- 1College of Plant Health and Medicine, Qingdao Agricultural University, Qingdao, China
- 2Key Laboratory of Analytical Chemistry for Biology and Medicine, Ministry of Education, Department of Chemistry, Wuhan University, Wuhan, China
- 3College of Applied Life Science, Jeju National University, Jeju, South Korea
- 4Marine Agricultural Research Center, Tobacco Research Institute, Chinese Academy of Agricultural Sciences, Qingdao, China
- 5Wuhan Institute of Biotechnology, Wuhan, China
Brassinosteroids (BRs) play an essential role in plant growth, development, and responses to diverse abiotic stresses. However, previous studies mainly analyzed how exogenous BRs influenced plant physiological reactions to drought stress, therefore, genetic evidences for the endogenous BRs-mediated regulation of plant responses still remain elusive. In this study, a key BRs biosynthetic gene, SoCYP85A1 was cloned from Spinacia oleracea, which has a complete open reading frame of 1,392 bp encoding a 464 amino acid peptide and shares high sequence similarities with CYP85A1 from other plants. The expression of SoCYP85A1 which was higher in leaf compared with root and stem, was induced by treatments of PEG6000, abscisic acid (ABA), low temperature and high salt. Increases in both SoCYP85A1 transcripts and endogenous BRs in transgenic tobacco which resulted in longer primary root and more lateral roots enhanced drought tolerance compared with wild types. The transgenic tobacco accumulated much lower levels of reactive oxygen species and malondialdehyde (MDA) than wild types did, accompanied by significantly higher content of proline and notably enhanced activities of antioxidant enzymes. Besides, transcriptional expressions of six stress-responsive genes were regulated to higher levels in transgenic lines under drought stress. Taken together, our results demonstrated that SoCYP85A1 involves in response to drought stress by promoting root development, scavenging ROS, and regulating expressions of stress-responsive genes.
Introduction
Abiotic stress such as drought, has been a primary factor adversely affecting plants quality and production in the natural environment (Farooq et al., 2009; Li et al., 2009). Plants have evolved intricate defense mechanisms to cope with water loss, which might involve physiological or molecular processes by regulating concentrations of phytohormones (Nemhauser et al., 2006; Gruszka, 2013; Xia et al., 2014) and expression of stress-related genes (Kodaira et al., 2011; Wang et al., 2015; Udawat et al., 2016).
Brassinosteroids as one of the most important phytohormones involve in various physiological processes (Choudhary et al., 2012). In addition of their roles in plant growth and development (Hu et al., 2000; Arteca and Arteca, 2001; Yu et al., 2004), BRs have recently been implicated in response to abiotic stresses (Kagale et al., 2007; Xia et al., 2009; Chung et al., 2014; Li et al., 2016a). Most previous studies focused on BRs regulations on plant response to abiotic stresses have been performed using exogenous BRs (Kurepin et al., 2008; Li and Feng, 2011; El-Mashad and Mohamed, 2012), however, the genetic mechanisms for endogenous BRs in response to stresses remain poorly understood (Chung et al., 2014; Zhou et al., 2014; Divi et al., 2016).
Brassinosteroids biosynthetic pathway has been annotated clearly by using GC-MS and genetic modified analysis in the last few years. To date, key enzymes such as DWF4, CPD, DET2, CYP90D1, CYP85A1, and CYP85A2 have been characterized to catalyze the conversion of the intermediates in the BRs biosynthesis pathway (Fujioka et al., 1997; Shimada et al., 2003; Kim et al., 2005; Ohnishi et al., 2012), which were all found to encode cytochrome P450 monooxygenases. Since the first BRs, BL was discovered in 1979 (Grove et al., 1979), over 70 BRs have been isolated and characterized (Tarkowská et al., 2016). Applications of exogenous BRs to plants result in various physiological effects, including promotion of germination and cell elongation, differentiation of tracheary element, enhancement of photomorphogenesis, pollen fertility and stress resistance (Clouse and Sasse, 1998; Steber and McCourt, 2001).
A number of BRs-deficient mutants in absence of BR biosynthesis genes represent typical characteristics with rounded leaves, shortened petioles, reduced fertility and delay in senescence (Vert et al., 2005). Similarly, BRs-deficient mutants in absence of BRs receptors exhibit the same abnormalities in the BRs metabolism, which demonstrated that BRs receptors are involved in BR signal transduction pathways. BRs signals are detected by BRs receptors such as BRASSINOSTEROID INSENSITIVE 1 (BRI1) in the cell membrane (Li and Chory, 1997; Wang et al., 2002). Within the past two decades, more than 20 different types of BRI1 mutants have been identified. Most of these weak mutants served as genetic screening materials, have made important contributions to BL synthesis, metabolism and signal transduction. It was recently reported that overexpression of GmBRI1b repressed the relatively high expression of genes involved in BRs biosynthesis, including DWF4, CPD, CYP85A1, and CYP85A2 in the transgenic bri1-5 mutant (Peng et al., 2016). A number of BRs signaling regulators, such as, BAK1 (Li et al., 2002), BRS1 (Li et al., 2001), BEN1 (Yuan et al., 2007), TCP1 (Guo et al., 2010) have been characterized by screening genetic suppressors of bri1-5 via the activation-tagging method. In detail, BAK1 and BRS1 have been confirmed to promote BRs signaling by interacting with and phosphorylating BRI1. BEN1 is responsible for BRs metabolism and overexpression of this gene could enhance the dwarf phenotype of bri1-5 (Yuan et al., 2007). TCP1 positively coordinates with the function of DWF4, a key enzyme in BRs biosynthesis, suggesting another level of regulation through which BRs mediate plant growth and development (Guo et al., 2010).
Since BRs biosynthetic pathway was analyzed gradually clearly, researchers have carried out to explore the biological functions of endogenous BRs via regulating expressions of related genes involved in BRs biosynthesis. Overexpression of CYP85A1 in tomato which increased the contents of 28-norCS and BL promoted seed germination, enhanced carotenoid content (Li et al., 2016a) and improved photosynthetic efficiency (Li et al., 2016b). These above reports mainly focused on plant growth and development influenced by overexpression of CYP85A1, however, researches on relationship between abiotic stress and CYP85A1 remained lack. Although physiological features between wild types of barley and semi-dwarf mutants of CYP85A1 were compared under drought stress, genetic mechanisms had not yet been revealed (Janeczko et al., 2016). In another recent report, overexpression of the BRs biosynthetic gene CYP90B1 in Brassica napus increased stress tolerance by regulating expressions of dehydration responsive genes (Sahni et al., 2016). However, genetic mechanisms how the CYP85A1 gene participates in plant response to drought stress remain still unknown.
There have different biosynthetic pathways of BRs among different plant species. In Arabidopsis and tomato, there are two pathways to biosynthesize CS, part of which is catalyzed to BL. While there is only one pathway in tobacco and rice, in which a small amount of CS turn into 3-epiCS (Fujioka et al., 1997; Hong et al., 2005). Besides, CYP85A1 is an enzyme to catalyze intermediate metabolites to produce CS (Shimada et al., 2001). Therefore, in order to explore the function of CYP85A1 and CS at the genetic level, we cloned this gene from spinach and generated CYP85A1-overexpressing tobacco. It was demonstrated that overexpression of CYP85A1 enhanced drought tolerance and root development of transgenic tobacco compared with wild types. In addition, we identified the molecular mechanism that CYP85A1 enhanced drought tolerance by eliminate ROS accumulation and modulating expression of stress-responsive genes.
Materials and Methods
Plant Materials and Stress Treatments
Spinach (Spinacia oleracea L.) seeds were sterilized and cultured in growth chambers under the condition of 16 h light/8 h dark cycle at 20°C with 50% relative humidity for 14 days. The materials were collected from the root, stem, and leaf, respectively, under the normal condition. For drought, salt or ABA treatment, the shoots were transferred to beakers containing fresh distilled water added with 20% PEG6000, 200 mM NaCl or 100 μM ABA, which were kept in the same growth chamber for designated time (0, 1, 3, 6 12, 24, and 48 h for drought and ABA stress; 0, 1, 3, 6 12, and 24 h for salt stress). Low temperature stress was carried out by transferring shoots to 4°C for 0, 1, 3, 6 12, 24, 48, and 72 h. For each treatment, 10 shoots were used, among which, 3 shoots were randomly selected at each time point. The materials from all treatments were frozen immediately in liquid nitrogen and stored at -80°C for RNA extraction.
Cloning and Bioinformatics Analysis of the SoCYP85A1 Gene
Total RNA was extracted from plant leaves using the Easy-BLUE kit (Intron Biotech, Korea). Reverse transcription to synthesize the first strand cDNA was performed with MaximeTM RT PreMix Kit (Intron biotech, Korea). Full-length cDNA of SoCYP85A1 was generated by rapid amplification of cDNA ends (RACE) technique using Gene-RACE Kit (Invitrogen, United States) according to the manufacturer’s instructions. The specific primers and nested primers were designed by the Primer 5.0 program based on the conservative sequences of CYP85A1 in other plants (Table 1). The PCR amplifications were performed using Ex-Taq DNA polymerase (Takara, Japan) with the template of the first strand cDNA. The PCR program consisted of a 5-min incubation at 94°C, 30 cycles of 30 s at 94°C, 30 s at 65°C, and 90 s at 72°C, followed by a 10-min extension at 72°C. The PCR product was purified by a Gel Extraction Kit (QIAGEN, Hilden, Germany) to ligate sub-cloned into the pMD18-T vector (TaKaRa, Japan) and then sequenced.
The full-length cDNA of SoCYP85A1 was analyzed by the BLAST algorithm1. Multiple alignments were carried out using the DNAMAN software between the deduced amino acid sequence of SoCYP85A1 and other CYP85A1 homologs from different plant species obtained from NCBI. The phylogenetic relationship tree was constructed by the neighbor-joining method using MEGA (version 7.0). Theoretical molecular weight and isoelectric point (pI) and were calculated with ProtParam tool2.
Analysis of the SoCYP85A1 Gene Expression by Quantitative Real-time PCR (qRT-PCR)
qRT-PCR was performed to evaluate expression levels of SoCYP85A1 under different treatments. RNA isolation (QIAGEN, Hilden, Germany) and cDNA synthesis (Takara, Japan) were performed based on the manufacturer’s instructions. The cDNA solution was used as templates for PCR amplifications with two pairs of specific primers of the SoCYP85A1 gene and Spinacia oleracea actin gene (GenBank: JN987183.1) as an internal control (Table 2).
qRT-PCR was conducted in a Real-time System (qTOWER Applied Biosystems, Jena, Germany) using SYBR Green PCR Master Mix (Takara, Japan) with following PCR program, 95°C for 5 min, followed by 40 cycles of 95°C for 15 s, and 62 (GSP2)/60 (Actin)°C for 1 min. The relative level of gene expression was calculated through the 2-ΔΔCt formula. Each sample was amplified by three biological replicates.
Transformation of Tobacco and Regeneration of Transgenic Plants
T0 generate transgenic tobacco plants expressing SoCYP85A1-GFP fusion protein, the target gene was inserted in the vector pB7WG2D, 1-GFP to create expression vector under the control of CAMV35S promotor and NOS terminator, which was transferred into Agrobacterium strain LBA4404. Transgenic tobacco plants were generated via the leaf disk transformation method (Horsch et al., 1985).
T0 transgenic seeds were germinated on MS medium containing glufosinate (5 mg/L). Putative transgenic plants were confirmed by PCR amplifications of the SoCYP85A1 gene to select T2 and T3 seeds. Ten lines were scanned by qRT-PCR to detect expressions of the SoCYP85A1 gene at the transcriptional level.
Assay for Drought Tolerance
Seeds of the three types as described above were cultured in pots containing the same amount of soil in growth chambers under the condition of 16 h light/8 h dark cycle at 28°C with 60% relative humidity. Six-week-old WT and transgenic lines were withheld water for 10 days to compare the drought tolerance. The leaves of 6-week-old seedlings exposed to water stress for 10 days were sampled to measure endogenous BRs, ABA, physiological indexes, and gene expressions.
Extraction and Quantification of Endogenous BRs
Quantification of endogenous BRs was performed as described previously (Ding et al., 2013).
Extraction and Quantification of Endogenous ABA Content
Quantification of endogenous ABA was carried out according to the following steps. In brief, about 0.1 g samples were grinded and then added 1 mL precooling reagent 1 at 4°C overnight. Samples were centrifuged at 8000 ×g for 10 min. Afterward, the supernatant was transferred into another tube and dried with nitrogen until there was 0.5 mL solution left, in which 0.5 mL reagent 2 was added to extract and decolored for three times. The upper ether phase was discarded and the lower one continued to be dried, which was dissolved in 0.5 mL methanol, filtered and used for HPLC analysis.
The samples were all measured on the HPLC apparatus (e2695, Waters, United States) coupled with reversed phase column of Kromasil C18. The flowing phase was consisted of methanol and water (2:3 v/v). The injection volume was 10 μL, of which flowing rate was 0.8 mL/min at column temperature of 35°C and wave length of 254 nm for 40 min.
Measurements of RWC, Water Loss Rate, Contents of H2O2, Proline, MDA, and Activities of Antioxidant Enzymes
Relative water content and water loss rate were measured as previous reports described (Du et al., 2010; Udawat et al., 2016). Contents of H2O2, proline, MDA, and activities of antioxidant enzymes (SOD, POD, and CAT) were spectrophotometrically determined with detection kits (H202-1-Y, A107, A003-3, A001-4, A084-3, A007-1, Jiancheng, Nanjing, China) according to the manufactural instructions.
qRT-PCR Analysis of the Downstream Genes Regulated by SoCYP85A1
The samples used for this assay were collected before and after treatments as described for drought/salt stress assay. RNA isolation and cDNA synthesis were performed as mentioned above. qRT-PCR was utilized to examined transcriptional levels of nine genes encoding enzymes involved in ABA biosynthesis (NtNCED1), polyamine biosynthesis (NtADC1 and NtSAMDC), ROS detoxification (NtAPX, NtCAT, NtGST, and NtSOD), as well as stress-responsive proteins (NtERD10C and NtLEA5). The NtActin gene was used as the internal control. The sequences of primers for qRT-PCR were list in Table 2.
Statistical Analysis
The experiments were conducted for three times, each of which contained three biological replicates. Data presented as the mean ± SD were analyzed by SPSS statistical software (ver.16.0, SPSS Inc., Chicago, IL, United States) via one-way analysis of variance. A Tukey’s test (P < 0.05) was applied for the significant difference statistical analysis.
Results
Cloning and Bioinformatics Analysis of SoCYP85A1
In this work, a new CYP450 gene was isolated by RACE from spinach and designated as SoCYP85A1 (GenBank Accession: KT900949). Sequence analysis presented that the SoCYP85A1 cDNA is 1669 bp with a 1392 ORF encoding a deduced protein of 464 amino acids with molecular weight of 54 kDa and isoelectric point of 9.66. Conserved domain of the SoCYP85A1 protein was analyzed and indicated that it belongs to p450 superfamily (Supplementary Figure 1A). Multiple alignments showed that the SoCYP85A1 protein presented a high sequence identify with other CYP85A1 proteins from Fagopyrum esculentum (BAO79854, 81%), Morus notabilis (XP_010099314, 80%), Arabidopsis thaliana (NP_851105, 80%), Medicago truncatula ((XP_013451102, 79%), Theobroma cacao (XP_007032850, 79%), Glycine soja (KHN08349, 78%), Nicotiana tabacum (NP_001312136, 78%) and Solanum lycopersicum ((NP_001234263, 76%) (Supplementary Figure 1B). The phylogenetic analysis revealed that SoCYP85A1 had a closer relationship with the CYP85A1 protein from Fagopyrum esculentum than those from other plant species (Supplementary Figure 1C).
Expression Patterns of SoCYP85A1 under Stress Treatments
qRT-PCR was performed to determine the expression patterns of SoCYP85A1 in different tissues under normal condition or various abiotic stresses. The data showed that expression of SoCYP85A1 was detected in all three tissues, which was higher in leaf compared with stem and root (Figure 1A).
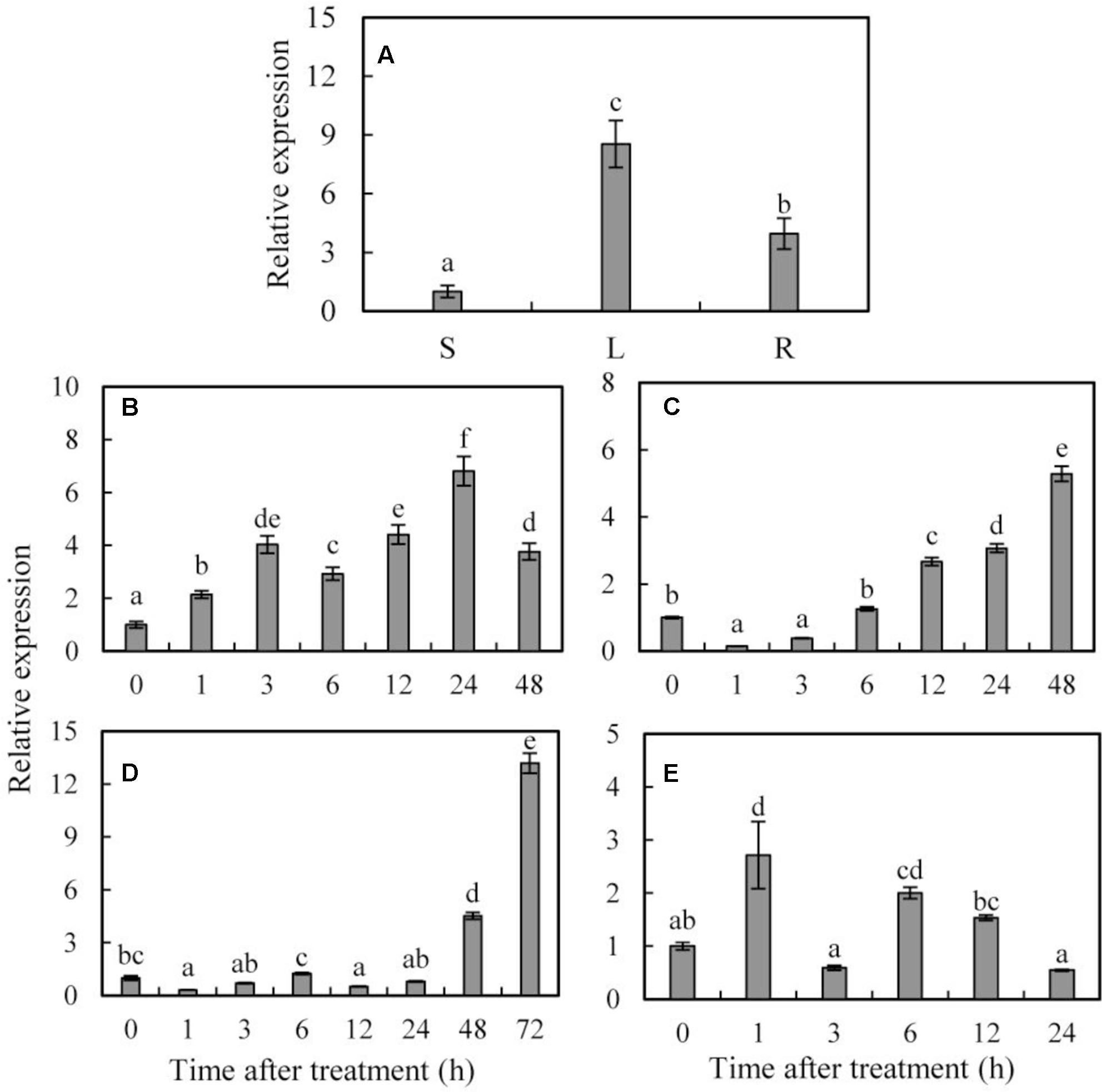
FIGURE 1. Organ expression assay of SoCYP85A1 and expression profiles of SoCYP85A1 under treatments with PEG, ABA, 4°C and NaCl. (A) Organ expression assay of SoCYP85A1 in spinach. The organs (stem, leaf, and root) are represented by S, L, and R, respectively. (B) 20% PEG6000; (C) 100 μM ABA; (D) 4°C; (E) 200 mM NaCl. The expression level of stem for organ expression assay and for stress assay at time point 0 were defined as 1. Error bars represent standard deviations (SD) for three independent replicates with different letters at P < 0.05 significant level.
The transcript level of SoCYP85A1 began to accumulate 1 h after PEG6000 treatment and reached maximum at 24 h, which was 6.81-fold of the initial level followed by a decrease at the end of the treatment (Figure 1B). When subjected to ABA stress, the SoCYP85A1 abundance was reduced in the first 3 h but gradually increased by 5.28-fold at 24 h (Figure 1C). Low temperature treatment led to a slight down-regulation first but a significant up-regulation by 4.52-fold at 24 h and 13.18-fold at 48 h, respectively (Figure 1D). During the high salt treatment, the transcription of SoCYP85A1 performed a slight increase and at the first 1 h, which was only 2.71-fold of that at 0 h (Figure 1E).
Regeneration of Transgenic Tobacco Plants Overexpressing SoCYP85A1
The ORF of SoCYP85A1 was overexpressed in tobacco under the control of 35S promotor of cauliflower mosaic virus (CaMV 35S). Two lines of T3 generation were confirmed by PCR analysis of genomic DNA with the specific primers for the SoCYP85A1 gene (Figure 2A). qRT-PCR was carried out to analyze expression levels in the two lines (Figure 2B). The WT and two lines (L2 and L8) were exposed to drought treatment to investigate the function of SoCYP85A1.
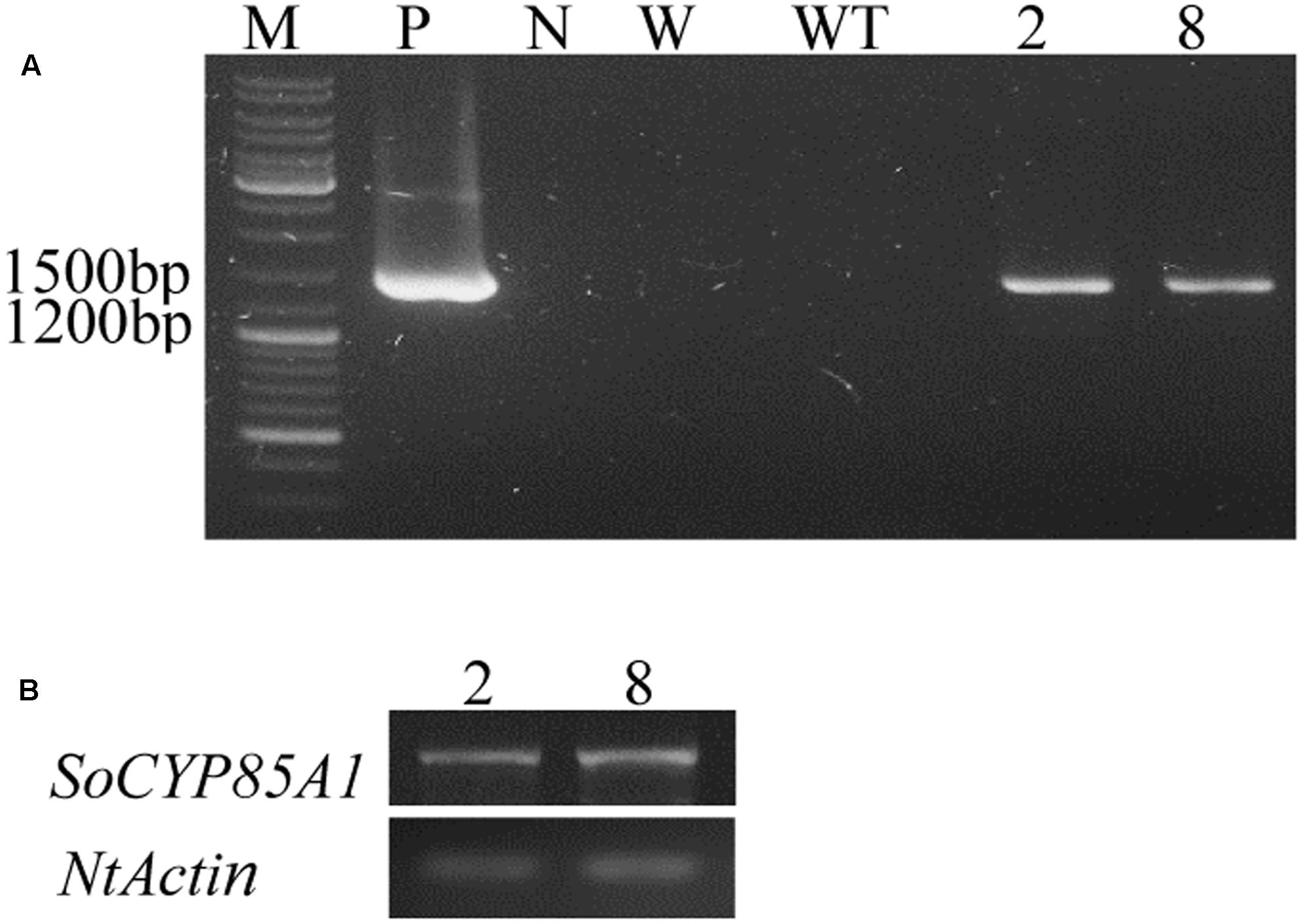
FIGURE 2. Molecular identifications of transgenic tobacco lines. (A) PCR analysis of genomic DNA with the specific primers for the SoCYP85A1 gene. PCR amplification of a 1,392 bp fragment of the SoCYP85A1 gene in transgenic lines. (B) Expressions of SoCYP85A1 in transgenic lines. M, molecular marker; P, plasmid; N, negative control; W, water; WT, wild types; 2 and 8, transgenic lines.
Overexpression of SoCYP85A1 Regulates Root Development and Enhances Drought Tolerance
Root development was changed between WT and the two transgenic lines at the stage of 6-week-old under the regular irrigation. Compared with WT, transgenic lines showed longer primary root and increased number of lateral root.
In order to investigate the role of SoCYP85A1 in the absence of water, seedlings of both WT and the two transgenic lines were subjected to withholding water for 10 days. It could be seen from Figure 3 that drought caused WT to develop more lateral roots, but length of primary root seemed to be no difference. Besides, leaves of WT curled and wilted seriously, even more of them withered to fall. However, exposed to lack of water, transgenic plants grew better than WT did, whose leaves appeared a little wilting. Furthermore, they developed longer primary root and more lateral roots.
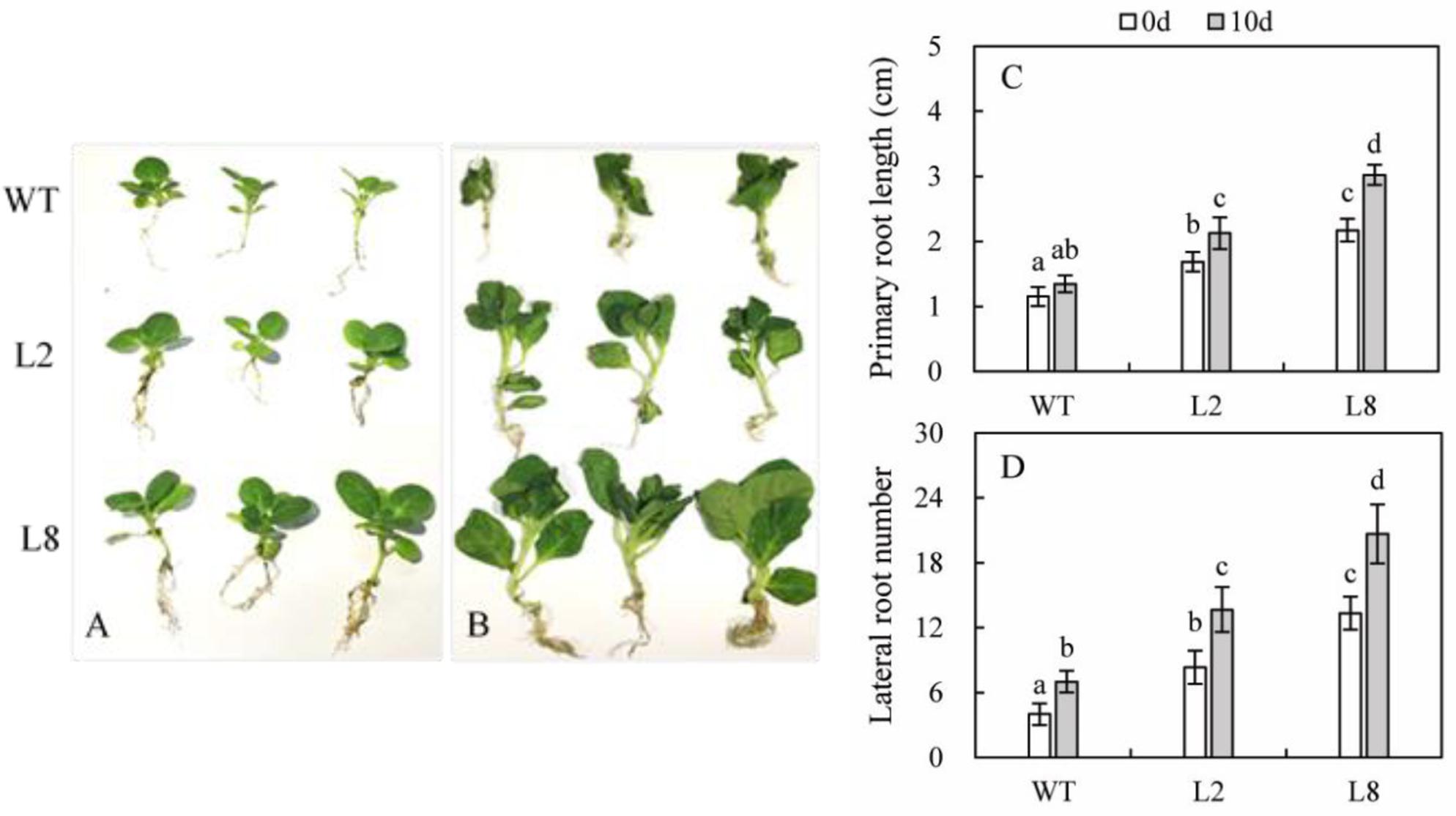
FIGURE 3. Effect of overexpression of SoCYP85A1 on root development before and after drought stress. Phenotypes of seedlings at 6-week-old under the normal conditions (A) and water loss for 10 d (B). Primary root length (C) and lateral root number (D) of seedlings at 6-week-old with and without water. Error bars represent SD and values with different letters are significant at P < 0.05.
Overexpression of SoCYP85A1 Increases SoCYP85A1 Transcript and CS Accumulation
qRT-PCR analysis indicated that the transcription of SoCYP85A1 was undetectable in WT with or without water. While, exposure to drought stress resulted in the transcriptional expression of SoCYP85A1 to be enhanced by more than 8 and 11 folds in L2 and L8, respectively (Figure 4A).
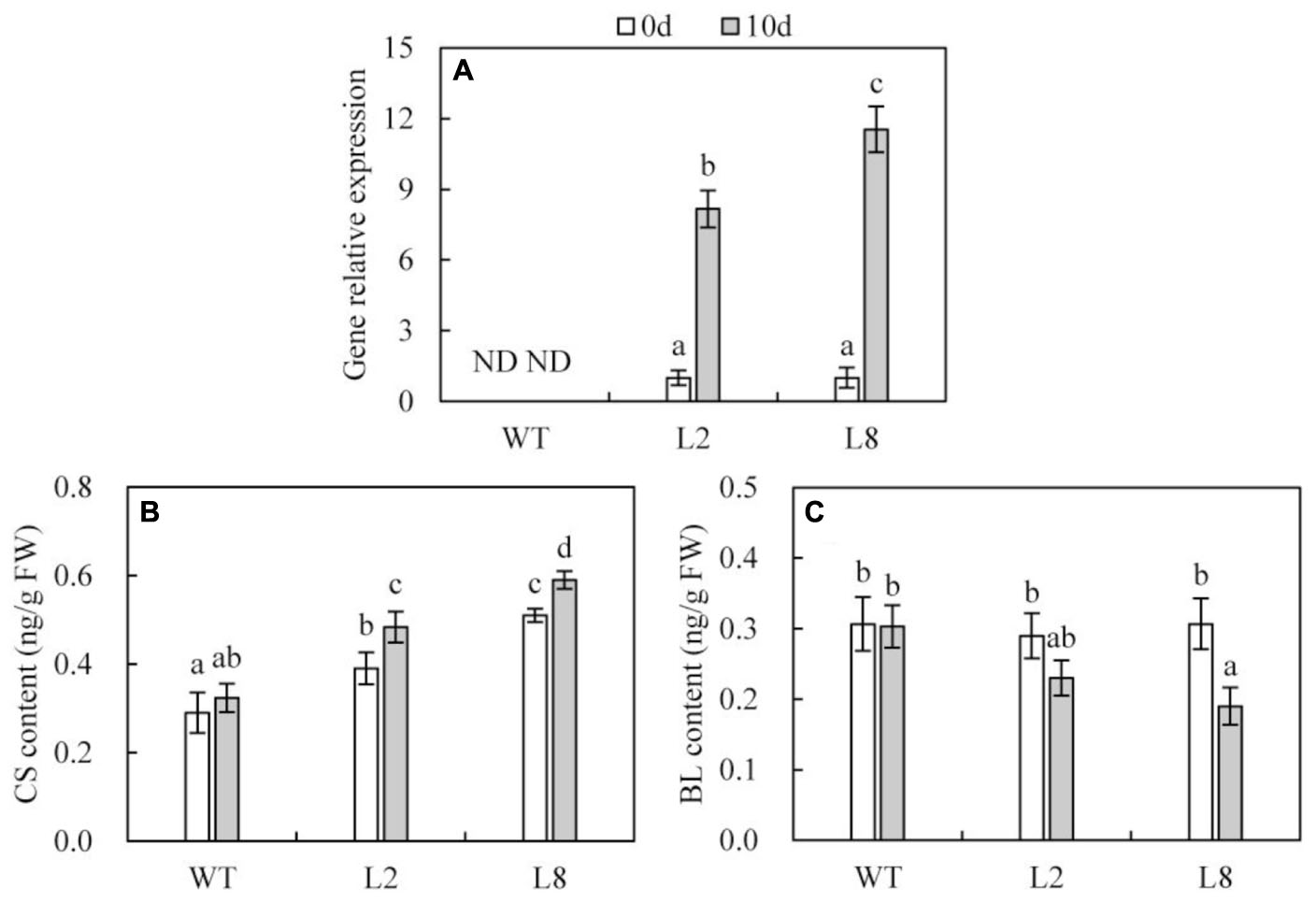
FIGURE 4. BRs biosynthesis capacity in WT and overexpressing lines (L2 and L8). (A) Transcriptional accumulations of the SoCYP85A1 gene in WT, L2 and L8 before and after 10-day drought stress. The expression level of L2 and L8 at time point 0 was defined as 1, respectively. (B) Detection of CS content. (C) Detection of BL content. CS, castasterone; BL, brassinolide; FW, fresh weight. The leaves from tobacco plants at 6-week-old stage were sampled for BRs analysis before and after 10-day water loss. Data are the means ± SD of three replicates and values with different letters are significant at P < 0.05.
Quantification of BRs metabolites revealed that overexpression of SoCYP85A1 induced more CS in transgenic lines than that in WT. Besides, subjected to drought stress, CS in WT just showed a slight increase, while transgenic lines performed a significant rise (Figure 4B). In contrast, BL contents had no significant differences between WT and the two transgenic lines before drought treatment. However, drought stress induced BL contents to decrease more notably in transgenic lines than in WT (Figure 4C). Additionally, other BRs metabolites including TE (teasterone), TY (typhasterol), 6-deoxoCS (6-deoxo castasterone) and 28-norCS (28-norcastasterone) were all undetectable in both WT and transgenic lines before and after water stress.
Drought Stress Resulted in Less Accumulation of ABA in Overexpressing SoCYP85A1 Plants
As is known to all that ABA plays an essential part in plant responses to various abiotic stresses, including drought stress (Lee and Luan, 2012). In order to identify whether transgenic plants enhanced drought tolerance was relevant to ABA accumulation, we determined the concentrations of this compound (Figure 5). The data indicated that both WT and the two transgenic lines contained similar level of ABA under the control condition. However, it should be pointed out that drought stress resulted in a significant reduction of ABA content in L2 and L8, compared with WT. ABA accumulation in transgenic lines exhibited a similar level before and after treatment. This result demonstrated that overexpression of SoCYP85A1 enhanced drought tolerance was not through ABA-dependent pathway.
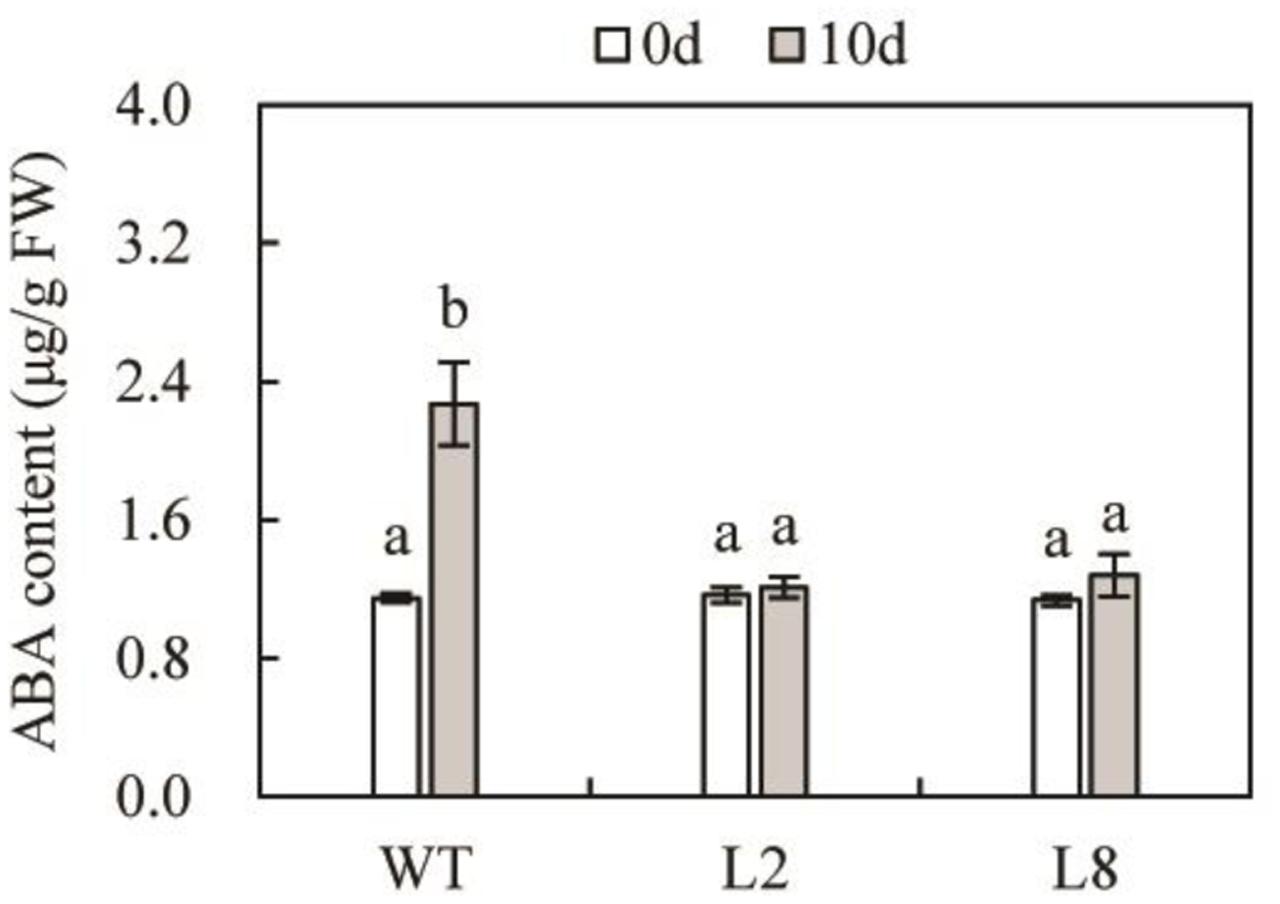
FIGURE 5. The endogenous contents of ABA in WT and transgenic lines under control and drought treatment. Error bars represent SD and values with different letters are significant at P < 0.05.
Overexpression of SoCYP85A1 Increases RWC, Proline and Decreases Water Loss Rate, MDA under Drought Treatment
To investigate the effects of the physiological status caused by overexpression of SoCYP85A1, the physiological measurements of RWC, water loss rate, proline, and MDA under drought treatment were conducted. RWC of the two transgenic lines was less reduced than that of WT. Meanwhile, water loss rate presented the opposite profile as RWC. The proline content of the two transgenic lines was higher than that of WT. In addition, MDA was induced lower accumulation in transgenic lines compared with WT under drought stress (Figure 6).
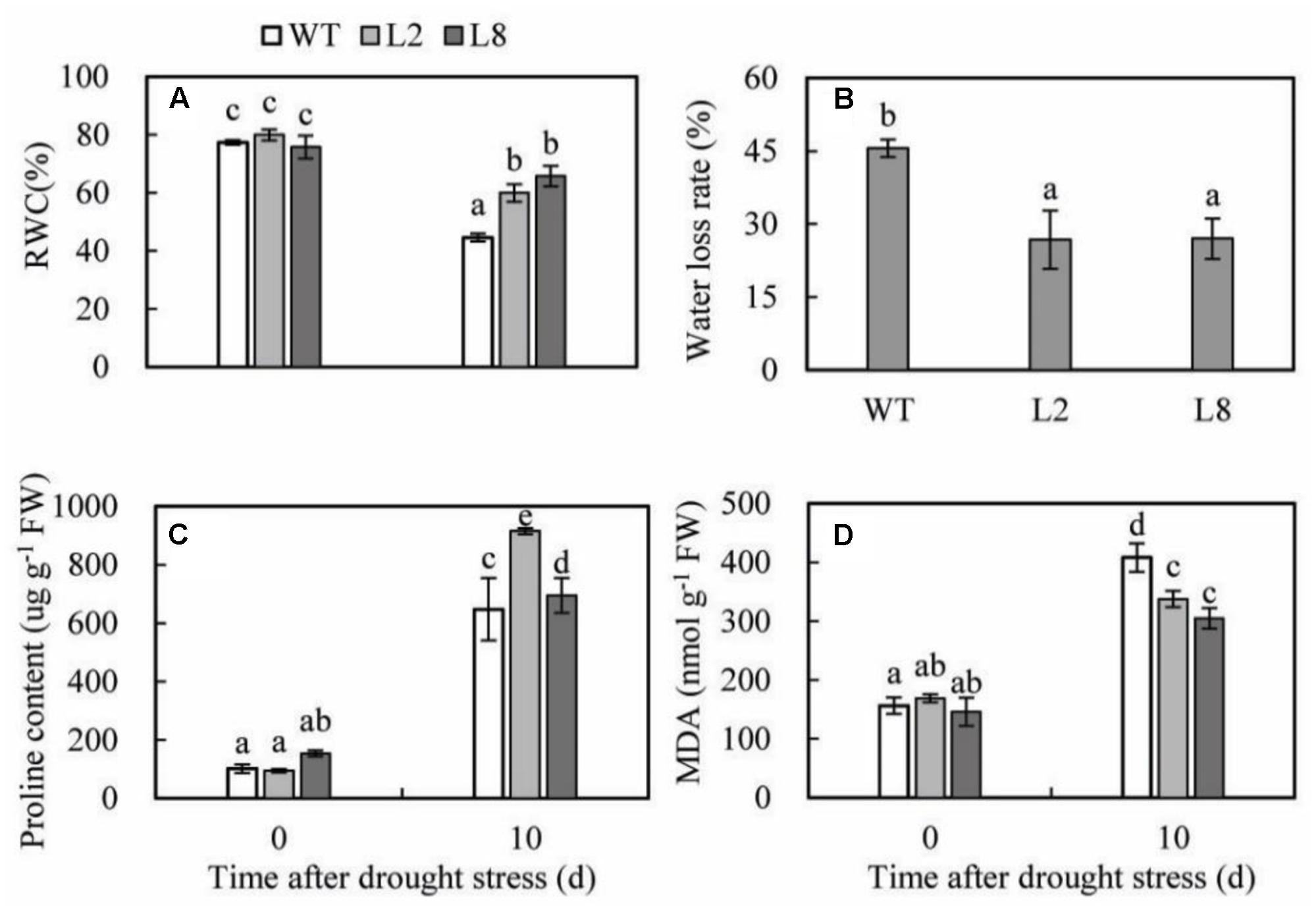
FIGURE 6. Analysis of the physiological indices in WT and transgenic lines (L2 and L8) under drought stress. Six-week-old seedlings were withheld water for 10 days to measure the value of RWC (A), water loss rate (B), proline content (C) and MDA (D). Error bars represent SD and values with different letters are significant at P < 0.05.
Overexpression of SoCYP85A1 Increases Antioxidant Enzyme Activities and Decreases the H2O2 Content under Drought Treatment
To demonstrate the abilities of scavenging ROS in the transgenic plants, the activities of the three key antioxidant enzymes (CAT, POD, and SOD), which play an important role in scavenging ROS, were detected in WT and transgenic lines before and after treatments as described above. The results presented that before treatments, there were no obvious differences between WT and transgenic plants in activities of CAT, POD, and SOD and the content of H2O2; however, after treatments, transgenic plants possessed significantly higher activities of CAT than WT did. Drought stress resulted in the increase of POD activity, which showed a remarkable rise in transgenic plants being 1.5 and 1.3 folds of that in WT, respectively. Drought treatment caused a significant decline in SOD activity of WT, which was significantly lower in transgenic plants. SOD activity of L2 and L8 was 1.39 and 1.36 folds of that in WT, respectively (Figure 7A). Besides, the H2O2 accumulation was lower in transgenic plants than in WT (Figure 7D), which performed a 41% and 20% increase in WT as compared to L2 and L8, respectively. These results indicated overexpression of SoCYP85A1 could reduce ROS accumulations by enhancing antioxidant enzyme activities under drought stress.
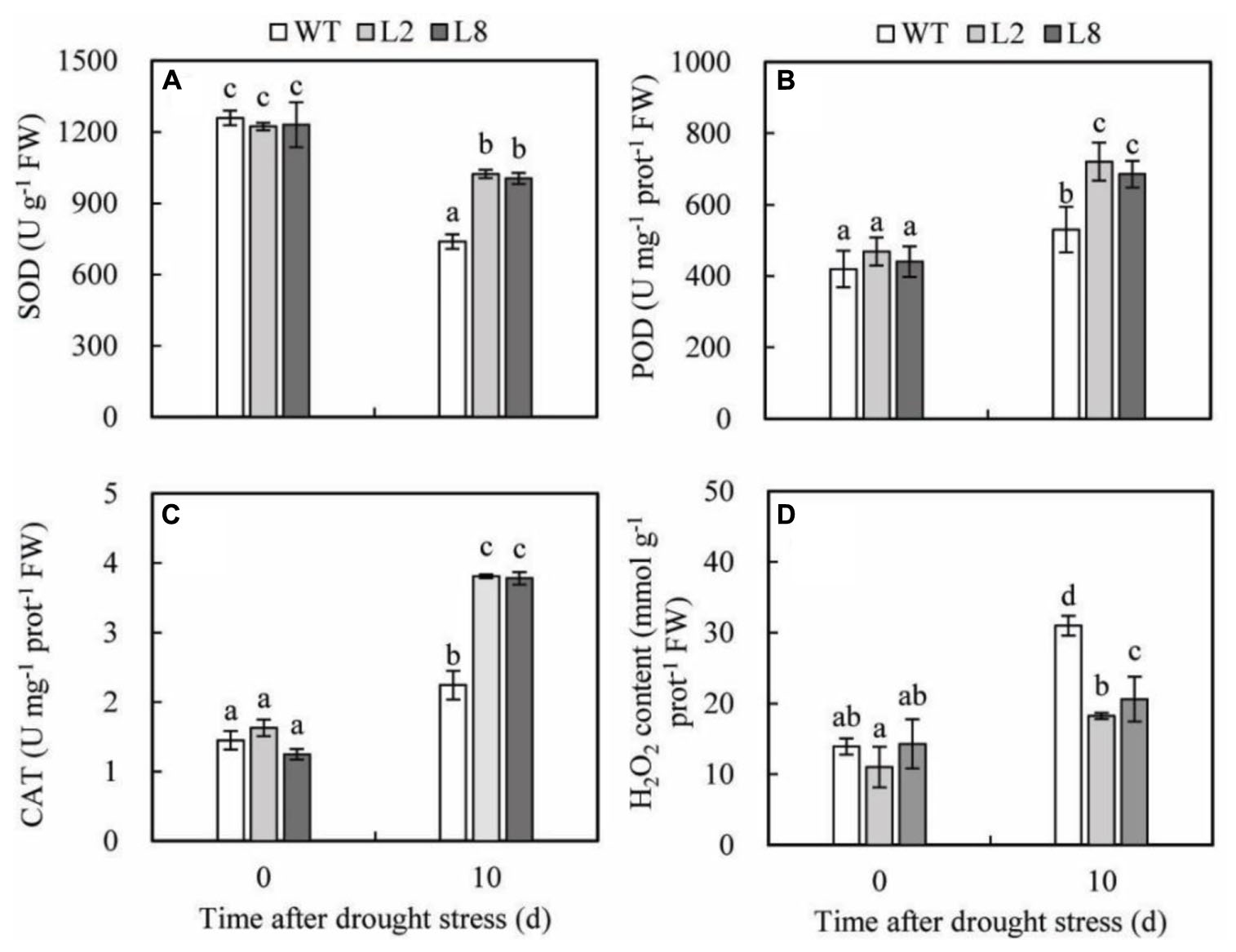
FIGURE 7. Analysis of three antioxidant enzymes’ activities and H2O2 accumulation in WT and transgenic lines (L2 and L8) under drought stress. Six-week-old seedlings were withheld water for 10 days to assess SOD (A), POD (B), CAT (C), and H2O2 (D). Error bars represent SD and values with different letters are significant at P < 0.05.
SoCYP85A1 Regulates Expressions of Stress-Relative Genes under Drought Treatment
To reveal the molecular mechanism that water loss enhanced drought resistance in transgenic plants, relative quantitative RT-PCR was carried out to investigate expressions of nine genes in both WT and transgenic plants before and after drought stress, which encode key enzymes involved in ABA biosynthesis (NtNCED1), polyamine biosynthesis (NtADC1 and NtSAMDC), ROS detoxification (NtAPX, NtCAT, NtGST and NtSOD), as well as stress-responsive proteins (NtERD10C and NtLEA5). Under the condition of withholding water, the transcript levels of NtAPX, NtCAT, NtGST, NtSOD, NtADC1, and NtLEA5 were obviously increased in transgenic plants compared with those in WT, whereas the transcript level of NtNCED1, NtSAMDC, and NtERD10C performed a slight rise (Figure 8). These results indicated that overexpression of SoCYP85A1 enhanced drought tolerance by regulating expressions of ROS and stress-responsive related genes.
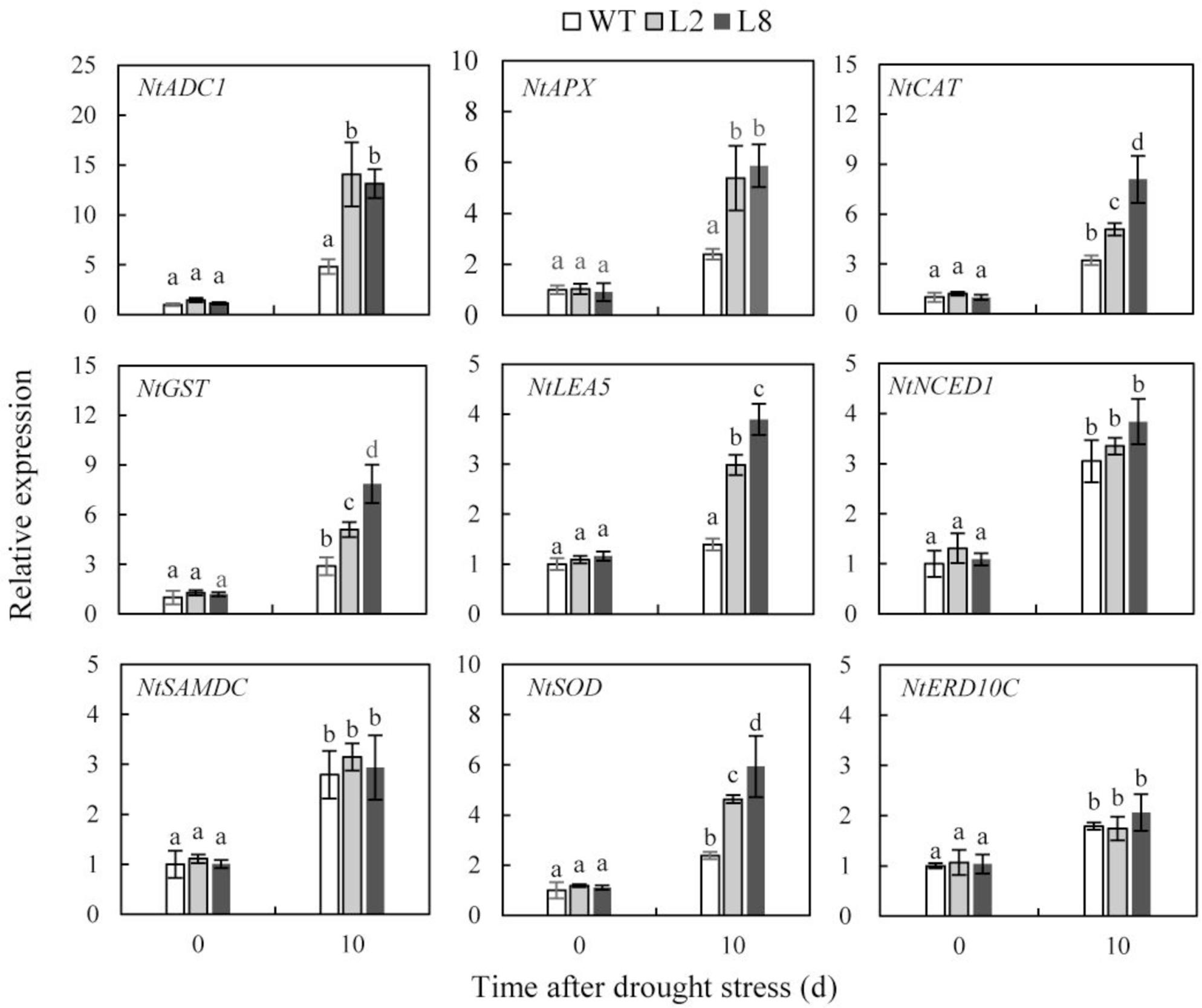
FIGURE 8. Expression levels of stress-relative genes in WT and transgenic lines (L2 and L8) under drought stress. Six-week-old seedlings were withheld water for 10 days. The tobacco leaves were sampled to extract the total RNA to synthesize cDNA. Expression levels of nine genes were detected. NtActin was used as the internal control. Error bars represent SD and values with different letters are significant at P < 0.05.
Discussion
Most previous researches applied exogenous BRs to analyze, thus the function of the endogenous BRs in the regulation of plant response to drought stress remain unknown and require further analysis (Llanes et al., 2016). Present studies have well documented that CYP85A1 is necessary to catalyze essential reactions for the production of CS, functional studies have been performed for CYP85A1 only in Arabidopsis, tomato as well as rice and there are few documentations in spinach. Besides, evidence for the relationships between CYP85A1 proteins and abiotic stresses remains limited. In this work, a gene encoding CYP85A1 from Spinacia oleracea was cloned by RACE. A highly conserved Cytochrome P450 domain was observed, indicating that CYP85A1 belongs to the P450 superfamily. Sequence multiple alignment demonstrated that SoCYP85A1 showed high degree of sequence identity with CYP85A1 of other plants retrieved from the database at amino acid level. The phylogenetic tree revealed the relationship between SoCYP85A1 and CYP85A1 from other plants, in which, SoCYP85A1 was closely related to CYP85A1 from Fagopyrum esculentum (BAO79854, 81%), Morus notabilis (XP_010099314, 80%) and Arabidopsis thaliana (NP_851105, 80%). However, current researches about CYP85A1 only limited on biocatalysis or regulations of plant growth and development (Castle et al., 2005; Kim et al., 2005; Pérez-España et al., 2011; Li et al., 2016a), no functional analyses on the relationships between CYP85A1 proteins and abiotic stresses of these three genes have been published to date.
qRT-PCR data showed that SoCYP85A1 had more abundant transcripts in leaves. However, previous studies indicated that Arabidopsis CYP85A1 mRNA mainly accumulated in roots (Bancos et al., 2002) or possessed higher transcriptional level in shoots (Shimada et al., 2003). The discrepancy between these results could attribute to the different species and different time of sampling. qRT-PCR analysis revealed that SoCYP85A1 was induced by PEG, NaCl, cold (4°C) and ABA (Figure 1), which implicated that SoCYP85A1 might play important roles in plant abiotic responses. NaCl data showed both, increase and decrease depending on the duration of treatment. We speculate that there might be a feedback mechanism in BRs biosynthesis caused by high salt stress. When the transcription of SoCYP85A1 increased at the first hour, BRs were induced to produce. However, when BRs accumulated to some extent, the expression of SoCYP85A1 was suppressed to be lower than that of the control at the third hour. While with the duration of stress, the content of BRs gradually reduced, which relieved the suppression of SoCYP85A1. As a result, the transcriptional level of SoCYP85A1 appeared to increase. This above course did not reciprocate until the transcription of SoCYP85A1 and the content of BRs reached a dynamic equilibrium. This hypothesis need to be validated on the basis of detecting the content of BRs under the high-salt treatment in our further research.
Compared with the other stresses, drought could cause more abundance of SoCYP85A1 at the transcriptional level, which prompted us to do further work on exploration of the potential role of this gene for enhancing drought tolerance. Transgenic tobacco were produced via Agrobacterium mediated transformation of SoCYP85A1 under the control of CaMV 35S promoter. The two selected transgenic lines (L2 and L8) exhibited more apparent resistance to drought stress through phenotypic morphology, concomitant with longer primary roots and more lateral roots of transgenic lines than those of WT under drought stress. Apart from drought stress, transgenic seedlings also displayed more tolerance to high-salt tolerance than WT did (data not shown). Our work was consistent with the recent report, in which overexpression of BRs biosynthetic gene DWF4 increased various stress tolerance (Sahni et al., 2016), implying that BRs biosynthetic genes could enhance plant tolerance to multiple stresses.
Manipulation of BRs biosynthesis has been recognized as a biotechnological target for enhancing crop stress tolerance (Divi and Krishna, 2009). Genetic analysis revealed that loss of CYP85A2 resulted in less BL accumulation and increased drought tolerance in Arabidopsis (Northey et al., 2016), which was inconsistent with previous studies that BL increased plant tolerance to drought. These inconsistent results might sound reasonable that any changes in BRs level resulted in differences of plant stress response (Xia et al., 2015). It could be worth noting that due to loss of CYP85A2, BL was sharply decreased, while CS (the precursor of the BL biosynthesis) was accumulated in abundance. The similar result was brought out in our study that transgenic seedlings overexpressing SoCYP85A1 induced significantly higher CS accumulations performed notably drought tolerance as compared to WT.
There have been increasing evidences that endogenous BRs have been employed to promote plant growth and development, including exogenous application of BRs, inhibitors of BR biosynthesis as well as genetic manipulations of BRs biosynthesis or signaling (Vriet et al., 2012). For instance, overexpression of CYP85A2 enhanced the growth of Arabidopsis with larger rosette leaves and longer petioles compared with WT, because higher level of BL induced by overexpression of CYP85A2 stimulated transgenic plants to hold significant advantages in morphological formation. (Kim et al., 2005). Our result in tobacco were in agreement with this finding. As shown in Figure 3, overexpression of SoCYP85A1 promoted growth and development of seedlings under normal conditions. It should be noticed that exposed to drought stress, this promotion seemed to be notable that transgenic lines possessed larger leaves, longer petioles as well as enhanced root morphology. Therefore, quantification of endogenous BRs was carried out in an effort to examine the influence of SoCYP85A1 in plant growth and development. The data showed that higher content of CS but not BL was detected in transgenic lines, so we speculated that CS might be a bioactive BRs to trigger the BRs signal transduction pathway to function BRs bioactivities. Besides, the significant increase of CS accumulation in transgenic lines by drought stress indicated that CS might be considered as the drought-stress-induced compound, which was consistent with the finding about Barely BRs mutant (Gruszka et al., 2016). In addition, shoot development was also promoted in transgenic lines, for which the reason might also be that overexpression of SoCYP85A1 resulted in higher content of CS. CS might be a bioactive BRs to function BRs bioactivities, one of which was stimulation of cell elongation. Therefore, shoot development was enhanced due to the increased content of endogenous CS in transgenic lines.
However, endogenous CS in transgenic Arabidopsis was not significantly affected by overexpression of CYP85A1 (Kim et al., 2005). The reasons for this inconformity might be that there existed different BRs biosynthesis pathways between Arabidopsis and tobacco. In the former there are two pathways to biosynthesize CS, part of which is catalyzed to BL. While there is only one pathway to generate CS in the latter, in which trace of CS only turns into 3-epiCS not BL (Suzuki et al., 1995; Fujioka et al., 1997; Hong et al., 2005). Therefore, BL that was detectable in tobacco might be biosynthesized in another pathway since there was a slight change of BL concentrations in WT and SoCYP85A1 overexpressing seedlings under the normal irrigation. On the other hand, a large accumulation of BL was commonly detected in reproductive organs such as siliques and seeds (Fujioka and Sakurai, 1997), which might indicated that a high level of BL was generated at particular developmental stages. It was well explained that in our result BL concentration was decreased by drought treatment since production of BL was not stimulated at young seedling stage as a result of drought stress. However, exposed to water loss plants generated a high level of CS regulating growth and development and enhancing drought tolerance, suggesting that CS functioned as a BRs bioactivity during seedling growth. Whether CS functions at other periods of tobacco needs to be confirmed. The similar finding had been reported in Arabidopsis that CS played an effective role as the bioactive BR that regulated the overall growth and development (Kim et al., 2005).
As is known to all that ABA plays an essential part in plant responses to various abiotic stresses, including drought stress (Lee and Luan, 2012). However, the mechanism that plants coordinate ABA and BRs remains unclear. When grown under optimal watering, both WT and transgenic lines accumulated similar concentrations of ABA. The similar result was also found in tomato that overexpression of CYP85A1 induced slightly but not significantly higher ABA accumulation over the WT (Li et al., 2016). It was unexpected that drought stress induced a significant increase in the accumulation of ABA in WT but a slight raise in transgenic lines, which exhibited well agreement with the transcriptional level of NtNECD1 involved in ABA biosynthesis. These results indicated that BRs biosynthesis do not affect the endogenous ABA accumulation under the control condition, while CS functions in negative regulation of ABA under drought stress. It could be an explanation for such regulation that CS and ABA act antagonistically on their target genes in BR signaling pathways particularly when stress responses (Chung et al., 2014).
Then the question was raised what other mechanisms might CYP85A1 confer the improvement of drought tolerance? To reveal it, we focused our spirit on the physiological differences between WT and transgenic tobacco under drought stress. The results showed that overexpression of SoCYP85A1 increased RWC and proline, decreased MDA and water loss, which indicated that less oxidative damage occurred in transgenic lines under drought stress. These data were consistent with the recent observation that barely semi-dwarf allelic mutants with an impaired activity of C6-oxidase produced less proline (Janeczko et al., 2016).
H2O2 level was also detected because it can reflect the degree of damage to plant cell (Miller et al., 2010). Less content of H2O2 in transgenic lines indicated transgenic lines could scavenge more ROS compared with WT. In order to detoxify ROS induced by drought stress, plants have evolved a complex antioxidant system (Miller et al., 2010), in which, efficient antioxidant enzymes protect plant cell from damages caused by stress through scavenging ROS (Gill and Tuteja, 2010). Among the enzymes, SOD provides the first line of defense against ROS by catalyzing the dismutation of H2O2, which is then scavenged by the coordinated action of CAT and POD (Blokhina et al., 2003). Our data revealed that activities of SOD, POD, and CAT in transgenic lines were similar as those in WT under normal conditions because ROS remained a low level without stress (Miller et al., 2010). However, after water loss, activities of the three enzymes were significantly higher in transgenic lines than in WT, implying that transgenic lines possessed more efficient detoxifying system when exposed to drought stress. Similar findings have been observed using other plants, such as tobacco, soybean, cucumber, and pistachio (Xu et al., 2011; Radhakrishnan and Lee, 2013; Shu et al., 2013; Kamiab et al., 2014).
To further investigate the mechanism that SoCYP85A1 enhanced drought tolerance at molecular level, qRT-PCR was carried out to detect transcriptional levels of the four different types of genes before and after drought stress. One type of genes including NtAPX, NtCAT, and NtSOD encodes antioxidant enzymes of POD, CAT and SOD, respectively. The transcriptional amount of the three genes were significantly upregulated in transgenic lines under drought stress. This result was consistent with the data of antioxidant enzyme activities described above, which indicated that the activities of the three enzymes might be regulated by SoCYP85A1 at transcriptional level. In this case, SoCYP85A1 might transcriptionally modulate the expression level of the three genes involved in ROS scavenging when it was overexpressed in tobacco.
The next type of genes involved in the polyamines synthesis was NtADC1 and NtSAMDC, which function in adaptive responses to various environmental stresses (Groppa and Benavides, 2008; Jang et al., 2009). Polyamines are thought to play an essential role in scavenging ROS homeostasis (Liu et al., 2015). It was interesting that in our results the transcription of NtADC1 was induced to be notably enhanced, while NtSAMDC remained a similar level after drought stress, suggesting that NtADC1 played a prominent role in accumulating less ROS in transgenic lines overexpressing SoCYP85A1under drought treatment.
Another type of gene was NtNECD1 that plays an essential role in ABA biosynthesis regulation (Huang et al., 2010). Our result showed that water stress induced expression of NtNECD1 a notable rise in both WT and transgenic lines. Whereas, it was noticeable that there was not prominent difference in expression level of NtNECD1 between WT and transgenic lines subjected to water loss, which exhibited an agreement with the quantification of ABA (Figure 7), implying that NtNECD1 was not transcriptionally upregulated by SoCYP85A1 in tobacco. Our work indicated that overexpression of SoCYP85A1 enhanced drought tolerance not by ABA-dependent pathway.
The last type of genes containing NtLEA5 and NtERD10C belong to the LEA protein family that protects and stabilizes macromolecules and/or cellular structures during plant stress responses (Xiong and Zhu, 2002; Liu et al., 2013). Our result showed that in the case of water loss, transcriptional level of NtLEA5 was obviously increased in L2 and L8 compared with that of WT, whereas that of NtERD10C changed slightly, which suggested that induction of a higher transcriptional level of NtLEA5 might be a more effective strategy to resist against drought stress in transgenic lines overexpressing SoCYP85A1.
In all, drought treatment led to upregulation of all types of genes in WT and transgenic lines. However, it was worthwhile noticing that mRNA levels of some genes were induced to be higher in transgenic lines than in WT, especially NtAPX, NtCAT, NtADC1, NtGST and NtSOD. These data demonstrated that the molecular mechanism of enhanced drought tolerance in transgenic lines relied on the inductions of ROS-related genes.
In addition, overexpression of SoCYP85A1 promoted growth and development of seedlings, especially after drought treatment, transgenic lines possessed larger leaves, longer petioles as well as enhanced root morphology. In this case, it was hard to compare the drought tolerance between WT and transgenic plants with significant different sizes. Therefore, we focused our spirits on physiological indicators. The results indicated that subjected to drought stress transgenic lines possessed higher content of proline, lower accumulation of MDA as well as a more efficient detoxifying system. Furtherly, ABA as another important indicator to measure drought tolerance was quantified in WT and transgenic plants with or without water. It should be kept in mind that ABA content in WT performed a significant increase in the absence of water, while water loss induced a slight rise in ABA accumulation of transgenic lines, suggesting that overexpression of SoCYP85A1 enhanced drought tolerance was not through ABA-depend pathway. Finally, to investigate the mechanism that SoCYP85A1 enhanced drought tolerance at molecular level, we carried out qRT-PCR to detect transcriptional levels of stress-responsive genes before and after drought stress. The data showed that expression level of ROS-related genes was notably enhanced in transgenic lines. These above results demonstrated overexpression of SoCYP85A1 could increase content of proline, reduce accumulation of MDA, establish a more efficient detoxifying system and upregulate transcripts of ROS-related genes to enhance drought tolerance.
Conclusion
A novel cytochrome p450 gene, SoCYP85A1, was upregulated by treatments of PEG, ABA, 4°C and NaCl. Overexpression of SoCYP85A1 enhanced drought tolerance in transgenic tobacco with increased CS content which promoted root development, accumulated more RWC and proline, less MDA and H2O2, improved activities of antioxidant enzymes, increased transcriptional expressions of ROS-related and stress-responsive genes under drought stress. Besides, activities of the antioxidant enzymes might be enhanced by SoCYP85A1 at transcriptional level. In addition, ABA quantification combined with expression level of NtNECD1 demonstrated that overexpression of SoCYP85A1 enhanced drought tolerance was not by ABA-dependent pathway. SoCYP85A1 might involve in plant resistance to drought stress via ABA-independent pathway, which could play the important role in the antioxidant pathway at the transcriptional level. However, the precision mechanisms underlying these results require a further analysis. Therefore, we have already commenced a deeper research, which focuses on transcriptomics and metabolomics studies to explore more target genes regulated by SoCYP85A1 and more kinds of phytohormone modulated by endogenous BRs in order to establish the relationship among physiological changes, differentially expressed genes and metabolite alterations under drought stress.
Author Contributions
WS and FD designed and performed part of the experiments; JD, XL, and YF detected BRs, enzyme activities and gene expressions. FD and DL cloned SoCYP85A1 gene. WS analyzed the data and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Shandong Province Natural Science Foundation (ZR2013CQ028), Qingdao Science and Technology Project (16-5-1-54-jch) and Research fund for the doctor Program of Higher Education (663/1111316).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2017.01909/full#supplementary-material
Abbreviations
ABA, abscisic acid; BL, brassinolide; BRs, brassinosteroids; CAT, catalase; CS, castasterone; LEA, late embryogenesis abundant; MDA, malondialdehyde; ORF, open reading frame; POD, peroxidase; QRT-PCR, quantitative real-time PCR; ROS, reactive oxygen species; RT-PCR, reverse transcription-PCR; RWC, relative water content; SOD, superoxide dismutase; WT, wild type.
Footnotes
References
Arteca, J. M., and Arteca, R. N. (2001). Brassinosteroid-induced exaggerated growth in hydroponically grown Arabidopsis plants. Physiol. Plant. 112,104–112. doi: 10.1034/j.1399-3054.2001.1120114.x
Bancos, S., Nomura, T., Sato, T., Molnar, G., Bishop, G. J., Koncz, C., et al. (2002). Regulation of transcript levels of the Arabidopsis cytochrome P450 genes involved in brassinosteroid biosynthesis. Plant Physiol. 130, 504–513. doi: 10.1104/pp.005439
Blokhina, O., Virolainen, E., and Fagerstedt, K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 91, 179–194. doi: 10.1093/aob/mcf118
Castle, J., Szekeres, M., Jenkins, G., and Bishop, G. J. (2005). Unique and overlapping expression patterns of Arabidopsis CYP85 genes involved in brassinosteroid C-6 oxidation. Plant Mol. Biol. 57, 129–140. doi: 10.1007/s11103-004-6851-7
Choudhary, S. P., Yu, J. Q., Yamaguchi-Shinozaki, K., Shinozaki, K., and Tran, L. S. (2012). Benefits of brassinosteroid crosstalk. Trends Plant Sci. 17, 594–605. doi: 10.1016/j.tplants.2012.05.012
Chung, Y. H., Kwon, S. I., and Choe, S. (2014). Antagonistic regulation of Arabidopsis growth by brassinosteroids and abiotic stresses. Mol. Cells 37, 795–803. doi: 10.14348/molcells.2014.0127
Clouse, S. D., and Sasse, J. M. (1998). Brassinosteroids: essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 427–451. doi: 10.1146/annurev.arplant.49.1.427
Ding, J., Mao, L. J., Wang, S. T., Yuan, B. F., and Feng, Y. Q. (2013). Determination of endogenous brassinosteroids in plant tissues using solid-phase extraction with double layered cartridge followed by high-performance liquid chromatography-tandem mass spectrometry. Phytochem. Anal. 24, 386–394. doi: 10.1002/pca.2421
Divi, U. K., and Krishna, P. (2009). Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol. 26, 131–136. doi: 10.1016/j.nbt.2009.07.006
Divi, U. K., Rahman, T., and Krishna, P. (2016). Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotechnol. J. 14, 419–432. doi: 10.1111/pbi.12396
Du, H., Wang, N., Cui, F., Li, X., Xiao, J., and Xiong, L. (2010). Characterization of the beta-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiol. 154, 1304–1318. doi: 10.1104/pp.110.163741
El-Mashad, A. A. A., and Mohamed, H. I. (2012). Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 249, 625–635. doi: 10.1007/s00709-011-0300-7
Farooq, M., Wahid, A., Kobayashi, N., Fujita, D., and Basra, S. M. A. (2009). Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 29, 185–212. doi: 10.1051/agro:2008021
Fujioka, S., Li, J., Choi, Y. H., Seto, H., Takatsuto, S., Noguchi, T., et al. (1997). The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell 9, 1951–1962. doi: 10.1105/tpc.9.11.1951
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Groppa, M. D., and Benavides, M. P. (2008). Polyamines and abiotic stress: recent advances. Amino Acids 34, 35–45. doi: 10.1007/s00726-007-0501-8
Grove, M. D., Spencer, G. F., Rohwedder, W. K., Mandava, N., Worley, J. F., Warthen, J. D., et al. (1979). Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281, 216–217. doi: 10.1038/281216a0
Gruszka, D. (2013). Brassinosteroid signaling pathway-new key players and interconnections with other signaling networks crucial for plant development and stress tolerance. Int. J. Mol. Sci. 14, 8740–8774. doi: 10.3390/ijms14058740
Gruszka, D., Janeczko, A., Dziurka, M., Pociecha, E., Oklestkova, J., and Szarejk, I. (2016). Barley brassinosteroid mutants provide an insight into phytohormonal homeostasis in plant reaction to drought stress. Front. Plant Sci. 7:1824. doi: 10.3389/fpls.2016.01824
Guo, Z., Fujioka, S., Blancaflor, E. B., Miao, S., Gou, X., and Li, J. (2010). TCP1 modulates brassinosteroid biosynthesis by regulating the expression of the key biosynthetic gene DWARF4 in Arabidopsis thaliana. Plant Cell 22, 1161–1173. doi: 10.1105/tpc.109.069203
Hong, Z., Ueguchitanaka, M., Fujioka, S., Takatsuto, S., Yoshida, S., Hasegawa, Y., et al. (2005). The rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell 17, 2243–2254. doi: 10.1105/tpc.105.030973
Horsch, R., Fry, J., Hoffmann, N., Eichholtz, D., Rogers, S. A., and Fraley, R. (1985). A simple and general method for transferring genes into plants. Science 227, 1229–1231. doi: 10.1126/science.227.4691.1229
Hu, Y., Bao, F., and Li, J. (2000). Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J. 24, 693–701. doi: 10.1046/j.1365-313x.2000.00915.x
Huang, X. S., Liu, J. H., and Chen, X. J. (2010). Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol. 10:230. doi: 10.1186/1471-2229-10-230
Janeczko, A., Gruszka, D., Pociecha, E., Dziurka, M., Filek, M., Jurczyk, B., et al. (2016). Physiological and biochemical characterisation of watered and drought-stressed barley mutants in the HvDWARF gene encoding C6-oxidase involved in brassinosteroid biosynthesis. Plant Physiol. Biochem. 99, 126–141. doi: 10.1016/j.plaphy.2015.12.003
Jang, E. K., Min, K. H., Kim, S. H., Nam, S. H., Zhang, S., Kim, Y. C., et al. (2009). Mitogen-activated protein kinase cascade in the signaling for polyamine biosynthesis in tobacco. Plant Cell Physiol. 50, 658–664. doi: 10.1093/pcp/pcp009
Kagale, S., Divi, U. K., Krochko, J. E., Keller, W. A., and Krishna, P. (2007). Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225, 353–364. doi: 10.1007/s00425-006-0361-6
Kamiab, F., Talaie, A., Khezri, S., and Javanshah, A. (2014). Exogenous application of free polyamines enhance salt tolerance of pistachio (Pistacia vera L.) seedlings. Plant Growth Regul. 72, 257–268. doi: 10.1007/s10725-013-9857-9
Kim, T. W., Hwang, J. Y., Kim, Y. S., Joo, S. H., Chang, S. C., Lee, J. S., et al. (2005). Arabidopsis CYP85A2, a cytochrome p450, mediates the baeyer-villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 17, 2397–2412. doi: 10.1105/tpc.105.033738
Kodaira, K. S., Qin, F., Tran, L. S. P., Maruyama, K., Kidokoro, S., Fujita, Y., et al. (2011). Arabidopsis Cys2/His2 zinc-finger proteins AZF1 and AZF2 negatively regulate abscisic acid-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol. 157, 742–756. doi: 10.1104/pp.111.182683
Kurepin, L. V., Qaderi, M. M., Back, T. G., Reid, D. M., and Pharis, R. P. (2008). A rapid effect of applied brassinolide on abscisic acid concentrations in Brassica napus leaf tissue subjected to short-term heat stress. Plant Growth Regul. 55, 165–167. doi: 10.1007/s10725-008-9276-5
Lee, S. C., and Luan, S. (2012). ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 35, 53–60. doi: 10.1111/j.1365-3040.2011.02426.x
Li, J., and Chory, J. (1997). A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90, 929–938. doi: 10.1016/S0092-8674(00)80357-8
Li, J., Lease, K. A., Tax, F. E., and Walker, J. C. (2001). BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 98, 5916–5921. doi: 10.1073/pnas.091065998
Li, J., Wen, J., Lease, K. A., Doke, J. T., Tax, F. E., and Walker, J. C. (2002). BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110, 213–222. doi: 10.1016/S0092-8674(02)00812-7
Li, K. R., and Feng, C. H. (2011). Effects of brassinolide on drought resistance of Xanthoceras sorbifolia seedlings under water stress. Acta Physiol. Plant. 33, 1293–1300. doi: 10.1007/s11738-010-066-0
Li, M. Q., Ahammed, G. J., Li, C. X., Bao, X., Yu, J. Q., Huang, C. L., et al. (2016). Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front. Plant Sci. 7:615. doi: 10.3389/fpls.2016.00615
Liu, Y., Wang, L., Xing, X., Sun, L., Pan, J., Kong, X., et al. (2013). ZmLEA3, a multifunctional group 3 LEA protein from maize (Zea mays L.), is involved in biotic and abiotic stresses. Plant Cell Physiol. 54, 944–959. doi: 10.1093/pcp/pct047
Li, X. J., Chen, X. J., Guo, X., Yin, L. L., Ahammed, G. J., Xu, C. J., et al. (2016a). DWARF overexpression induces alteration in phytohormone homeostasis, development, architecture and carotenoid accumulation in tomato. Plant Biotechnol. J. 14, 1021–1033. doi: 10.1111/pbi.12474
Li, X. J., Guo, X., Zhou, Y. H., Shi, K., Zhou, J., Yu, J. Q., et al. (2016b). Overexpression of a brassinosteroid biosynthetic gene Dwarf enhances photosynthetic capacity through activation of calvin cycle enzymes in tomato. BMC Plant Biol. 16:33. doi: 10.1186/s12870-016-0715-6
Li, Y., Ye, W., Wang, M., and Yan, X. (2009). Climate change and drought: a risk assessment of crop-yield impacts. Clim. Res. 39, 31–46. doi: 10.3354/cr00797
Liu, J. H., Wang, W., Wu, H., Gong, X. Q., and Moriguchi, T. (2015). Polyamines function in stress tolerance: from synthesis to regulation. Front. Plant Sci. 6:827. doi: 10.3389/fpls.2015.00827
Llanes, A., Andrade, A., Alemano, S., and Luna, V. (2016). Alterations of endogenous hormonal levels in plants under drought and salinity. Am. J. Plant Sci. 7, 1357–1371. doi: 10.4236/ajps.2016.79129
Miller, G., Suzuki, N., Ciftci-Yilmaz, S., and Mittler, R. (2010). Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 33, 453–457. doi: 10.1111/j.1365-3040.2009.02041.x
Nemhauser, J. L., Hong, F., and Chory, J. (2006). Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126, 467–475. doi: 10.1016/j.cell.2006.05.050
Northey, J. G., Liang, S., Jamshed, M., Deb, S., Foo, E., Reid, J. B., et al. (2016). Farnesylation mediates brassinosteroid biosynthesis to regulate abscisic acid responses. Nat. Plants 25:16114. doi: 10.1038/nplants.2016.114
Ohnishi, T., Godza, B., Watanabe, B., Fujioka, S., Hategan, L., Ide, K., et al. (2012). CYP90A1/CPD, a brassinosteroid biosynthetic cytochrome P450 of Arabidopsis, catalyzes C-3 oxidation. J. Biol. Chem. 287, 31551–31560. doi: 10.1074/jbc.M112.392720
Peng, S. N., Tao, P., Xu, F., Wu, A. P., Huo, W. G. and Wang, J. X. (2016). Functional characterization of soybean Glyma04g39610 as a brassinosteroid receptor gene and evolutionary analysis of soybean brassinosteroid receptors. Int. J. Mol. Sci. 17, 897. doi: 10.3390/ijms17060897
Pérez-España, V. H., Sánchez-León, N., and Vielle-Calzada, J. P. (2011). CYP85A1 is required for the initiation of female gametogenesis in Arabidopsis thaliana. Plant Signal. Behav. 6, 321–326. doi: 10.4161/psb.6.3.13206
Radhakrishnan, R., and Lee, I. J. (2013). Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J. Plant Growth Regul. 32, 22–30. doi: 10.1007/s00344-012-9274-8
Sahni, S., Prasad, B. D., Liu, Q., Grbic, V., Sharpe, A., Singh, S. P., et al. (2016). Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 6:28298. doi: 10.1038/srep28298
Shimada, Y., Fujioka, S., Miyauchi, N., Kushiro, M., Takatsuto, S., Nomura, T., et al. (2001). Brassinosteroid-6-oxidases from Arabidopsis and tomato catalyze multiple c-6 oxidations in brassinosteroid biosynthesis. Plant Physiol. 126, 770–779.
Shimada, Y., Goda, H., Nakamura, A., Takatsuto, S., Fujioka, S., and Yoshida, S. (2003). Organ-specific expression of brassinosteroid-biosynthetic genes and distribution of endogenous brassinosteroids in Arabidopsis. Plant Physiol. 131, 287–297. doi: 10.1104/pp.013029
Shu, S., Yuan, L. Y., Guo, S. R., Sun, J., and Yuan, Y. H. (2013). Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol. Biochem. 63, 209–216. doi: 10.1016/j.plaphy.2012.11.028
Steber, C. M., and McCourt, P. (2001). A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 125, 763–769. doi: 10.1104/pp.125.2.763
Suzuki, H., Fujioka, S., Takatsuto, S., Yokota, T., Murofushi, N., and Sakurai, A. (1995). Biosynthesis of brassinosteroids in seedlings of Catharanthus roseus, Nicotiana tabacum, and Oryza sativa. Biosci. Biotechnol. Biochem. 59, 168–172. doi: 10.1271/bbb.59.168
Tarkowská, D., Novák, O., Oklestkova, J., and Strnad, M. (2016). The determination of 22 natural brassinosteroids in a minute sample of plant tissue by UHPLC–ESI–MS/MS. Anal. Bioanal. Chem. 408, 6799–6812. doi: 10.1007/s00216-016-9807-2
Udawat, P., Jha, R. K., Sinha, D., Mishra, A., and Jha, B. (2016). Overexpression of a cytosolic abiotic stress responsive universal stress protein (SBUSP) mitigates salt and osmotic stress in transgenic tobacco plants. Front. Plant Sci. 7:518. doi: 10.3389/fpls.2016.00518
Vert, G., Nemhauser, J. L., Geldner, N., Hong, F., and Chory, J. (2005). Molecular mechanisms of steroid hormone signaling in plants. Annu. Rev. Cell Dev. Biol. 21, 177–201. doi: 10.1146/annurev.cellbio.21.090704.151241
Vriet, C., Russinova, E., and Reuzeaua, C. (2012). Boosting crop yields with plant steroids. Plant Cell 24, 842–857. doi: 10.1105/tpc.111.094912
Wang, X. T., Zeng, J., Li, Y., Rong, X. L., Sun, J. T., Sun, T., et al. (2015). Expression of TaWRKY44, a wheat WRKY gene, in transgenic tobacco confers multiple abiotic stress tolerances. Front. Plant Sci. 6:615. doi: 10.3389/fpls.2015.00615
Wang, Z. Y., Nakano, T., Gendron, J., He, J., Chen, M., Vafeados, D., et al. (2002). Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2, 505–513. doi: 10.1016/S1534-5807(02)00153-3
Xia, X. J., Gao, C. J., Song, L. X., Zhou, Y. H., and Yu, J. Q. (2014). Role of H2O2 dynamics in brassinosteroid-induced stomatal closure and opening in Solanum lycopersicum. Plant Cell Environ. 37, 2036–2050. doi: 10.1111/pce.12275
Xia, X. J., Wang, Y. J., Zhou, Y. H., Tao, Y., Mao, W. H., Shi, K., et al. (2009). Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 150, 801–814. doi: 10.1104/pp.109.138230
Xia, X. J., Zhou, Y. H., Shi, K., Zhou, J., Foyer, C. H., and Yu, J. Q. (2015). Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 66, 2839–2856. doi: 10.1093/jxb/erv089
Xiong, L., and Zhu, J. K. (2002). Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 25, 131–139. doi: 10.1046/j.13653040.2002.00782.x
Xu, S., Hu, J., Li, Y., Ma, W., Zheng, Y., and Zhu, S. J. (2011). Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regul. 63, 279–290. doi: 10.1007/s10725-010-9528-z
Yu, J. Q., Huang, L. F., Hu, W. H., Zhou, Y. H., Mao, W. H., Ye, S. F., et al. (2004). A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J. Exp. Bot. 55, 1135–1143. doi: 10.1093/jxb/erh124
Yuan, T., Fujioka, S., Takatsuto, S., Matsumoto, S., Gou, X., He, K., et al. (2007). BEN1, a gene encoding a dihydroflavonol 4-reductase (DFR)-like protein, regulates the levels of brassinosteroids in Arabidopsis thaliana. Plant J. 51, 220–233. doi: 10.1111/j.1365-313X.2007.03129.x
Keywords: drought stress tolerance, transgenic tobacco, SoCYP85A1, brassinosteroids, stress-responsive genes, ROS
Citation: Duan F, Ding J, Lee D, Lu X, Feng Y and Song W (2017) Overexpression of SoCYP85A1, a Spinach Cytochrome p450 Gene in Transgenic Tobacco Enhances Root Development and Drought Stress Tolerance. Front. Plant Sci. 8:1909. doi: 10.3389/fpls.2017.01909
Received: 16 May 2017; Accepted: 23 October 2017;
Published: 09 November 2017.
Edited by:
Viswanathan Chinnusamy, Indian Agricultural Research Institute (ICAR), IndiaReviewed by:
Xin Deng, Institute of Botany, Chinese Academy of Sciences, ChinaSusanne Hoffmann-Benning, Michigan State University, United States
Copyright © 2017 Duan, Ding, Lee, Lu, Feng and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenwen Song, songwenwen2002@163.com
 Fangmeng Duan1
Fangmeng Duan1 Jun Ding
Jun Ding Xueli Lu
Xueli Lu Yuqi Feng
Yuqi Feng Wenwen Song
Wenwen Song
