- 1Research and Innovation Centre, Fondazione Edmund Mach, Genomics and Biology of Fruit Crop Department, San Michele all'Adige, Italy
- 2Center Agriculture Food Environment, University of Trento, San Michele all'Adige, Italy
- 3Research and Innovation Centre, Fondazione Edmund Mach, Computational Biology Platform, San Michele all'Adige, Italy
- 4Technology Transfer Centre, Fondazione Edmund Mach, Experiment and Technological Services Department, San Michele all'Adige, Italy
- 5Institute for Sustainable Plant Protection—CNR, Grugliasco, Italy
Terpenoids, especially monoterpenes, are major aroma-impact compounds in grape and wine. Previous studies highlighted a key regulatory role for grapevine 1-deoxy-D-xylulose 5-phosphate synthase 1 (VvDXS1), the first enzyme of the methylerythritol phosphate pathway for isoprenoid precursor biosynthesis. Here, the parallel analysis of VvDXS1 genotype and terpene concentration in a germplasm collection demonstrated that VvDXS1 sequence has a very high predictive value for the accumulation of monoterpenes and also has an influence on sesquiterpene levels. A metabolic engineering approach was applied by expressing distinct VvDXS1 alleles in the grapevine model system “microvine” and assessing the effects on downstream pathways at transcriptional and metabolic level in different organs and fruit developmental stages. The underlying goal was to investigate two potential perturbation mechanisms, the former based on a significant over-expression of the wild-type (neutral) VvDXS1 allele and the latter on the ex-novo expression of an enzyme with increased catalytic efficiency from the mutated (muscat) VvDXS1 allele. The integration of the two VvDXS1 alleles in distinct microvine lines was found to alter the expression of several terpenoid biosynthetic genes, as assayed through an ad hoc developed TaqMan array based on cDNA libraries of four aromatic cultivars. In particular, enhanced transcription of monoterpene, sesquiterpene and carotenoid pathway genes was observed. The accumulation of monoterpenes in ripe berries was higher in the transformed microvines compared to control plants. This effect is predominantly attributed to the improved activity of the VvDXS1 enzyme coded by the muscat allele, whereas the up-regulation of VvDXS1 plays a secondary role in the increase of monoterpenes.
Introduction
Isoprenoids, also known as terpenoids, are the largest family of plant natural compounds with many biological functions including growth and development (gibberellic acid, abscisic acid, brassinosteroids, and cytokinins), photosynthesis (chlorophylls, carotenoids, plastoquinones), defense as well as interaction with the environment (monoterpenes, sesquiterpenes, and diterpenes) (Dudareva et al., 2004; Aharoni et al., 2005; Tholl, 2006). From a human perspective, isoprenoids are also of commercial interest (Aharoni et al., 2005). Some of them play a direct role in the fruit quality, such as the monoterpenes linalool, geraniol, nerol that are the major aromatic determinants in Muscat grape varieties, others are used as flavors and fragrances in foods and cosmetics (e.g., menthol, nootkatone, and sclareol) or for medical applications (e.g., taxol, artemisinin, and glycyrrhizin). Isoprenoids derive from both the mevalonate pathway (MVA), which is active in the cytosol, and the plastidial 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway through the formation of the common precursor intermediates isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) (Laule et al., 2003). The MVA route is responsible for the formation of sesquiterpenes, triterpenes, sterols, and the prenyl chain of ubiquinone, while the MEP pathway is involved in the biosynthesis of isoprene, monoterpenes, diterpenes, carotenoids, the phytyl side chain of chlorophyll and the prenyl chain of plastoquinone (Eisenreich et al., 2004). In spite of this compartmentalization, a crosstalk between the cytosolic and plastidial pathways has been demonstrated in Arabidopsis, which takes place preferentially from the chloroplast to the cytoplasm (Laule et al., 2003; Dudareva et al., 2013).
The ecological and commercial importance of terpenoids makes their manipulation through metabolic engineering an attractive challenge, as proved by intensive research in recent years (Farhi et al., 2011; Dong et al., 2013; Houshyani et al., 2013; Lange and Ahkami, 2013). Although highly appealing from a biotechnological viewpoint, this goal is not easy to achieve (McCaskill and Croteau, 1997). Metabolic pathways are controlled at multiple levels and any form of perturbation can have wide-ranging effects at the whole system level (Capell and Christou, 2004). Therefore, the modulation of key regulatory enzymes may result in an altered production of various metabolites.
Studies from the model plant Arabidopsis thaliana (Mandel et al., 1996; Estévez et al., 2000, 2001) suggested that the control of the MEP pathway flux is primarily exerted by the first enzyme of the route, 1-deoxy-D-xylulose 5-phosphate synthase (DXS). The key role of DXS in the plastidial isoprenoid biosynthesis was subsequently proved in other plant species, including Lycopersicon esculentum (Lois et al., 2000; Enfissi et al., 2005), Whitania somnifera (Jadaun et al., 2017), Solanum tuberosum (Morris et al., 2006), Lavandula latifolia (Muñoz-Bertomeu et al., 2006), Catharanthus roseus (Peebles et al., 2011), Daucus carota (Simpson et al., 2016), Salvia sclarea (Vaccaro et al., 2014), Salvia milthiorrhiza (Zhou et al., 2016). It was further demonstrated that the MEP pathway is controlled by tight feedback regulation of the reaction catalyzed by DXS (Wolfertz et al., 2004; Flores-Pérez et al., 2008; Wright et al., 2014).
In several plant species DXS is encoded by more than a single gene and each isoform displays differential expression during development and in specific organs, suggesting a non-redundant function (Rodríguez-Concepción and Boronat, 2002; Khemvong and Suvachittanont, 2005; Kim et al., 2005; Phillips et al., 2007; Cordoba et al., 2011; Han et al., 2013; Saladié et al., 2014; Xu et al., 2014).
Multiple DXS gene isoforms were also predicted in grapevine, where VvDXS1 is located on chromosome 5, three VvDXS2 isoforms (VvDXS2A, VvDXS2B, and VvDXS2C) located on chromosomes 15, 11, and 7 respectively, and VvDXS3 on chromosome 4 (Battilana et al., 2009). Over the last few years, VvDXS1 was discovered to co-localize with the major QTL (quantitative trait locus) for monoterpene content in mature grape berry (Battilana et al., 2009) and a non-neutral dominant mutation in this gene causing an amino acid exchange from K (Lysine) to N (Asparagine) at position 284 of the protein was found to be significantly associated with muscat-flavored grapevine varieties (Emanuelli et al., 2010). This mutation was shown to improve the enzymatic catalytic efficiency in vitro and to cause a dramatic increase of glycosylated monoterpenes in transgenic tobacco overexpressing the VvDXS1 N284 allele (Battilana et al., 2011).
In the present study the functional analysis of VvDXS1 was carried out for the first time in grapevine using the microvine model system (Chaïb et al., 2010) modified to ectopically express either the mutated (N284) or the non-mutated (K284) form of the gene. A TaqMan array tool was developed in order to simultaneously evaluate the expression pattern of a hundred terpenoid biosynthetic genes in transformed microvines at various stages during berry development. This allowed to investigate how a potentially enhanced MEP pathway flux may perturb isoprenoid metabolism. In addition, the content of free and bound monoterpenes, as well as sesquiterpenes, was assessed in mature berries. The results are discussed with reference to natural variation of VvDXS1 and terpene concentration in the grapevine (Vitis vinifera L.) germplasm.
Materials and Methods
Plant Material and Gene Transfer
Agrobacterium tumefaciens (A.t.)-mediated gene transfer was performed on embryogenic calli of “microvine 04C023V0006” (derived from a cross between “Grenache” and the original L1 mutant microvine, Chaïb et al., 2010), “Chardonnay” and “Brachetto” genotypes according to Dalla Costa et al. (2014). Experiments were carried out using A.t. strain EHA105 (Hood et al., 1993) carrying a pK7WG2 plant binary vector (Karimi et al., 2002), with the muscat (M) or the neutral (N) allele of VvDXS1 under the control of the CaMV-35S promoter. The two forms of the gene differ for one nucleotide substitution at position 1822 (G in the neutral allele or T in the muscat allele), which results in the substitution of Lysine with Asparagine at position 284 in the protein sequence. As selectable marker the neomycin phosphotransferase II (nptII) gene was used, which confers resistance to kanamycin.
Transformed and wild-type in vitro plantlets were acclimatized in a growth chamber (94.5 μmol·m−2·s−1 cool white light and 16 h-light photoperiod, at 25°C and 70% humidity) and subsequently transferred to the greenhouse.
Allele Discrimination by VvDXS1 Amplicon Digestion and Sequencing
The PCR was performed in a 20 μl reaction volume containing 100 ng of leaf DNA, 0.25 mM dNTPs, 0.3 μM of each primer (Fw: ATTGCTGTCATAGGTGATGGAG; Rv: CTGTTGTCTTGGTACTCTTAAC), 1X Taq Buffer Advanced (5 Prime, Hilden, Germany) and 1 unit of 5 Prime Taq DNA Polymerase (5 Prime, Hilden, Germany). The initial denaturation at 95°C for 5 min was followed by 35 cycles of 30 s at 95°C, 30 s at 58°C, and 30 s at 68°C, with a final extension of 7 min at 68°C. The obtained amplicon (423 bp) was digested with the FastDigest StyI restriction enzyme (Thermo Fischer Scientific, Waltham, MA, USA) to detect SNP1822 G/T (StyI recognizes the restriction site when G is present) or sequenced with the 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
Estimation of Transgene Insertion Copies through Southern Blot and qPCR
Digoxigenin-labeled probes for VvDXS1 and nptII genes were obtained with the PCR Dig Probe Synthesis Kit (Roche Diagnostics, Indianapolis, IN, USA), using the following primers: VvDXS1-SB-fw = ATGGCTCTCTGTACGCTCTCA and VvDXS1-SB-rv = AGTTGTTTCAGCTCCTTGACAG; nptII-SB-fw = GAAGGGACTGGCTGCTATTG and nptII-SB-rv = AATATCACGGGTAGCCAACG. For each sample, 10 μg of DNA was digested with the Fastdigest® restriction enzymes XbaI and EcoRI (Thermo Fischer Scientific, Waltham, MA, USA) for VvDXS1 probing and with HindIII for nptII probing. Digestion products were precipitated, resuspended in 30 μl Milli-Q water and separated overnight on 0.9% agarose gel (0.5X TBE) at 50 V. Membrane blotting and hybridization were performed following Roche user's manual. The autoradiographic film was exposed overnight before development.
Quantitative real-time PCR (qPCR) amplification was performed on genomic DNA in 96-well reaction plates on the iCycler iQ Thermocycler (Biorad, Hercules, CA, USA) according to the method by Dalla Costa et al. (2009).
Gene Expression Analysis
Total RNA was isolated from grape leaves, flowers and fruits at different development stages using the Spectrum™ Plant Total RNA Kit (Sigma Aldrich, St. Louis, MO, USA) and quantified with the spectrophotometer NanoDrop ND-8000 (NanoDrop Technologies, Wilmington, DE, USA). Following DNase treatment, 2 μg of RNA were retro-transcribed into cDNA with the SuperScript® III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) and oligo(dT) (or random primers for applications with TaqMan array cards). The qPCR was carried out as described in Supplementary Materials.
TaqMAN Array Card Development and Assay
A set of terpenoid biosynthetic genes were selected for loading onto the TaqMan array card based on their expression in aromatic grapevine cultivars. For this purpose, four normalized cDNA libraries were obtained using the extracted RNA from ripening berries of the cultivars Gewürztraminer (TRA), Malvasia di Candia aromatica (MAL), Moscato Bianco (MOB) and Rhein Riesling (RIE). The normalized cDNA libraries were then sequenced with a 454 GS FLX Titanium system (Roche, Indianapolis, IN, USA) and filtered high quality reads were aligned to the 12x V1 version of the Vitis vinifera genome (details are provided in Supplementary Materials). In parallel, a total of 180 genes involved in terpenoid biosynthesis in grapevine were retrieved from KEGG (http://www.genome.jp/kegg-bin/show_organism?org=vvi) by searching for the following ko terms: vvi00900 (terpenoid backbone biosynthesis), vvi00902 (monoterpenoid biosynthesis), vvi00904 (diterpenoid biosynthesis), vvi00905 (brassinosteroid biosynthesis), vvi00906 (carotenoid biosynthesis) and vvi00909 (sesquiterpenoid and triterpenoid biosynthesis). An additional 20 genes were selected based on their co-localization with grapevine QTLs for monoterpenoid content (Battilana et al., 2009) or peculiar expression in ripening berries of aromatic grapes (unpublished data). All the above mentioned genes were checked for their expression in the normalized cDNA libraries and were manually investigated in order to precisely define their gene structure, allelic and possible splicing variants.
TaqMan array (TA) cards (Applied Biosystems, Foster City, CA, USA) are 384-well microfluidic cards with eight ports, each containing 48 connected wells. Primers and probes are preloaded and dried onto the wells by the manufacturer at the following concentrations: 9 × 10−7 mol/L for primer, 2 × 10−7 mol/L for probe. All the probes are conjugated at 5′ to a reporter 6-carboxyfluorescein (FAM) and at 3′ to a non-fluorescent quencher (NFQ) with the minor groove binder (MGB) moiety attached to the molecule. In the TaqMan array card developed in our study (Aromix), four samples (for details see Results) can be assessed simultaneously for 83 targets connected with the terpene metabolism (Table S1). The card also features five endogenous genes, as well as the manufacturer's card control PCR. Probes and primers were designed by the manufacturer based on the gene sequences supplied by the authors. All TA cards were run on the ViiA™ 7 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Each of the eight ports of the card was loaded with a 100 μl solution obtained by mixing 50 μl of 1:20 diluted cDNA with 50 μl of TaqMan Gene Expression Master Mix 2X (Applied Biosystems, Foster City, CA, USA). The cards were centrifuged twice at 1,200 rpm for 1 min and sealed, the loading ports were excised and the cards were placed in the thermal cycler. The following cycling conditions were used: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 30 s followed by 60°C for 1 min. Results were processed with qbasePLUS software (Biogazelle, Zwijnaarde, Belgium; Hellemans et al., 2007) and normalized by the reference genes selected from qbasePLUS (actin and glyceraldehyde-3-phosphate dehydrogenase).
Terpenoid Quantification in Grapevine Germplasm and in Microvine Plants Transformed with VvDXS1
Approximately 90 grapevine cultivars representing Muscat, herbaceous, or other distinct flavored cultivars, as well as non-aromatic ones were analyzed at technological maturity (harvest time) for monoterpene and sesquiterpene content in whole berries. Berry flavor phenotype as assessed merely by tasting and the VvDXS1 genotype were previously reported for the majority of these accessions (Emanuelli et al., 2014).
For each microvine line, berry number and size, total leaf area and photosynthetic activity were evaluated in four biological replicates 1 month before berry sampling. Targeted cluster thinning or leaf pruning were carried out in order to set a similar ratio between total leaf area and number of berries for all the plants under analysis (Table S2). Clusters were collected at technological maturity (18–20.5 degrees Brix,°Bx) and berry skin was separated from the pulp. Skins were grounded to a fine powder in liquid nitrogen and used for monoterpene and sesquiterpene analysis.
Eleven monoterpenes were extracted from the starting plant material and quantified in their free and glycosidically bound form by solid phase extraction (SPE) and high-resolution gas chromatography-mass spectrometry (HRGC-MS), as previously described by Battilana et al. (2011). A non-targeted analysis was carried out to profile the sesquiterpenes.
Results
Characterization of Transformed Plants
Genetically modified plants were obtained from microvine (Mi), Chardonnay (C) and Brachetto (B) genotypes (Figure 1, Figure S1). Microvines were chosen, since they have short generation cycles and continuous flowering, which ensure fruit production and significantly reduce the time required for genetic studies in grapevine (Chaïb et al., 2010). The identity of the VvDXS1 integrated allele (neutral or muscat form) was confirmed by sequencing. The number of T-DNA integration copies as calculated by real-time PCR and Southern blot is shown in Figures 1A,B, Figure S1A. One T-DNA integration copy was detected for Mi-N4, two for C-M5, C-M6 and B-N8, while Mi-M1 and the remaining lines transformed with the neutral allele presented a multi-copy insertion.
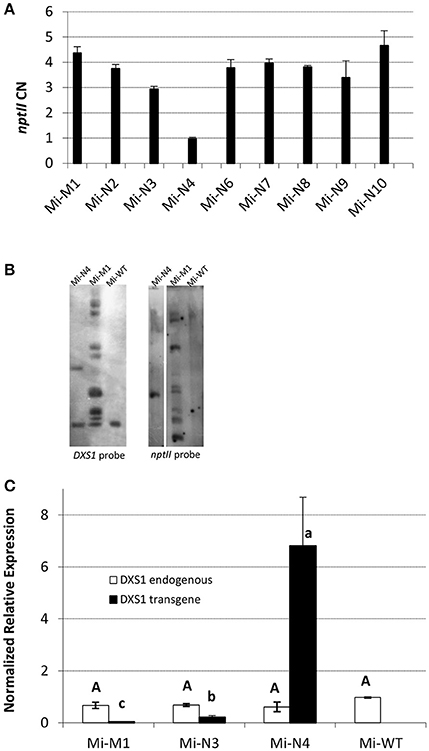
Figure 1. Molecular characterization of in vitro transformed microvines. (A,B) Determination of T-DNA integration copies by real-time PCR for nptII quantification (copy number (CN) values are the mean ± standard error (SE) of two biological replicates analyzed in two separate PCR sessions) (A) and by Southern blot with VvDXS1 and nptII probes (B). (C) Transcription profile of endogenous (white) and transgenic (black) VvDXS1 in the leaf tissue. Expression values are the mean ± SE of three biological replicates. Uppercase and lowercase lettering on the bars indicate different subsets according to ANOVA and Tukey's HSD post-hoc tests (P < 0.05). WT, wild-type; M, VvDSX1 muscat allele; N, VvDXS1 neutral allele.
The qPCR expression analysis proved that VvDXS1 transgene was transcribed in the leaves of all transformed lines, although the level of expression was variable across the lines. Conversely, endogenous VvDXS1 was stably expressed (Figure 1C, Figures S1B, S2). After this evaluation, Chardonnay and Brachetto plants—which were not expected to produce fruits at least in the short-term—were not further investigated and were kept for future experiments. Several biological replicates of each transformed and WT microvine line were transferred to soil and successfully acclimatized in the greenhouse in 2013, 2014, and 2015 (Figure 2). Data on flowering time and berry development were collected for Mi-M1, Mi-N3, Mi-N4, and Mi-WT acclimatized in January 2014 (Table 1). The first microvine plants began to bloom about 150 days after acclimatization and anthesis occurred on average 197 days after acclimatization in 11 out of the 14 observed plants. Eight microvine plants reached veraison and maturity, respectively 281 and 309 days (on average) after acclimatization. Modified plants did not show any evident phenotypic difference in vitro or in greenhouse conditions, compared to the control plants (Table S2).

Figure 2. A shoot of microvine 04C023V0006 with flowers and berries at different developmental stages.
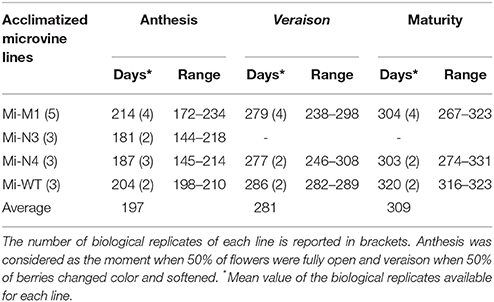
Table 1. Time required to reach anthesis, veraison and maturity starting from acclimatization, for three transformed microvine lines and control microvine (WT).
VvDXS1 Expression in Various Tissues and Developmental Stages
The expression of both endogenous and transgenic VvDXS1 in different plant tissues and during fruit development was investigated in Mi-N4, the microvine line with the highest transcription of the single copy transgene in leaves, and in Mi-M1, the only microvine line transformed with the muscat allele (Figure 1). The mRNA level of VvDXS1 transgene was significantly higher in the leaf in comparison to flower at anthesis (stage E-L 21 of the modified E-L system by Coombe, 1995) and berry at pre-veraison (E-L 32), veraison (E-L 35) and maturity (E-L 38). Following berry development, the transgene transcript level decreased from pre-veraison to veraison and returned to increase after veraison. A similar trend was observed for the endogenous VvDXS1 mRNA, even though the recovery following veraison was not detected (Figure 3, Figure S3). A comparable time-course profile, with the minimum point of the convexity corresponding to the veraison stage, was also obtained from the total VvDXS1 expression analysis in three microvine lines (Figure S4).
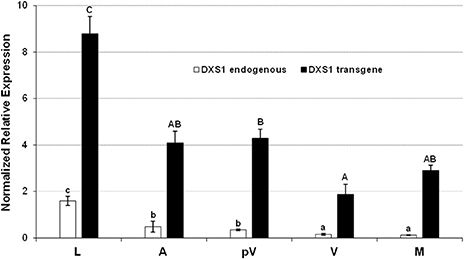
Figure 3. Expression analysis of endogenous (white) and transgenic (black) VvDXS1 in different organs and berry developmental stages of the line Mi-N4. The same cDNAs employed for the TaqMan card assay were assessed from anthesis onwards. Expression values are the mean ± SE of two biological replicates analyzed in two separate PCR sessions (in the case of leaves, four biological replicates were considered). Uppercase and lowercase lettering on the bars indicate different subsets according to ANOVA and Tukey's HSD post-hoc tests (P < 0.05). L, leaves; A, flowers at anthesis; pV, berries at pre-veraison stage; V, berries at veraison stage; M, berries at technological maturity (18 °Brix).
Development of a Tool to Quantify the Transcriptional Profile of Terpenoid Genes in Grapevine (Aromix_Taqman Array Card)
In order to define a minimal gene set that could describe the transcriptional perturbation of terpenoid biosynthesis, a manual selection of candidate genes actually expressed during berry ripening in four aromatic grapevine cultivars was performed. By combining the cDNA libraries of the four varieties a total of 21246 genes was found to be expressed (17603, 18032, 17724, and 18198 in MAL, MOB, RIE, and TRA libraries, respectively), of which 14875 were common to all four cultivars (data not shown).
Two-hundred genes involved in terpenoid biosynthesis were checked for their expression in the normalized cDNA libraries and a final set of 78 genes (including five endogenous genes) was selected to be included in the TaqMan array card. For 10 of these genes two specific probes were designed in order to discriminate between splicing variants, which resulted in a total of 88 probes (Table S1).
The relative expression of these genes was evaluated in Mi-M1, Mi-N4, and Mi-WT plants during the 2014 growing season at four phenological stages (Table S3). In addition to the five endogenous genes, 64 terpenoid biosynthetic genes were expressed in the plants under study, of which 47 were expressed in all the investigated phenological stages while 17 were expressed only in some stages. Finally, nine genes on the TaqMan array could not be amplified, the majority of which (six) coded for terpene synthases.
Overall, the genes belonging to the MEP and the MVA pathways showed an evident up-regulation in the transformed lines at anthesis and veraison and a moderate down-regulation in the pre-veraison stage compared to WT. This general trend was also observed for the genes involved in the metabolism of carotenoids while the monoterpene and sesquiterpene biosynthetic genes were more highly expressed at anthesis. Based on ANOVA and t-tests, 24 genes showed significantly different expression (P < 0.05) among the analyzed plants during the four phenological stages (12 at anthesis, nine at veraison, four at pre-veraison and three at maturity) (Figure 4).
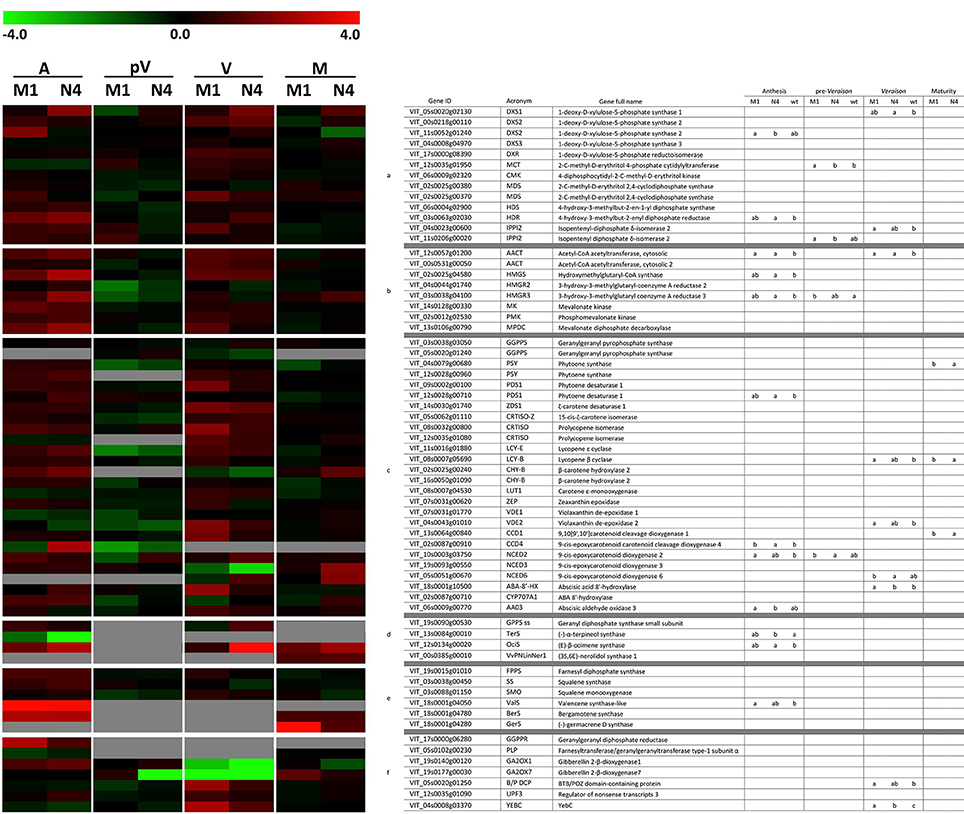
Figure 4. Relative expression matrix of the terpenoid pathway genes assayed by the Aromix_TaqMan array card in transformed and WT microvines during fruit development. Columns represent the transformed lines (M1 = Mi-M1, N4 = Mi-N4) at different stages (A, anthesis; pV, pre-veraison; V, veraison; M, maturity), while rows represent the genes grouped into six blocks related to terpenoid pathways: (a) MEP, (b) MVA, (c) carotenoids/apocarotenoids, (d) monoterpenes, (e) sesquiterpenes and (f) others (chlorophyll, gibberellin, isoprene, tocopherol, etc). Each element of the matrix indicates the log2 expression fold change in Mi-M1 or Mi-N4 lines compared to WT by means of a color code (heatmap). To this purpose, the raw Cq values of each line were considered stage by stage. The associated relative expression, which is the mean of two biological replicates both analyzed in duplicate, was calculated with the qBasePLUS software and is reported in Table S3 (at maturity only one WT biological replicate was available). Gray spaces correspond to not detectable transcripts. The heatmaps were generated with TM4 Multi experiment viewer (MeV) software (Saeed et al., 2003). The letters in the last four columns indicate different subsets according to ANOVA and LSD tests (anthesis, pre-veraison, veraison) and t-test (maturity) (P < 0.05).
The simultaneous analysis of splicing variants of ten genes did not highlight relevant differences in most cases. However, for VIT_04s0079g00680 (PSY) and VIT_12s0057g01200 (AACT) only one splicing variant was expressed throughout berry development. A differential profile was also observed when both isoforms were expressed at least in one stage, as for VIT_06s0009g00770 (AAO3) and VIT_18s0001g10500 (ABA 8′-HX) (Figure S5, Table S3).
The expression data derived from the TaqMan card assay were validated with sybr-green real-time PCR on a set of relevant genes belonging to the MEP-, MVA-, carotenoid-, sesquiterpene-, and monoterpene biosynthetic pathways (Table S4). A strong correlation between the two sets of measurements was observed, with Pearson correlation coefficient (R) values approximately or >0.9 for seven genes and between 0.8 and 0.7 for three genes (Figure S4). Difference in expression levels between transformed and WT lines was the most notable at anthesis, and therefore a further expression analysis on a set of modulated genes (VvDXS1, VvAACT, VvHMGS, VvHMGR3, VvOciS, VvFPPS, VvValS) was repeated by qPCR on flowers collected in triplicate in 2016. The significant differences found in 2014 were confirmed in 2016 (data not shown).
Effect of the VvDXS1 Mutation on the Accumulation of Terpenoids
Terpenoid Content in the Grapevine Germplasm
The concentration of monoterpenes and sesquiterpenes in the grapevine collection is shown in Figures 5A,B. The mean content of monoterpenes (in their free and glycosidically bound fractions and as a total) proved to be significantly different (p = 0.000) between homozygotes T/T or heterozygotes G/T for SNP1822 and wild-type homozygotes G/G, as assessed by both ANOVA and Kruskal-Wallis analyses. In particular, out of 70 accessions homozygous or heterozygous for T at position 1822, 62 had a total monoterpene content higher than 4 mg/kg of berries, which is typical of intensely flavored muscats in the classification scheme by Mateo and Jiménez (2000), while only eight cultivars (e.g., Italia and Perlette) showed a monoterpene content ranging from 1 to 4 mg/kg, which is attributed to non-muscat but aromatic varieties by the same authors. In contrast, all 17 homozygotes 1822 G/G proved to be neutral with a monoterpene content lower than 1 mg/kg of berries (Figure 5A). A similar, though lower, effect of the VvDXS1 genotype was observed on the sesquiterpene content (Figure 5B), which represents a novel finding.
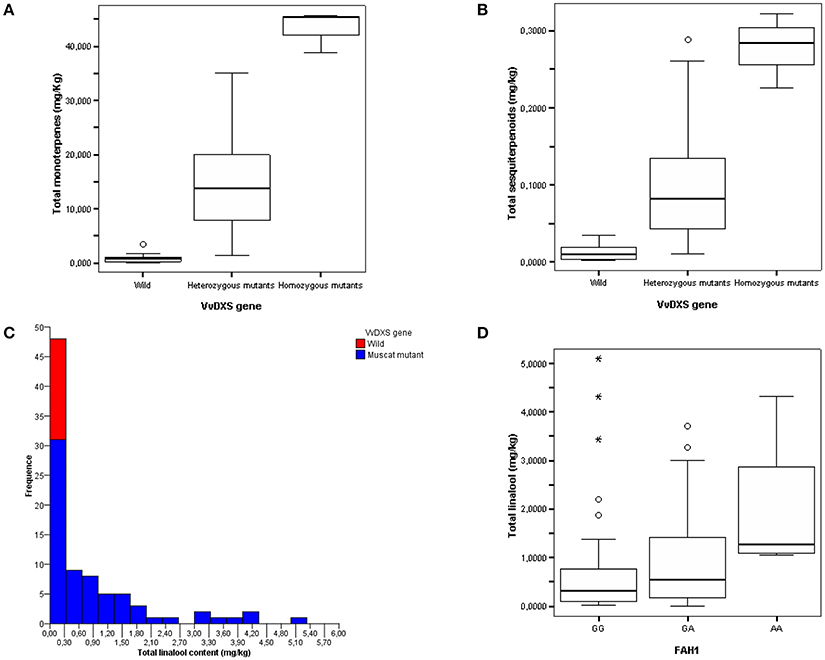
Figure 5. Total monoterpene (A) and sesquiterpene (B) content in the FEM aromatic core collection classified according to the VvDXS1 genotype at position 1822. Plants heterozygous or homozygous for T are indicated as mutants, while the homozygotes G/G correspond to the wild-type. Total linalool content in the whole FEM aromatic core collection classified according to the VvDXS1 genotype at position 1822 (C) and in a subset of accessions with the muscat mutation at SNP1822 classified according to the genotype at the marker FAH1 on chromosome 10 (Battilana et al., 2009; Emanuelli et al., 2011) (D).
Terpenoid Content in Mature Berries of Transformed and WT Microvine Plants
The monoterpene and sesquiterpene content was investigated in mature berry skins of transformed and control microvines cultivated in the greenhouse. Both free and bound monoterpenes were present at significantly higher levels in Mi-M1 compared to Mi-N4 and Mi-WT lines (Figure 6). Total bound monoterpenes (2500 μg/Kg for Mi-M1, 991 μg/Kg for Mi-N4, and 529 μg/Kg for Mi-WT) were more abundant than the total free component, which showed values near to 500 μg/Kg in Mi-M1 and far below in Mi-N4 and Mi-WT. The most abundant compounds were geranic acid (82% of both total free and bound monoterpenes in the Mi-M1 line), followed by citronellol, geraniol and nerol. Linalool was not present in any line (data not shown). Significant (P < 0.05) differences among lines were found for free geranic acid, bound geranic acid and citronellol, with the Mi-M1 line showing the highest level (Figure S6). No sesquiterpenes were detected in ripe berry skins of transformed and control microvines (data not shown). Samples were further evaluated for VvDXS1 (transgene and endogenous gene) expression. A significantly decreased level was observed in Mi-M1 compared to Mi-N4 (six times less, Figure 6) confirming the significant differences seen between the two transformed lines in the leaf tissue (Figure 1C).
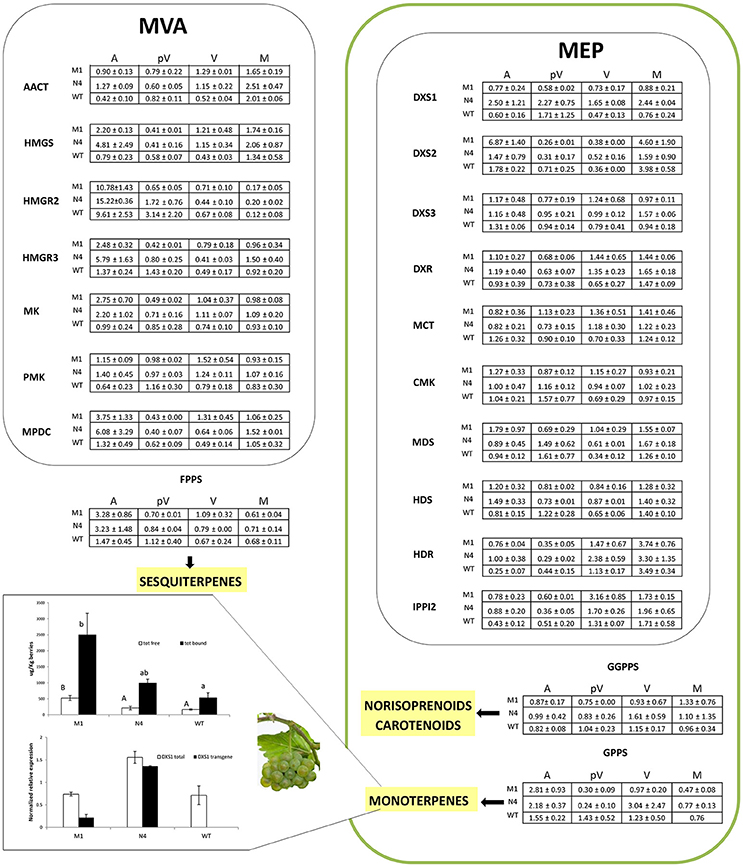
Figure 6. Transcription profile of specific genes of the MEP and MVA pathways during berry development (see matrix) and monoterpene content vs. VvDXS1 transcript level in mature fruits (see histograms). For the time-course expression profiles, the raw Cq values from TaqMan card assay were elaborated according to Hellemans et al. (2007) and numbers in the cells are the mean of two biological replicates ± SE. The full gene name corresponding to each acronym can be retrieved in Figure 4. Monoterpene content was assessed in berry skin of fruits collected at technological maturity in 2016 (Table S2) and concentration values in the upper histogram are the mean ± SE of four biological replicates. The letters on the bars indicate different subsets according to ANOVA and Tukey's HSD post-hoc tests (P < 0.05). A portion of the skin powder grounded for metabolic analysis was used to quantify VvDXS1 expression and values in the lower histogram are the mean ± SE of two (for WT) or three (for M1 and N4) biological replicates. M1 = Mi-M1, N4 = Mi-N4, A, flowers at anthesis; pV, berries at pre-veraison; V, berries at veraison; M, berries at maturity.
Discussion
The VvDXS1 Genotype Is Highly Predictive of Terpene Content in Grapevine
The first indications of the role that VvDXS1 plays in the genetic control of monoterpene biosynthesis derived from the analysis of segregating progenies (Battilana et al., 2009; Duchêne et al., 2009) and were subsequently confirmed by a genetic association study based on berry taste (Emanuelli et al., 2010). In the present work a grapevine germplasm collection was considered, for which VvDXS1 genotype and terpene content (as assessed by chemical methods) were analyzed in parallel. This analysis clearly highlighted that sequence variation of VvDXS1 has a very high predictive value for the accumulation of monoterpenes and, as a novel finding, also of sesquiterpenes. The strength of such genetic effect is even more evident if considering that the investigated plants have been grown under field conditions and have been influenced by uncontrolled environmental factors.
Transformed Microvines Carrying Distinct VvDXS1 Alleles Are Reported for the First Time
A metabolic engineering approach was adopted for studying the regulatory role of VvDXS1 on terpenoid metabolism in grapevine, given its relevance for the quality of grapes and wines. Besides Chardonnay and Brachetto, which are frequently used cultivars amenable to genetic transformation (Iocco et al., 2001; Dalla Costa et al., 2009; Dhekney et al., 2011; Perrone et al., 2012), the microvine genotype was also employed. Several genetic experiments have used the microvine (Luchaire et al., 2017) since it was presented as a grapevine model system for functional genomic studies (Chaïb et al., 2010), however no studies based on engineered microvine plants have been published so far. The present work was focused on the microvine transformation with different VvDXS1 alleles. The underlying goal was to investigate two potential perturbation mechanisms, the former based on a significant over-expression of the wild-type allele and the latter on the ex-novo expression of an enzyme with increased catalytic efficiency from the mutated allele. Here, we obtained a total of 13 transformed lines (nine microvines, two Chardonnay and two Brachetto) carrying the mutated or non-mutated form of VvDXS1 gene with integrations ranging from a single copy to multiple copies (Figure 1, Figures S1, S2).
A high level of transgene expression was measured in the lines Mi-N4, C-M5, C-M6, and B-N1. Conversely, a low expression rate was observed in the other lines, likely due to multi-copy insertions that may produce silencing effects or to the “position effect,” which is related to the genomic region where the T-DNA copies are integrated. Both causes of epigenetic silencing are well documented in literature (Matzke and Matzke, 1998; De Buck et al., 2007; Tang et al., 2007) and nowadays they represent two of the major concerns in plant genetic engineering (Rajeevkumar et al., 2015).
The period required by 04C023V0006 microvine plants for flowering in the greenhouse (on average 197 days in Table 1) was four times longer than that reported by Chaïb et al. (2010) for two different microvine genotypes. Alternatively, the time needed to reach fruit maturity (berry sugar content >18 °Brix) was consistent with that observed by Chaïb (i.e., 16 weeks post anthesis). Comparing microvine with the conventional grapevine genotypes, we confirmed that the time necessary for flowering is dramatically reduced while fruit ripening times are very similar (data not shown). Moreover, the VvDXS1 insertion did not affect the phenology or the morphology of the microvine plants (Table 1, Table S2).
VvDXS1 Is Spatially and Developmentally Regulated in the Transformed Microvine Lines
The spatio-temporal analysis of the VvDXS1 transcript in microvine confirmed that this gene is not expressed in a steady-state level in different grapevine organs and during fruit ripening but it is highly modulated as previously observed in Moscato Bianco (Battilana et al., 2011). Moreover, the VvDXS1 expression trend in the transformed lines Mi-M1 and Mi-N4 was similar to that in the WT plant (Figure 6, Figure S4). The modulation of VvDXS1 was even more evident when the expression profile of the endogenous gene and transgene were simultaneously analyzed in Mi-M1 and Mi-N4 lines (Figure 3, Figure S3). Under these conditions, the transgene proved to be controlled by the same organ-specific and developmental signals as the endogenous counterpart. In particular, a significantly higher expression was detected in the leaf with respect to the other tissues, an outcome which may be explained by the strong requirement of chlorophyll and carotenoids during leaf maturation (Estévez et al., 2000). Regarding the berry developmental stages, a significant increase was assessed after veraison, confirming the findings of Battilana et al. (2011). Such data may indicate additional levels of gene expression regulation occurring post-transcriptionally or with a feedback mechanism, as reported for other plant species (Hemmerlin et al., 2012).
The Integration of VvDXS1 Alleles in Transformed Microvines Perturbs Several Terpenoid Pathways at the Transcriptional Level
The TaqMan array, which was developed ad hoc to harbor as many as possible truly expressed genes, allowed to evaluate the transcription profile of a number of terpenoid pathway genes in the same technical conditions. Four important stages in the grape reproductive life were considered (Figure 4).
Flowering
It has been demonstrated that plants at anthesis emit a plethora of volatile terpenes to attract pollinating insects and for the protection of floral tissues from microbial pathogens or herbivores (Tholl, 2006). The overexpression of Vv-N-DXS1 in Mi-N4 and, to a lesser extent, of Vv-M-DXS1 in Mi-M1 at flowering time resulted in a significant up-regulation of HDR (VIT_03s0063g02030), which codes for the ultimate enzyme of the MEP pathway. Experimental evidence in several plants suggests that, in addition to DXS, this gene may have a rate-limiting role in IPP and DMAPP synthesis (Botella-Pavía et al., 2004; Page et al., 2004; Cordoba et al., 2009; Vranová et al., 2012) and in grapevine its expression has been reported to closely parallel the veraison-initiated accumulation of monoterpenes (Martin et al., 2012; Wen et al., 2015). On the contrary, DXR (VIT_17s0000g08390), another putative regulatory gene (Carretero-Paulet et al., 2006; Wungsintaweekul et al., 2008), showed no expression variation in transformed lines compared to WT. This conforms to the observation that the rate-limiting role of DXR varies among plant species and in different conditions (Cordoba et al., 2009). The expression of other MEP pathway genes were not significantly affected in transformed microvines, which is in agreement with the minimal effects of DXS overexpression on transcript levels of MEP pathway genes in Arabidopsis plants under natural conditions (Wright et al., 2014). Surprisingly, three important genes of the cytosolic MVA pathway, AACT (VIT_12s0057g01200), HMGS (VIT_02s0025g04580), and HMGR3 (VIT_03s0038g04100), exhibited strong up-regulation in the transformed lines, especially in line Mi-N4, compared to the WT. This outcome was unexpected as the recently reported examples of exchange events between the two pathways concerned only precursor intermediates like IPP, GPP (geranyl diphosphate), and FPP (farnesyl diphosphate) (Hemmerlin et al., 2012; May et al., 2013; Pazouki and Niinemets, 2016). In addition, in the literature there is no supporting evidence for a transcriptional co-regulation of the two pathways (Wille et al., 2004; Vranová et al., 2013). At most, a cross-talk regulation, if any, is expected to occur at a post-transcriptional level (Hemmerlin et al., 2012). Our observation adds to recent reports that indicate that there is deviation from the general notion that synthesis of sesquiterpenes and triterpenes occur via the MVA pathway whereas monoterpenes and diterpenes are synthetized via the MEP pathway, and instead suggests that these pathways are integrated rather than mutually exclusive (Chaurasiya et al., 2012).
Analysis of isoprenoid pathways downstream of IPP and DMAPP revealed a slight but widespread up-regulation of carotenoid, monoterpene and sesquiterpene pathway genes in transformed lines compared to the control. Several genes involved in carotenoid metabolism were induced, although not significantly, in both Mi-N4 and Mi-M1 lines with respect to WT. Similarly, overexpression of DXS in other plant species resulted in an increased expression of genes responsible for carotenogenesis (e.g., phytoene synthase) and a higher carotenoid content (Estévez et al., 2001; Morris et al., 2006; Henriquez et al., 2016; Simpson et al., 2016). Moreover, a recent genome-wide association study investigating the variation for carotenoid concentration in maize grain identified DXS1 as a candidate gene (Suwarno et al., 2015). It is also interesting to observe the significantly higher level of CCD4 transcript (VIT_02s0087g00910) in Mi-N4 line compared to WT. CCD4 is a member of carotenoid cleavage dioxygenases (CCDs), which are involved in norisoprenoid production. Norisoprenoids are found in flowers and fruits of many plants and possess aromatic properties together with low odor thresholds (Schmidt et al., 2006; Ebeler and Thorngate, 2009). They also contribute to floral and fruity aroma in Muscat cultivars and in Riesling-type varieties (Baumes et al., 2002). Pertaining to monoterpenes, a strong up-regulation was observed for ocimene synthase (VIT_12s0134g00020) in both transformed lines but more prominently in Mi-N4. In grapevine, ocimene synthase is specifically expressed in flower buds and to a lesser extent in open flowers (Lücker et al., 2004; Matarese et al., 2014). Regarding sesquiterpenes, a significant up-regulation was noticed for valencene synthase (VIT_18s0001g04050), which generates one of the major volatiles emitted from flowers of white and red varieties (Lücker et al., 2004; Martin et al., 2009).
Our findings seem to indicate that the transcription of genes which are physiologically “turned on” during anthesis is strongly enhanced in transformed plants with a potentially stronger MEP pathway flux. However, while it is reasonable to connect the enhanced expression of carotenoid-, apo-carotenoid-, and monoterpene pathway genes to the increased MEP pathway flux, the cause of an enhanced expression of MVA and, as a consequence, of sesquiterpene genes remains unknown and shall be the subject of further investigation.
Pre-veraison
At pre-veraison a diffused down-regulation of MEP and MVA pathway genes was observed in transformed lines compared to WT. A similar pattern was seen for carotenoid genes while monoterpene and sesquiterpene synthase transcripts were poorly detected in all the analyzed plants.
Veraison and Maturity
At veraison, most of the genes were up-regulated in the transformed lines respect to WT. According to Coombe and McCarthy (2000), at this stage several physiological and biochemical processes are initiated, and during the subsequent ripening phase major aromatic compounds including terpenes and norisoprenoids are synthesized. As discussed for the anthesis stage, MEP and MVA pathway genes showed a widespread rise in the transformed lines compared to the control. Regarding the carotenoid pathway, many genes were modulated: the first genes of the route showed a high expression in transformed lines while a more heterogeneous pattern was detected for the genes responsible for carotenoid degradation.
At maturity, MEP and MVA pathway genes were poorly modulated between the transformed lines and the control. Regarding sesquiterpenes, germacrene-D-synthase (VIT_18s0001g04280) was strongly expressed in Mi-M1 compared to WT.
Overexpression of VvDXS1 Increases the Level of Monoterpenes in Ripe Berries with the Form N284 Being More Effective
The microvine system has allowed to evaluate the metabolic profile of fruits engineered for an important regulatory gene of terpenoid metabolism. We detected significant differences between transformed and control grapes in the accumulation of monoterpenes at harvest time. In particular, the total monoterpene content was 1.7- and 4.4-fold in Mi-N4 and Mi-M1 lines with respect to WT microvines, with ratios ranging from 1.3 to 1.9 (Mi-N4) and from 3.2 to 4.7 (Mi-M1) for free and glycosidically bound monoterpenes, respectively (Figure 6). These values are similar, albeit often higher in the case of Mi-M1, to those reported in previous experiments assessing the enhancement of isoprenoid compounds upon DXS overexpression in other plants (Lois et al., 2000; Estévez et al., 2001; Enfissi et al., 2005; Carretero-Paulet et al., 2006; Morris et al., 2006; Muñoz-Bertomeu et al., 2006; Peebles et al., 2011; Vaccaro et al., 2014; Wright et al., 2014; Shi et al., 2016; Simpson et al., 2016; Zhou et al., 2016; Jadaun et al., 2017).
Even if we cannot exclude an effect of the gai mutation on terpene biosynthesis (Hong et al., 2012; Murcia et al., 2017), our findings conform with the idea that DXS1 ectopic expression can raise the metabolic flux through the MEP pathway, thereby improving the formation of isoprenoids. Comparing Mi-N4 with WT plants, a close relationship may be observed between the increase in DXS1 total transcripts (2.2-fold) and its end products (1.7-fold increase in total monoterpene content) (Figure 6). Oppositely, in the case of Mi-M1 line, a weak expression of the DXS1 mutated allele resulted in an important gain in total monoterpenes (4.4-fold increase) (Figure 6). This outcome is in line with the results of Battilana et al. (2011) who found improved catalytic performances of the muscat DXS1 enzyme in comparison with the neutral form resulting in enhanced monoterpene biosynthesis.
The effect played by VvDXS1 on sesquiterpene content in the FEM aromatic core collection could not be confirmed in the microvine lines analyzed here, as they did not accumulate sesquiterpenes in ripe berry skins. A possible explanation is that the microvines have been grown in a greenhouse (according to the extant restrictions on GMOs in Italy), whereas the germplasm collection is planted in open field. Although the investigated microvines proved to be a convenient model system to detect differences in terpenoid pathway genes and monoterpene content among lines, it is evident that such system maintained in the greenhouse does not reproduce exactly the environmental conditions present in the vineyard (especially the exposure to light). Moreover, additional genes might play a limiting role in terpene production in the microvine genetic background. Both facts might also explain the lower monoterpene content of the transformed microvines (Figure 6) compared to the monoterpene content of the germplasm accessions with the muscat mutation (Figure 5A). An effect of VvDXS1 on sesquiterpene biosynthesis cannot be excluded in flowers, which represent the only tissue where a significant modulation of sesquiterpene pathway genes was observed (Figure 4). However, the quantification of sesquiterpenes in flowers was out of the aim of the present study.
Prospects for the Bioengineering of Isoprenoid Biosynthesis in Grapevine
The metabolic engineering approach adopted in the present work provided new insights into the functional effect of VvDXS1 alleles on terpenoid metabolism in grapevine. In order to optimize this basic approach from a biotechnological point of view, one should keep in mind some important aspects. First, DXS1 is post-transcriptionally regulated both at the level of gene expression (e.g., the DXS down-regulation by PSY activity and carotenoid synthesis) and of protein abundance/activity (e.g., the feedback inhibition of DXS1 activity by IPP and DMAPP), which is especially important in vivo to rapidly link the pathway with environmental and physiological challenges or metabolic fluctuations (Lois et al., 2000; Banerjee et al., 2013; Hemmerlin, 2013; Ghirardo et al., 2014; Pokhilko et al., 2015; Rodríguez-Concepción and Boronat, 2015). Secondly, the MEP pathway flux may be diverted via cross-talk with the MVA route (Hemmerlin et al., 2012; Pazouki and Niinemets, 2016) or via export of intermediates like methylerythritol cyclodiphoshate (MecPP), as observed in transformed Arabidopsis plants overexpressing DXS (Xiao et al., 2012; Wright et al., 2014; González-Cabanelas et al., 2015), and hydroxymethylbutenyl diphosphate (HMbPP) (Ward et al., 2011). Finally, other limiting steps in the MEP-pathway or in upstream and downstream pathways may exist, which includes the need for coordination between plant development and secondary metabolite production in order to not compete for carbon sources (Estévez et al., 2001; Enfissi et al., 2005; Muñoz-Bertomeu et al., 2006; Rodríguez-Concepción and Boronat, 2015; Shi et al., 2016; Zeng et al., 2016). In this regard, the analysis of additional metabolites (e.g., carotenoids and chlorophylls) could point out potentially competing reactions. The involvement of multiple key enzymes might also explain the lack of linalool in the microvines under investigation; in particular, terpene synthases or other genes in the confidence interval of the previously identified linalool-specific QTL on chromosome 10 (Battilana et al., 2009; Emanuelli et al., 2011; Costantini et al., 2017) might play a limiting role in linalool production in combination with VvDXS1, as highlighted in the grapevine germplasm (Figures 5C,D) and reported in other plants (Zeng et al., 2016).
Conclusion
The genetic transformation of microvine plants with VvDXS1 causes a significant perturbation in downstream pathways both at the transcriptional and metabolic level with no evident effect on plant morphology and phenology. The increased production of monoterpenes in the transformed lines with respect to the control may be predominantly attributable to the increased activity of the VvDXS1 enzyme with the K284N mutation and to a lesser extent due to VvDXS1 up-regulation. This gene is therefore an effective target for improving metabolic flux in the monoterpene biosynthetic route and accumulating more aroma-active compounds in the grape berry. Moreover, our experiment has shown a potential effect of VvDXS1 on the sesquiterpene pathway. The continuation of this study will enable the evaluation of the VvDXS1 gain-of-function mutation on the level of additional metabolites in the microvine model system under control and stress conditions.
Author Contributions
FE and MSG designed the project; FE and SL developed the TaqMan array tool; LDC performed microvine transformation, and contributed with MT to management and characterization of plant materials at morphological and transcriptional level; PM-S and SL genotyped the FEM aromatic core collection and prepared the samples for metabolic analysis; SM, DS, and RL provided the metabolic data; EC and AC did the bioinformatic analysis; IG was responsible for Brachetto and Chardonnay engineering with a contribution by LC; LDC, FE, MT, PM-S, LC, MM, and MSG took part in data interpretation; LDC drafted the work; LC, FE, and MSG revised it critically. All the authors approved the final version of this text.
Funding
This work was supported by the Autonomous Province of Trento (Accordo di Programma) and the European Union's Horizon 2020 research and innovation programme (grant agreement number 652615, VITISMART: Toward a sustainable viticulture).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Mark Thomas for providing the somatic embryos of microvine. They are also grateful to Xiaoguang Yu, Massimo Bertamini, and Valentino Poletti for technical assistance in chemical analysis and plant management and to Jessica Vervalle for grammatical revision.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2017.02244/full#supplementary-material
References
Aharoni, A., Jongsma, M. A., and Bouwmeester, H. J. (2005). Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 10, 594–602. doi: 10.1016/j.tplants.2005.10.005
Banerjee, A., Wu, Y., Banerjee, R., Li, Y., Yan, H., and Sharkey, T. D. (2013). Feedback inhibition of deoxy-D-xylulose-5-phosphate synthase regulates the methylerythritol 4-phosphate pathway. J. Biol. Chem. 288, 16926–16936. doi: 10.1074/jbc.M113.464636
Battilana, J., Costantini, L., Emanuelli, F., Sevini, F., Segala, C., Moser, S., et al. (2009). The 1-deoxy-d-xylulose 5-phosphate synthase gene co-localizes with a major QTL affecting monoterpene content in grapevine. Theor. Appl. Genet. 118, 653–669. doi: 10.1007/s00122-008-0927-8
Battilana, J., Emanuelli, F., Gambino, G., Gribaudo, I., Gasperi, F., Boss, P. K., et al. (2011). Functional effect of grapevine 1-deoxy-D-xylulose 5-phosphate synthase substitution K284N on Muscat flavour formation. J. Exp. Bot. 62, 5497–5508. doi: 10.1093/jxb/err231
Baumes, R., Wirth, J., Bureau, S., Gunata, Y., and Razungles, A. (2002). Biogeneration of C13-norisoprenoid compounds: experiments supportive for an apo-carotenoid pathway in grapevines. Anal. Chim. Acta 458, 3–14. doi: 10.1016/S0003-2670(01)01589-6
Botella-Pavía, P., Besumbes, O., Phillips, M. A., Carretero-Paulet, L., Boronat, A., and Rodríguez-Concepción, M. (2004). Regulation of carotenoid biosynthesis in plants: evidence for a key role of hydroxymethylbutenyl diphosphate reductase in controlling the supply of plastidial isoprenoid precursors. Plant J. 40, 188–199. doi: 10.1111/j.1365-313X.2004.02198.x
Capell, T., and Christou, P. (2004). Progress in plant metabolic engineering. Curr. Opin. Biotechnol. 15, 148–154. doi: 10.1016/j.copbio.2004.01.009
Carretero-Paulet, L., Cairó, A., Botella-Pavía, P., Besumbes, O., Campos, N., Boronat, A., et al. (2006). Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants overexpressing deoxyxylulose 5-phosphate reductoisomerase. Plant Mol. Biol. 62, 683–695. doi: 10.1007/s11103-006-9051-9
Chaïb, J., Torregrosa, L., MacKenzie, D., Corena, P., Bouquet, A., and Thomas, M. R. (2010). The grape microvine-A model system for rapid forward and reverse genetics of grapevines. Plant J. 62, 1083–1092. doi: 10.1111/j.1365-313X.2010.04219.x
Chaurasiya, N. D., Sangwan, N. S., Sabir, F., Misra, L., and Sangwan, R. S. (2012). Withanolide biosynthesis recruits both mevalonate and DOXP pathways of isoprenogenesis in Ashwagandha Withania somnifera L. (Dunal). Plant Cell Rep. 31, 1889–1897. doi: 10.1007/s00299-012-1302-4
Coombe, B. G. (1995). Growth stages of the grapevine: adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1, 104–110. doi: 10.1111/j.1755-0238.1995.tb00086.x
Coombe, B. G., and McCarthy, M. G. (2000). Dynamics of grape berry growth and physiology of ripening. Aust. J. Grape Wine Res. 6, 131–135. doi: 10.1111/j.1755-0238.2000.tb00171.x
Cordoba, E., Porta, H., Arroyo, A., San Román, C., Medina, L., Rodríguez-Concepción, M., et al. (2011). Functional characterization of the three genes encoding 1-deoxy-D-xylulose 5-phosphate synthase in maize. J. Exp. Bot. 62, 2023–2038. doi: 10.1093/jxb/erq393
Cordoba, E., Salmi, M., and León, P. (2009). Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. J. Exp. Bot. 60, 2933–2943. doi: 10.1093/jxb/erp190
Costantini, L., Kappel, C. D., Trenti, M., Battilana, J., Emanuelli, F., Sordo, M., et al. (2017). Drawing links from transcriptome to metabolites: the evolution of aroma in the ripening berry of Moscato Bianco (Vitis vinifera L.). Front. Plant Sci. 8:780. doi: 10.3389/fpls.2017.00780
Dalla Costa, L., Pinto-Sintra, A. L., Campa, M., Poletti, V., Martinelli, L., and Malnoy, M. (2014). Development of analytical tools for evaluating the effect of T-DNA chimeric integration on transgene expression in vegetatively propagated plants. Plant Cell Tissue Organ Cult. 118, 471–484. doi: 10.1007/s11240-014-0499-z
Dalla Costa, L., Vaccari, I., Mandolini, M., and Martinelli, L. (2009). Elaboration of a reliable strategy based on real-time PCR to characterize genetically modified plantlets and to evaluate the efficiency of a marker gene removal in grape (Vitis).spp. J. Agric. Food Chem. 57, 2668–2677. doi: 10.1021/jf802740m
De Buck, S., Peck, I., De Wilde, C., Marjanac, G., Nolf, J., De Paepe, A., et al. (2007). Generation of single-copy T-DNA transformants in Arabidopsis by the CRE/loxP recombination-mediated resolution system. Plant Physiol. 145, 1171–1182. doi: 10.1104/pp.107.104067
Dhekney, S. A., Li, Z. T., and Gray, D. J. (2011). Grapevines engineered to express cisgenic Vitis vinifera thaumatin-like protein exhibit fungal disease resistance. Vitr. Cell. Dev. Biol. 47, 458–466. doi: 10.1007/s11627-011-9358-3
Dong, L., Miettinen, K., Goedbloed, M., Verstappen, F. W. A., Voster, A., Jongsma, M. A., et al. (2013). Characterization of two geraniol synthases from Valeriana officinalis and Lippia dulcis: similar activity but difference in subcellular localization. Metab. Eng. 20, 198–211. doi: 10.1016/j.ymben.2013.09.002
Duchêne, E., Butterlin, G., Claudel, P., Dumas, V., Jaegli, N., and Merdinoglu, D. (2009). A grapevine (Vitis vinifera L.) deoxy-D-xylulose synthase gene colocates with a major quantitative trait loci for terpenol content. Theor. Appl. Genet. 118, 541–552. doi: 10.1007/s00122-008-0919-8
Dudareva, N., Klempien, A., Muhlemann, K., and Kaplan, I. (2013). Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 198, 16–32. doi: 10.1111/nph.12145
Dudareva, N., Pichersky, E., and Gershenzon, J. (2004). Biochemistry of plant volatiles. Plant Physiol. 135, 1893–1902. doi: 10.1104/pp.104.049981
Ebeler, S. E., and Thorngate, J. H. (2009). Wine chemistry and flavor: looking into the crystal glass. J. Agric. Food Chem. 57, 8098–8108. doi: 10.1021/jf9000555
Eisenreich, W., Bacher, A., Arigoni, D., and Rohdich, F. (2004). Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell. Mol. Life Sci. 61, 1401–1426. doi: 10.1007/s00018-004-3381-z
Emanuelli, F., Battilana, J., Costantini, L., and Grando, M. S. (2011). “Molecular breeding of grapevine for aromatic quality and other traits relevant to viticulture,” in Breeding for fruit quality, eds M. A. Jenks and P. J. Bebeli (Chichester: Wiley), 247–260. doi: 10.1002/9780470959350.ch11
Emanuelli, F., Battilana, J., Costantini, L., Le Cunff, L., Boursiquot, J.-M., This, P., et al. (2010). A candidate gene association study on muscat flavor in grapevine (Vitis vinifera L.). BMC Plant Biol. 10:241. doi: 10.1186/1471-2229-10-241
Emanuelli, F., Sordo, M., Lorenzi, S., Battilana, J., and Grando, M. S. (2014). Development of user-friendly functional molecular markers for VvDXS gene conferring muscat flavor in grapevine. Mol. Breed. 33, 235–241. doi: 10.1007/s11032-013-9929-6
Enfissi, E. M., Fraser, P. D., Lois, L. M., Boronat, A., Schuch, W., and Bramley, P. M. (2005). Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnol. J. 3, 17–27. doi: 10.1111/j.1467-7652.2004.00091.x
Estévez, J. M., Cantero, A., Reindl, A., Reichler, S., and León, P. (2001). 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J. Biol. Chem. 276, 22901–22909. doi: 10.1074/jbc.M100854200
Estévez, J. M., Cantero, A., Romero, C., Kawaide, H., Jiménez, L. F., Kuzuyama, T., et al. (2000). Analysis of the expression of CLA1, a gene that encodes the 1-deoxyxylulose 5-phosphate synthase of the 2-C-methyl-D-erythritol-4-phosphate pathway in Arabidopsis. Plant Physiol. 124, 95–104. doi: 10.1104/pp.124.1.95
Farhi, M., Marhevka, E., Masci, T., Marcos, E., Eyal, Y., Ovadis, M., et al. (2011). Harnessing yeast subcellular compartments for the production of plant terpenoids. Metab. Eng. 13, 474–481. doi: 10.1016/j.ymben.2011.05.001
Flores-Pérez, U., Sauret-Güeto, S., Gas, E., Jarvis, P., and Rodríguez-Concepción, M. (2008). A mutant impaired in the production of plastome-encoded proteins uncovers a mechanism for the homeostasis of isoprenoid biosynthetic enzymes in Arabidopsis plastids. Plant Cell 20, 1303–1315. doi: 10.1105/tpc.108.058768
Ghirardo, A., Wright, L. P., Bi, Z., Rosenkranz, M., Pulido, P., Rodríguez-Concepción, M., et al. (2014). Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiol. 165, 37–51. doi: 10.1104/pp.114.236018
González-Cabanelas, D., Wright, L. P., Paetz, C., Onkokesung, N., Gershenzon, J., Rodríguez-Concepción, M., et al. (2015). The diversion of 2-C-methyl-D-erythritol-2,4-cyclodiphosphate from the 2-C-methyl-d-erythritol 4-phosphate pathway to hemiterpene glycosides mediates stress responses in Arabidopsis thaliana. Plant J. 82, 122–137. doi: 10.1111/tpj.12798
Han, M., Heppel, S. C., Su, T., Bogs, J., Zu, Y., An, Z., et al. (2013). Enzyme inhibitor studies reveal complex control of methyl-D-erythritol 4-phosphate (MEP) pathway enzyme expression in Catharanthus roseus. PLoS ONE 8:e62467. doi: 10.1371/journal.pone.0062467
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F., and Vandesompele, J. (2007). qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8:R19. doi: 10.1186/gb-2007-8-2-r19
Hemmerlin, A. (2013). Post-translational events and modifications regulating plant enzymes involved in isoprenoid precursor biosynthesis. Plant Sci. 203–204, 41–54. doi: 10.1016/j.plantsci.2012.12.008
Hemmerlin, A., Harwood, J. L., and Bach, T. J. (2012). A raison d'être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid Res. 51, 95–148. doi: 10.1016/j.plipres.2011.12.001
Henriquez, M. A., Soliman, A., Li, G., Hannoufa, A., Ayele, B. T., and Daayf, F. (2016). Molecular cloning, functional characterization and expression of potato (Solanum tuberosum) 1-deoxy-D-xylulose 5-phosphate synthase 1 (StDXS1) in response to Phytophthora infestans. Plant Sci. 243, 71–83. doi: 10.1016/j.plantsci.2015.12.001
Hong, G.-J., Xue, X.-Y., Mao, Y.-B., Wang, L.-J., and Chen, X.-Y. (2012). Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 24, 2635–2648. doi: 10.1105/tpc.112.098749
Hood, E. E., Gelvin, S. B., Melchers, L. S., and Hoekema, A. (1993). New Agrobacterium helper plasmids for gene-transfer to plants. Transgenic Res. 2, 208–218. doi: 10.1007/BF01977351
Houshyani, B., Assareh, M., Busquets, A., Ferrer, A., Bouwmeester, H. J., and Kappers, I. F. (2013). Three-step pathway engineering results in more incidence rate and higher emission of nerolidol and improved attraction of Diadegma semiclausum. Metab. Eng. 15, 88–97. doi: 10.1016/j.ymben.2012.10.002
Iocco, P., Franks, T., and Thomas, M. R. (2001). Genetic transformation of major wine grape cultivars of Vitis vinifera L. Transgenic Res. 10, 105–112. doi: 10.1023/A:1008989610340
Jadaun, J. S., Sangwan, N. S., Narnoliya, L. K., Singh, N., Bansal, S., Mishra, B., et al. (2017). Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera: active involvement of plastid isoprenogenic pathway in their biosynthesis. Physiol. Plant. 159, 381–400. doi: 10.1111/ppl.12507
Karimi, M., Inzé, D., and Depicker, A. (2002). GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7, 193–195. doi: 10.1016/S1360-1385(02)02251-3
Khemvong, S., and Suvachittanont, W. (2005). Molecular cloning and expression of a cDNA encoding 1-deoxy-D-xylulose-5-phosphate synthase from oil palm Elaeis guineensis Jacq. Plant Sci. 169, 571–578. doi: 10.1016/j.plantsci.2005.05.001
Kim, B.-R., Kim, S.-U., and Chang, Y.-J. (2005). Differential expression of three 1-deoxy-D: -xylulose-5-phosphate synthase genes in rice. Biotechnol. Lett. 27, 997–1001. doi: 10.1007/s10529-005-7849-1
Lange, B. M., and Ahkami, A. (2013). Metabolic engineering of plant monoterpenes, sesquiterpenes and diterpenes-current status and future opportunities. Plant Biotechnol. J. 11, 169–196. doi: 10.1111/pbi.12022
Laule, O., Fürholz, A., Chang, H.-S., Zhu, T., Wang, X., Heifetz, P. B., et al. (2003). Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 100, 6866–6871. doi: 10.1073/pnas.1031755100
Lois, L. M., Rodríguez-Concepción, M., Gallego, F., Campos, N., and Boronat, A. (2000). Carotenoid biosynthesis during tomato fruit development: regulatory role of 1-deoxy-D-xylulose 5-phosphate synthase. Plant J. 22, 503–513. doi: 10.1046/j.1365-313x.2000.00764.x
Luchaire, N., Rienth, M., Romieu, C., Nehe, A., Chatbanyong, R., Houel, C., et al. (2017). Microvine: a new model to study grapevine growth and developmental patterns and their responses to elevated temperature. Am. J. Enol. Vitic. 68, 283–292. doi: 10.5344/ajev.2017.16066
Lücker, J., Bowen, P., and Bohlmann, J. (2004). Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (−)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 65, 2649–2659. doi: 10.1016/j.phytochem.2004.08.017
Mandel, M. A., Feldmann, K. A., Herrera-Estrella, L., Rocha-Sosa, M., and León, P. (1996). CLA1, a novel gene required for chloroplast development, is highly conserved in evolution. Plant J. 9, 649–658. doi: 10.1046/j.1365-313X.1996.9050649.x
Martin, D. M., Chiang, A., Lund, S. T., and Bohlmann, J. (2012). Biosynthesis of wine aroma: transcript profiles of hydroxymethylbutenyl diphosphate reductase, geranyl diphosphate synthase, and linalool/nerolidol synthase parallel monoterpenol glycoside accumulation in Gewürztraminer grapes. Planta 236, 919–929. doi: 10.1007/s00425-012-1704-0
Martin, D. M., Toub, O., Chiang, A., Lo, B. C., Ohse, S., Lund, S. T., et al. (2009). The bouquet of grapevine (Vitis vinifera L. cv. Cabernet Sauvignon) flowers arises from the biosynthesis of sesquiterpene volatiles in pollen grains. Proc. Natl. Acad. Sci. U.S.A. 106, 7245–7250. doi: 10.1073/pnas.0901387106
Matarese, F., Cuzzola, A., Scalabrelli, G., and D'Onofrio, C. (2014). Expression of terpene synthase genes associated with the formation of volatiles in different organs of Vitis vinifera. Phytochemistry 105, 12–24. doi: 10.1016/j.phytochem.2014.06.007
Mateo, J. J., and Jiménez, M. (2000). Monoterpenes in grape juice and wines. J. Cromatogr. A 881, 557–567. doi: 10.1016/S0021-9673(99)01342-4
Matzke, A. J., and Matzke, M. A. (1998). Position effects and epigenetic silencing of plant transgenes. Curr. Opin. Plant Biol. 1, 142–148. doi: 10.1016/S1369-5266(98)80016-2
May, B., Lange, M. B., and Wüst, M. (2013). Biosynthesis of sesquiterpenes in grape berry exocarp of Vitis vinifera L.: evidence for a transport of farnesyl diphosphate precursors from plastids to the cytosol. Phytochemistry 95, 135–144. doi: 10.1016/j.phytochem.2013.07.021
McCaskill, D., and Croteau, R. (1997). Prospects for the bioengineering of isoprenoid biosynthesis. Adv. Biochem. Eng. Biotechnol. 55, 107–146. doi: 10.1007/BFb0102064
Morris, W. L., Ducreux, L. J. M., Hedden, P., Millam, S., and Taylor, M. A. (2006). Overexpression of a bacterial 1-deoxy-D-xylulose 5-phosphate synthase gene in potato tubers perturbs the isoprenoid metabolic network: implications for the control of the tuber life cycle. J. Exp. Bot. 57, 3007–3018. doi: 10.1093/jxb/erl061
Muñoz-Bertomeu, J., Arrillaga, I., Ros, R., and Segura, J. (2006). Up-regulation of 1-deoxy-D-xylulose-5-phosphate synthase enhances production of essential oils in transgenic spike lavender. Plant Physiol. 142, 890–900. doi: 10.1104/pp.106.086355
Murcia, G., Fontana, A., Pontin, M., Baraldi, R., Bertazza, G., and Piccoli, P. N. (2017). ABA and GA3 regulate the synthesis of primary and secondary metabolites related to alleviation from biotic and abiotic stresses in grapevine. Phytochemistry 135, 34–52. doi: 10.1016/j.phytochem.2016.12.007
Page, J. E., Hause, G., Raschke, M., Gao, W., Schmidt, J., Zenk, M. H., et al. (2004). Functional analysis of the final steps of the 1-deoxy-D-xylulose 5-phosphate (DXP) pathway to isoprenoids in plants using virus-induced gene silencing. Gene 134, 1401–1413. doi: 10.1104/pp.103.038133
Pazouki, L., and Niinemets, Ü. (2016). Multi-substrate terpene synthases: their occurrence and physiological significance. Front. Plant Sci. 7:1019. doi: 10.3389/fpls.2016.01019
Peebles, C. A., Sander, G. W., Hughes, E. H., Peacock, R., Shanks, J. V., and San, K. Y. (2011). The expression of 1-deoxy-D-xylulose synthase and geraniol-10-hydroxylase or anthranilate synthase increases terpenoid indole alkaloid accumulation in Catharanthus roseus hairy roots. Metab. Eng. 13, 234–240. doi: 10.1016/j.ymben.2010.11.005
Perrone, I., Gambino, G., Chitarra, W., Vitali, M., Pagliarani, C., Riccomagno, N., et al. (2012). The grapevine root-specific aquaporin VvPIP2;4N controls root hydraulic conductance and leaf gas exchange under well-watered conditions but not under water stress. Plant Physiol. 160, 965–977. doi: 10.1104/pp.112.203455
Phillips, M. A., Walter, M. H., Ralph, S. G., Dabrowska, P., Luck, K., Urós, E. M., et al. (2007). Functional identification and differential expression of 1-deoxy-D-xylulose 5-phosphate synthase in induced terpenoid resin formation of Norway spruce (Picea abies). Plant Mol. Biol. 65, 243–257. doi: 10.1007/s11103-007-9212-5
Pokhilko, A., Bou-Torrent, J., Pulido, P., Rodríguez-Concepción, M., and Ebenhöh, O. (2015). Mathematical modelling of the diurnal regulation of the MEP pathway in Arabidopsis. New Phytol. 206, 1075–1085. doi: 10.1111/nph.13258
Rajeevkumar, S., Anunanthini, P., and Sathishkumar, R. (2015). Epigenetic silencing in transgenic plants. Front. Plant Sci. 6:693. doi: 10.3389/fpls.2015.00693
Rodríguez-Concepción, M., and Boronat, A. (2002). Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 130, 1079–1089. doi: 10.1104/pp.007138
Rodríguez-Concepción, M., and Boronat, A. (2015). Breaking new ground in the regulation of the early steps of plant isoprenoid biosynthesis. Curr. Opin. Plant Biol. 25, 17–22. doi: 10.1016/j.pbi.2015.04.001
Saeed, A. I., Sharov, V., White, J., Li, J., Liang, W., and Bhagabati, N. (2003). TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34, 374–378.
Saladié, M., Wright, L. P., Garcia-Mas, J., Rodríguez-Concepción, M., and Phillips, M. A. (2014). The 2-C-methylerythritol 4-phosphate pathway in melon is regulated by specialized isoforms for the first and last steps. J. Exp. Bot. 65, 5077–5092. doi: 10.1093/jxb/eru275
Schmidt, H., Kurtzer, R., Eisenreich, W., and Schwab, W. (2006). The carotenase AtCCD1 from Arabidopsis thaliana is a dioxygenase. J. Biol. Chem. 281, 9845–9851. doi: 10.1074/jbc.M511668200
Shi, M., Luo, X., Ju, G., Li, L., Huang, S., Zhang, T., et al. (2016). Enhanced diterpene tanshinone accumulation and bioactivity of transgenic Salvia miltiorrhiza hairy roots by pathway engineering. J. Agric. Food Chem. 64, 2523–2530. doi: 10.1021/acs.jafc.5b04697
Simpson, K., Quiroz, L. F., Rodríguez-Concepción, M., and Stange, C. R. (2016). Differential contribution of the first two enzymes of the MEP pathway to the supply of metabolic precursors for carotenoid and chlorophyll biosynthesis in carrot (Daucus carota). Front. Plant Sci. 7:1344. doi: 10.3389/fpls.2016.01344
Suwarno, W. B., Pixley, K. V., Palacios-Rojas, N., Kaeppler, S. M., and Babu, R. (2015). Genome-wide association analysis reveals new targets for carotenoid biofortification in maize. Theor. Appl. Genet. 128, 851–864. doi: 10.1007/s00122-015-2475-3
Tang, W., Newton, R. J., and Weidner, D. A. (2007). Genetic transformation and gene silencing mediated by multiple copies of a transgene in eastern white pine. J. Exp. Bot. 58, 545–554. doi: 10.1093/jxb/erl228
Tholl, D. (2006). Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 9, 297–304. doi: 10.1016/j.pbi.2006.03.014
Vaccaro, M., Malafronte, N., De Tommasi, N., and Leone, A. (2014). Enhanced biosynthesis of bioactive abietane diterpenes by overexpressing AtDXS or AtDXR genes in Salvia sclarea hairy roots. Plant Cell Tissue Organ Cult. 119, 65–77. doi: 10.1007/s11240-014-0514-4
Vranová, E., Coman, D., and Gruissem, W. (2012). Structure and dynamics of the isoprenoid pathway network. Mol. Plant 5, 318–333. doi: 10.1093/mp/sss015
Vranová, E., Coman, D., and Gruissem, W. (2013). Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 64, 665–700. doi: 10.1146/annurev-arplant-050312-120116
Ward, J. L., Baker, J. M., Llewellyn, A. M., Hawkins, N. D., and Beale, M. H. (2011). Metabolomic analysis of Arabidopsis reveals hemiterpenoid glycosides as products of a nitrate ion-regulated, carbon flux overflow. Proc. Natl. Acad. Sci. U.S.A. 108, 10762–10767. doi: 10.1073/pnas.1018875108
Wen, Y.-Q., Zhong, G.-Y., Gao, Y., Lan, Y.-B., Duan, C.-Q., and Pan, Q.-H. (2015). Using the combined analysis of transcripts and metabolites to propose key genes for differential terpene accumulation across two regions. BMC Plant Biol. 15:240. doi: 10.1186/s12870-015-0631-1
Wille, A., Zimmermann, P., Vranová, E., Fürholz, A., Laule, O., Bleuler, S., et al. (2004). Sparse graphical Gaussian modeling of the isoprenoid gene network in Arabidopsis thaliana. Genome Biol. 5:R92. doi: 10.1186/gb-2004-5-11-r92
Wolfertz, M., Sharkey, T. D., Boland, W., and Kühnemann, F. (2004). Rapid regulation of the methylerythritol 4-phosphate pathway during isoprene synthesis. Plant Physiol. 135, 1939–1945. doi: 10.1104/pp.104.043737
Wright, L. P., Rohwer, J. M., Ghirardo, A., Hammerbacher, A., Ortiz-Alcaide, M., Raguschke, B., et al. (2014). Deoxyxylulose 5-phosphate synthase controls flux through the methylerythritol 4-phosphate pathway in Arabidopsis. Plant Physiol. 165, 1488–1504. doi: 10.1104/pp.114.245191
Wungsintaweekul, J., Sirisuntipong, T., Kongduang, D., Losuphanporn, T., Ounaroon, A., Tansakul, P., et al. (2008). Transcription profiles analysis of genes encoding 1-deoxy-D-xylulose 5-phosphate synthase and 2C-methyl-D-erythritol 4-phosphate synthase in plaunotol biosynthesis from Croton stellatopilosus. Biol. Pharm. Bull. 31, 852–856. doi: 10.1248/bpb.31.852
Xiao, Y., Savchenko, T., Baidoo, E. E. K., Chehab, W. E., Hayden, D. M., Tolstikov, V., et al. (2012). Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149, 1525–1535. doi: 10.1016/j.cell.2012.04.038
Xu, Y., Liu, J., Liang, L., Yang, X., Zhang, Z., Gao, Z., et al. (2014). Molecular cloning and characterization of three cDNAs encoding 1-deoxy-D-xylulose-5-phosphate synthase in Aquilaria sinensis (Lour.) Gilg. Plant Physiol. Biochem. 82, 133–141. doi: 10.1016/j.plaphy.2014.05.013
Zeng, X., Liu, C., Zheng, R., Cai, X., Luo, J., Zou, J., et al. (2016). Emission and accumulation of monoterpene and the key terpene synthase (TPS) associated with monoterpene biosynthesis in Osmanthus fragrans Lour. Front. Plant Sci. 6:1232. doi: 10.3389/fpls.2015.01232
Keywords: functional SNP, gain-of-function mutation, microvine, monoterpene, sesquiterpene, TaqMan card, Vitis vinifera, VvDSX1 alleles
Citation: Dalla Costa L, Emanuelli F, Trenti M, Moreno-Sanz P, Lorenzi S, Coller E, Moser S, Slaghenaufi D, Cestaro A, Larcher R, Gribaudo I, Costantini L, Malnoy M and Grando MS (2018) Induction of Terpene Biosynthesis in Berries of Microvine Transformed with VvDXS1 Alleles. Front. Plant Sci. 8:2244. doi: 10.3389/fpls.2017.02244
Received: 15 September 2017; Accepted: 20 December 2017;
Published: 17 January 2018.
Edited by:
Claudio Bonghi, Università degli Studi di Padova, ItalyReviewed by:
Dinesh A. Nagegowda, Central Institute of Medicinal and Aromatic Plants (CIMAP), IndiaAxel Schmidt, Max Planck Institute for Chemical Ecology (MPG), Germany
Copyright © 2018 Dalla Costa, Emanuelli, Trenti, Moreno-Sanz, Lorenzi, Coller, Moser, Slaghenaufi, Cestaro, Larcher, Gribaudo, Costantini, Malnoy and Grando. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Costantini, laura.costantini@fmach.it
M. Stella Grando, stella.grando@unitn.it
†Present Address: Davide Slaghenaufi, Department of Biotechnology, University of Verona, Verona, Italy
‡These authors have contributed equally to this work.
 Lorenza Dalla Costa
Lorenza Dalla Costa Francesco Emanuelli
Francesco Emanuelli Massimiliano Trenti
Massimiliano Trenti Paula Moreno-Sanz
Paula Moreno-Sanz Silvia Lorenzi
Silvia Lorenzi Emanuela Coller
Emanuela Coller Sergio Moser
Sergio Moser Davide Slaghenaufi
Davide Slaghenaufi Alessandro Cestaro
Alessandro Cestaro Roberto Larcher
Roberto Larcher Ivana Gribaudo
Ivana Gribaudo Laura Costantini
Laura Costantini Mickael Malnoy
Mickael Malnoy M. Stella Grando
M. Stella Grando