departments of China and other countries to support fundamental and applied research on bacterial wilt, call for special funding programs for the expansion of this research community, and maintain the high level of research.
(v) China Plant Bacterial Wilt Symposium: organize the meeting every 2 years by universities or institutes to reunite Ralstonia spp. research community. The 2nd CPBW will be hosted by Plant Protection Institute of CAAS in Beijing in 2018.
Author Contributions
Organization: GJ and ZW; Curation: GJ, ZW, JX, HC, YZ, XS, AM, WD, and BL; Writing – original draft: GJ and ZW; Writing – review and editing: JX, HC, YZ, XS, AM, WD, and BL.
Funding
This research was financially supported the National Natural Science Foundation of China (41671248 to ZW, 31272008 to JX, 31200067 and 31670082 to YZ), the Young Elite Scientist Sponsorship Program by CAST (2015QNSC001, ZW), the Natural Science Foundation of Jiangsu Province (BK20170085, ZW), the key project of the China National Tobacco Corporation (110201502019 and 110201601025 LS-05 to WD and GJ), the Fundamental Research Funds for the Central Universities (2013PY080 to HC), and the Initial Project Fund from Huazhong Agricultural University (12012 to HC). AM is supported by funds from the Chinese Academy of Sciences and the Chinese 1000 Talents Program.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Special acknowledgment to the main organizers (WD, BL, and Jie Feng) and sponsors (Southwest University Chongqing, Tobacco Science Research Institute and Zhejiang Longwan Chemicals Co., Ltd.) of First China Bacterial Wilt Symposium. Zhaofu Li was acknowledged for the visualization of Figure 1. We wish to apologize to colleagues whose work in China could not be cited due to space limitations.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01549/full#supplementary-material
References
Cai, Y., Liao, Z., Zhang, J., Kong, W., and He, C. (2003). Effect of ecological organic fertilizer on tomato bacterial wilt and soil microbial diversities. J. Appl. Ecol. 14, 349–353.
Cao, J. (1982). Investigation report of bacterial wilt in Eucalyptus saligna and E. grandis. Guangxi For. Sci. 4, 30–31.
Chai, X., and Dai, H. (1994). Occurrence and control of Mulberry bacterial wilt in Zhejiang Province. Bull. Seric. 25, 53.
Champoiseau, P. G., Jones, J. B., and Allen, C. (2009). Ralstonia solanacearum Race 3 biovar 2 causes tropical losses and temperate anxieties. Plant Health Prog. 10. doi: 10.1094/PHP-2009-0313-01-RV
Chen, B., Jiang, H., Liao, B., and Ren, X. (2007). Progress on groundnut genetic enhancement for bacterial wilt resistance. Chin. Agric. Sci. Bull. 23, 369–372. doi: 10.1094/PHP-2009-0313-01-RV
Chen, Q., Wong, Q., and Hu, F. (2004). Effects of avirulent strains of Ralstonia solanacearum on tomato bacterial wilt. Chin. J. Biol. Control 20, 42–44.
Chen, R., Zhu, X., Wang, Z., Guo, Z., Dong, H., Wang, L., et al. (1997). A report of investigating and studying tobacco infectious diseases of 16 main tobacco producing provinces (regions) in China. Chin. Tob. Sci. 4, 1–7.
Chen, Y., He, L., and Xu, J. (2005). Detection of bacterial wilt infection in potato using PCR. J. Plant Prot. 32, 129–132.
Chen, Z., and Zhang, X. (2000). Value of ecosystem services in China. Chin. Sci. Bull. 45, 870–876. doi: 10.1007/BF02886190
Dang, F., Lei, Y., Guan, D., Wang, Z., and He, S. (2013). Identification and evaluation of resistance to bacterial wilt in pepper. Plant Sci. J. 31, 378–384. doi: 10.3724/SP.J.1142.2013.40378
Deng, H., and Nan, Y. (1979). Preliminary report on two strains of olive and peanut bacterial wilt. Pract. For. Technol. 10, 19–22.
Deng, R., Deng, K., He, T., Lei, Z., and Chen, E. (2014). Progresses of main diseases resistance breeding in potato. Southwest China J. Agric. Sci. 27, 1337–1342.
Elphinstone, M. (2005). “The current bacterial wilt situation: a global overview,” in Bacterial Wilt Disease and the Ralstonia Solanacearum Species Complex, eds C. Allen, P. Piror, and A. C. Hayward (St. Paul, MN: APS Press), 9–28.
Fan, H. (1987). Adjusting farmland microclimate to control the bacterial wilt in pepper. Bimon. Xinjiang Meteorol. 10, 23.
Fang, S., Gu, G., Chen, Y., Huang, C., and Chen, S. (2013). Colonization and infection of Ralstonia solanacearum in weed roots. Acta Tabacaria Sin. 19, 72–81. doi: 10.1128/mBio.00875-13
Fegan, M., and Prior, P. (2005). “How complex is the Ralstonia solanacearum species complex,” in Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex, eds C. Allen, P. Prior, and A. C. Hayward (St. Paul, MN: APS Press), 449–461.
Gong, M., Wang, R., Du, G., and Zheng, S. (2013). Effect of lime on the disease incidence of tobacco bacterial wilt. Plant Dr. 26, 44–45.
Gu, Y., Hou, Y., Huang, D., Hao, Z., Wang, X., Wei, Z., et al. (2016). Application of biochar reduces Ralstonia solanacearum infection via effects on pathogen chemotaxis, swarming motility, and root exudate adsorption. Plant Soil 415, 269–281. doi: 10.1007/s11104-016-3159-8
Guo, J. H., Qi, H. Y., Guo, Y. H., Ge, H. L., Gong, L. Y., Zhang, L. X., et al. (2004). Biocontrol of tomato wilt by plant growth-promoting rhizobacteria. Biol. Control 29, 66–72. doi: 10.1016/S1049-9644(03)00124-5
Hayward, A. C. (1991). Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 29, 65–87. doi: 10.1146/annurev.py.29.090191.000433
He, K., Yang, S. Y., Li, H., Wang, H., and Li, Z. L. (2014). Effects of calcium carbonate on the survival of Ralstonia solanacearum in soil and control of tobacco bacterial wilt. Eur. J. Plant Pathol. 140, 665–675. doi: 10.1007/s10658-014-0496-4
He, L. Y., Sequeira, L., and Kelman, A. (1983). Characteristics of strains of Pseudomonas solanacearum from China. Plant Dis. 67, 1357–1361. doi: 10.1094/PD-67-1357
Hu, J., Wei, Z., Friman, V. P., Gu, S. H., Wang, X. F., Eisenhauer, N., et al. (2016). Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. mBio 7:e1790–16. doi: 10.1128/mBio.01790-16
Hua, J., Zhang, C., and He, L. (1985). A preliminary study on strains of Pseudomonas solanacearum of potato in China. Acta Phytopathol. Sin. 15, 181–184.
Huang, F., Chen, Y., Zhou, X., Li, S., Li, Y., and Wu, Z. (1997). Study on integrated control of tobacco bacterial wilt. Guangxi Agric. Sci. 1, 32–35.
Huang, J., Wei, Z., Tan, S., Mei, X., Yin, S., Shen, Q., et al. (2013). The rhizosphere soil of diseased tomato plants as a source for novel microorganisms to control bacterial wilt. Appl. Soil Ecol. 72, 79–84. doi: 10.1016/j.apsoil.2013.05.017
Huang, T., Zhao, X. A., and Jiang, X. (2009). Study on effect of different rootstocks on resistance to bacterial wilt in tomato. J. Chang Veg. 05x, 55–56. doi: 10.3865/j.issn.1001-3547.2009.10.021
Huang, W., Zhang, H., Xu, J., Wang, S., Kong, X., Ding, W., et al. (2017). Loop-mediated isothermal amplification method for the rapid detection of Ralstonia solanacearum phylotype I Mulberry strains in China. Front. Plant Sci. 8:76. doi: 10.3389/fpls.2017.00076
Huang, Y., and Lei, D. (2013). Study on bacterial wilt resistance of tomato grafted by different rootstocks. Acta Agric. Jiangxi 25, 73–75.
Huang, Z. (1991). Discussion on the causes of cucumber bacterial wilt in suburbs of Shanghai. Shanghai Veg. 16, 36–38.
Hwang, L., Chen, Y. S., and Hwang, H. Y. (1956). A preliminary study of sweet potato wilt and its control. Acta Phytopathol. Sin. 2, 97–111.
Jiang, Y., Li, B., Liu, P., Liao, F., Weng, Q., and Chen, Q. (2016). First report of bacterial wilt caused by Ralstonia solanacearum on fig trees in China. For. Pathol. 46, 256–258. doi: 10.1111/efp.12267
Lai, W., Jiang, Z., Tan, B., Wu, G., Chen, J., Guan, W., et al. (1982). Identification of Mulberry bacterial wilt pathogens. J. South China Agric. Univ. 3, 66–73.
Lei, J., Duan, J., Ma, H., Li, J., Li, H., and Yang, Z. (2010). Screening, identification and optimized fermentation condition of an actinomycete strain against Pseudomonas solanacearum. Chin. J. Appl. Environ. Biol. 16, 79–83. doi: 10.3724/SP.J.1145.2010.00079
Li, W., Lv, L., and Wei, C. (2014). Research advance in bacterial wilt resistance of eggplant. Guangdong Agric. Sci. 41, 91–94.
Li, Y., Feng, J., Liu, H., Wang, L., Hsiang, T., Li, X., et al. (2016). Genetic diversity and pathogenicity of Ralstonia solanacearum causing tobacco bacterial wilt in China. Plant Dis. 100, 1288–1296. doi: 10.1094/PDIS-04-15-0384-RE
Liang, Z. C., and Wang, T. Z. (1982). Resistance measurements of Casuarina equisetifolia L. to bacterial wilt disease. Trop. For. 1, 31–34.
Liao, B. (2005). “A broad review and perspective on breeding for resistance to bacterial wilt,” in Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex, eds C. Allen, P. Prior, and A. C. Hayward (St. Paul, MN: American Phytopathological Society), 225–238.
Liao, B. (2014). “Peanut Breeding,” in Genetics, Genomics and Breeding of Peanuts, eds N. Mallikarjuna and R. K. Varsheney (Boca Raton, FL: CRC Press), 61–78.
Lin, C. H., Chuang, M. H., and Wang, J. F. (2015). First report of bacterial wilt caused by Ralstonia solanacearum on Chard in Taiwan. Plant Dis. 99, 282–282. doi: 10.1094/PDIS-07-14-0715-PDN
Liu, L., Sun, C., Liu, S., Chai, R., Huang, W., Liu, X., et al. (2015). Bioorganic fertilizer enhances soil suppressive capacity against bacterial wilt of tomato. PLOS ONE 10:e0121304. doi: 10.1371/journal.pone.0121304
Liu, M., Zhang, M., Ji, J., Yin, F., Zhang, Y., Tu, Y., et al. (2005). Advances in research bacterial wilt of ginger in China. Chin. Agric. Sci. Bull. 21, 337–340.357.
Liu, W. Y., Chung, K. M. K., Wong, C. F., Jiang, J. W., Hui, R. K. H., and Leung, F. C. C. (2012). Complete genome sequence of the endophytic Enterobacter cloacae subsp. cloacae strain ENHKU01. J. Bacteriol. 194, 5965. doi: 10.1128/JB.01394-1312
Liu, Y., Fan, J., and Li, Y. (2012). Research progress on tobacco breeding resistant to bacterial wilt. Acta Tabacaria Sin. 18, 93–99.
Liu, Y., Wu, D., Liu, Q., Zhang, S., Tang, Y., Jiang, G., et al. (2017). The sequevar distribution of Ralstonia solanacearum in tobacco-growing zones of China is structured by elevation. Eur. J. Plant Pathol. 147, 541–551. doi: 10.1007/s10658-016-1023-1026
Lu, Z., Peng, L., Dng, H., Zuo, X., Peng, J., and Jiang, X. (2013). Screening and identifying of antagonistic actinomycetes against Ralstonia solanacearum. Chin. Tob. Sci. 34, 54–58.
Milling, A., Meng, F., Denny, T. P., and Allen, C. (2009). Interactions with hosts at cool temperatures, not cold tolerance, explain the unique epidemiology of Ralstonia solanacearum race 3 biovar 2. Phytopathology 99, 1127–1134. doi: 10.1094/PHYTO-99-10-1127
Ouyang, X., Chao, Z., Bai, Z., Wu, Y., Chen, Q., Tian, M., et al. (2015). Preliminary study on grafting cultivation of tobacco with eggplant. Hunan Agric. Sci. 1, 25–26. doi: 10.16498/j.cnki.hnnykx.2015.05.010
Pan, Z. C., Xu, J., Prior, P., Xu, J. S., Zhang, H., Chen, K. Y., et al. (2013). Development of a specific molecular tool for the detection of epidemiologically active mulberry causing-disease strains of Ralstonia solanacearum phylotype I (historically race 5-biovar 5) in China. Eur. J. Plant Pathol. 137, 377–391. doi: 10.1007/s10658-013-0249-9
Prior, P., Ailloud, F., Dalsing, B. L., Remenant, B., Sanchez, B., and Allen, C. (2016). Genomic and proteomic evidence supporting the division of the plant pathogen Ralstonia solanacearum into three species. BMC Genomics 17:90. doi: 10.1186/s12864-016-2413-z
Qian, Y., Wang, X., Wang, D., Zhang, L., Zu, C., Gao, Z., et al. (2013). The detection of QTLs controlling bacterial wilt resistance in tobacco (N. tabacum L.). Euphytica 192, 259–266. doi: 10.1007/s10681-012-0846-842
Qiao, J., Chen, Z., Liang, X., Liu, Y., and Liu, Y. (2015). Colonization of Bacillus subtilis Bs916 on tomato root. Jiangsu J. Agric. Sci. 31, 229–234.
Ran, L. X., Liu, C. Y., Wu, G. J., van Loon, L. C., and Bakker, P. A. H. M. (2005). Suppression of bacterial wilt in Eucalyptus urophylla by fluorescent Pseudomonas spp. in China. Biol. Control 32, 111–120. doi: 10.1016/j.biocontrol.2004.08.007
Safni, I., Cleenwerck, I., De Vos, P., Fegan, M., Sly, L., and Kappler, U. (2014). Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis subsp. nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R. solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum sp. nov. Int. J. Syst. Evol. Microbiol. 64, 3087–3103. doi: 10.1099/ijs.0.066712-66710
Sichuan Academy of Forestry (1977). Study of bacterial wilt control on common olive. Sci. Silvae Sin. 13, 61–66.
Sun, D. R., Chen, C. R., and Wang, Y. R. (1981). Resistance evaluation of bacterial wilt (Pseudomonas solanacearum E. F. Smith) of peanut (Arachis hypogaea L.) in the People’s Republic of China. Proc. Am. Peanut Res. Educ. Soc. 13, 21–28.
Sun, S., Wu, H. X., and Wang, J. (2013). Research review on the bacterial wilt of Casuarina. For. Pest Dis. 32, 29–34.
Sun, S. K., and Huang, J. W. (1985). Formulated soil amendment for controlling Fusarium wilt and other soilborne diseases. Plant Dis. 69, 917–920. doi: 10.1094/PD-69-917
Tan, Q. Q., Yuan, J., Yang, X. H., Chen, X., Wang, L. S., and Wu, S. P. (2014). Identification of resistance to Phytophtora blight and bacterial wilt in pepper varieties in Guizhou Province Regional Trial. Seed 33, 82–85.
Wang, D., Shen, H., and Ran, L. (2015). Biocontrol of bacterial wilt in Eucalyptus urophylla and growth promotion by Bacillus subtilis strain CN181. Hebei J. For. Orchard Res. 30, 331–334.
Wang, H., Chen, Y., Wang, S., Li, C., Su, X., and Li, S. (2010). Control effects of chloropicrin soil fumigation on tobacco weeds and soil-borne diseases. Chin. Agric. Sci. Bull. 26, 244–248.
Wang, L., Wang, B., Zhao, G., Cai, X., Jabaji, S., Seguin, P., et al. (2017). Genetic and pathogenic diversity of Ralstonia solanacearum causing potato brown rot in China. Am. J. Potato Res. 94, 403–416. doi: 10.1007/s12230-017-9576-2
Wang, X., Wei, Z., Li, M., Wang, X., Shan, A., Mei, X., et al. (2017). Parasites and competitors suppress bacterial pathogen synergistically due to evolutionary trade-offs. Evolution 71, 733–746. doi: 10.1111/evo.13143
Wei, Z., Huang, J., Yang, T., Jousset, A., Xu, Y., Shen, Q., et al. (2017). Seasonal variation in the biocontrol efficiency of bacterial wilt is driven by temperature-mediated changes in bacterial competitive interactions. J. Appl. Ecol. doi: 10.1111/1365-2664.12873 [Epub ahead of print].
Wei, Z., Huang, J. F., Hu, J., Gu, Y. A., Yang, C. L., Mei, X. L., et al. (2015). Altering transplantation time to avoid periods of high temperature can efficiently reduce bacterial wilt disease incidence with tomato. PLOS ONE 10:e0139313. doi: 10.1371/journal.pone.0139313
Wei, Z., Yang, X., Yin, S., Shen, Q., Ran, W., and Xu, Y. (2011). Efficacy of Bacillus-fortified organic fertiliser in controlling bacterial wilt of tomato in the field. Appl. Soil Ecol. 48, 152–159. doi: 10.1016/j.apsoil.2011.03.013
Wu, Q., and Liang, Z. (1988). Identification and pathogenic tests of the causal organism of the bacterial wilt of Eucalyptus. J. South China Agric. Univ. 9, 59–67.
Wu, Y. F., Cheng, A. S., Lin, C. H., and Chen, C. Y. (2013). First report of bacterial wilt caused by Ralstonia solanacearum on Roselle in Taiwan. Plant Dis. 97, 1375–1375. doi: 10.1094/PDIS-02-13-0186-PDN
Wu, Z. H., Xie, Y. J., Luo, L. F., and Zhang, W. Y. (2007). Advances in research on bacterial wilt Caused by Ralstonia solanacearum in Eucalyptus spp. in China. For. Res. 20, 569–575.
Xie, S., Ruan, H., Du, Y., Lin, L., Ma, H., and Wang, W. (2009). Genetic diversity of Ralstonia solanacearum on peanuts in Fujian. Fujian J. Agric. Sci. 24, 351–354.
Xiong, S., Sun, C., Shi, C., Jiang, X., and Peng, L. (2014). Screening and Identifying of antagonistic actinomycetes against Ralstonia solanacearum in tomato. North. Hortic. 5, 114–117.
Xu, J., Pan, Z. C., Prior, P., Xu, J. S., Zhang, Z., Zhang, H., et al. (2009). Genetic diversity of Ralstonia solanacearum strains from China. Eur. J. Plant Pathol. 125, 641–653. doi: 10.1007/s10658-009-9512-9515
Xue, F., Yan, T., Yang, L., and Qiao, J. (2010). Influences of organic fertilizer application on soil biological properties. Chin. J. Eco Agric. 18, 1372–1377. doi: 10.3724/SP.J.1011.2010.01372
Xue, Q. Y., Chen, Y., Li, S. M., Chen, L. F., Ding, G. C., Guo, D. W., et al. (2009). Evaluation of the strains of Acinetobacter and Enterobacter as potential biocontrol agents against Ralstonia wilt of tomato. Biol. Control 48, 252–258. doi: 10.1016/j.biocontrol.2008.11.004
Xue, Q. Y., Yin, Y. N., Yang, W., Heuer, H., Prior, P., Guo, J. H., et al. (2011). Genetic diversity of Ralstonia solanacearum strains from China assessed by PCR-based fingerprints to unravel host plant- and site-dependent distribution patterns. FEMS Microbiol. Ecol. 75, 507–519. doi: 10.1111/j.1574-6941.2010.01026.x
Yang, T., Wei, Z., Friman, V. P., Xu, Y., Shen, Q., Kowalchuk, G. A., et al. (2017). Resource availability modulates biodiversity-invasion relationships by altering competitive interactions. Environ. Microbiol. 19, 2984–2991. doi: 10.1111/1462-2920.13708
Yang, W., Xu, Q., Liu, H. X., Wang, Y. P., Wang, Y. M., Yang, H. T., et al. (2012). Evaluation of biological control agents against Ralstonia wilt on ginger. Biol. Control 62, 144–151. doi: 10.1016/j.biocontrol.2012.05.001
Yang, Y., Liu, J., Yang, C., Gong, H., Feng, D., and Xie, B. (2008). Control of solanaceae vegetable bacterial wilt with avirulent hrp-mutants. Acta Phytophylacica Sin. 35, 433–437.
Yao, G., Zhang, F., and Li, Z. (1994). Control of bacterial wilt with soil amendment. Chin. J. Biol. Control 10, 106–109.
Yin, G., Wang, X., Zhang, Y., Pan, G., and Yang, Q. (2005). Research progress on tomato bacterial wilt and resistance breeding in China. J. Yunnan Agric. Univ. 20, 163–167.
Yu, S. L., Wang, C. T., Yang, Q. L., Zhang, D. X., Zhang, X. Y., Cao, Y. L., et al. (2011). Peanut Genetics and Breeding in China. Shanghai: Shanghai Science and Technology Press.
Zhang, P., Wei, Z., Zhu, Z., Gao, X., Deng, K., Ran, W., et al. (2013). Effect of a bio-organic fertilizer on microbial flora and Ralstonia solanacearum population in rhizosphere soils of continuous cropping tomato and pepper. J. Nanjing Agric. Univ. 36, 77–82.
Zhang, Y., Huang, Z., Guan, G., Ding, W., Tang, Y., and Li, J. (2007). Influence of different agricultural ecological regulation and control measures on tobacco bacterial wilt. Chin. Tob. Sci. 28, 49–52.
Zhao, Y., Zhang, C., Chen, H., Yuan, M., Nipper, R., Prakash, C. S., et al. (2016). QTL mapping for bacterial wilt resistance in peanut (Arachis hypogaea L.). Mol. Breed. 36, 13. doi: 10.1007/s11032-015-0432-0
Zheng, H., Lin, J., Gao, Y., Guo, W., and Huang, R. (1992). Preliminary study on the resistance of common Casuarina equisetifolia to bacterial wilt and its physiological and biochemical mechanism. J. Fujian For. Sci. Technol. 1, 9–13.
Zheng, S., Ding, W., Du, G., Yang, L., Liu, X., and Zhang, Y. (2014). Control efficacy and action mechanism of mineral nutrition on tobacco bacterial wilt. Sci. Agric. Sin. 47, 1099–1110.
Zheng, X., Deng, H., Liu, Q., Chen, Z., Chen, Y., and Li, H. (2007). Strains and genetic diversity of Ralstonia solanacearum isolated from tobacco in Guangdong Province. J. Huazhong Agric. Univ. 26, 463–468.
Zheng, X., Song, B., Tan, X., and Chen, H. (2014). Identification of potato bacterial wilt pathogens. Chin. Potato J. 28, 83–89. doi: 10.1371/journal.pone.0096027
Keywords: bacterial wilt, China, distribution, host range, diversity
Citation: Jiang G, Wei Z, Xu J, Chen H, Zhang Y, She X, Macho AP, Ding W and Liao B (2017) Bacterial Wilt in China: History, Current Status, and Future Perspectives. Front. Plant Sci. 8:1549. doi: 10.3389/fpls.2017.01549
Received: 29 May 2017; Accepted: 24 August 2017;
Published: 11 September 2017.
Edited by:
Essaid Ait Barka, University of Reims Champagne-Ardenne, FranceReviewed by:
Carlos Alberto Lopes, Brazilian Agricultural Research Corporation, BrazilAna Maria Bocsanczy, University of Florida, United States
Copyright © 2017 Jiang, Wei, Xu, Chen, Zhang, She, Macho, Ding and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Wei, weizhong@njau.edu.cn
 Gaofei Jiang
Gaofei Jiang Zhong Wei
Zhong Wei Jin Xu3
Jin Xu3 Huilan Chen
Huilan Chen Yong Zhang
Yong Zhang Xiaoman She
Xiaoman She Alberto P. Macho
Alberto P. Macho Wei Ding
Wei Ding Boshou Liao
Boshou Liao