- Department of Agronomy and Horticulture, University of Nebraska-Lincoln, Lincoln, NE, United States
Alkaline soils comprise 30% of the earth and have low plant-available iron (Fe) concentration, and can cause iron deficiency chlorosis (IDC). IDC causes soybean yield losses of $260 million annually. However, it is not known whether molecular responses to IDC are equivalent to responses to low iron supply. IDC tolerant and sensitive soybean lines provide a contrast to identify specific factors associated with IDC. We used RNA-seq to compare gene expression under combinations of normal pH (5.7) or alkaline pH (7.7, imposed by 2.5 mM bicarbonate, or pH 8.2 imposed by 5 mM bicarbonate) and normal (25 μM) or low (1 μM) iron conditions from roots of these lines. Thus, we were able to treat pH and Fe supply as separate variables. We also noted differential gene expression between IDC sensitive and tolerant genotypes in each condition. Classical iron uptake genes, including ferric-chelate reductase (FCR) and ferrous transporters, were upregulated by both Fe deficiency and alkaline stress, however, their gene products did not function well at alkaline pH. In addition, genes in the phenylpropanoid synthesis pathway were upregulated in both alkaline and low Fe conditions. These genes lead to the production of fluorescent root exudate (FluRE) compounds, such as coumarins. Fluorescence of nutrient solution increased with alkaline treatment, and was higher in the IDC tolerant line. Some of these genes also localized to previously identified QTL regions associated with IDC. We hypothesize that FluRE become essential at alkaline pH where the classical iron uptake system does not function well. This work could result in new strategies to screen for IDC tolerance, and provide breeding targets to improve crop alkaline stress tolerance.
Introduction
Iron (Fe) is an essential micronutrient for plants. Iron deficient plants increase root Fe uptake capacity by one of two strategies (Kobayashi and Nishizawa, 2012); grasses use a chelation-uptake strategy, while non-grass monocots and dicots employ what is classically considered a reduction-uptake strategy. In the reduction-uptake strategy, Fe deficient roots acidify the rhizosphere with H+-ATPases, encoded by the AHA or HA genes, they reduce ferric iron [Fe(III)] to ferrous iron [Fe(II)] with ferric-chelate reductase (FCR) proteins encoded by the FRO genes, and they take up iron with transporters encoded by IRT genes (Eide et al., 1996; Robinson et al., 1999; Santi and Schmidt, 2009).
While Fe is usually abundant in soil, its solubility is low in alkaline soils, which can lead to iron deficiency chlorosis (IDC), a functional Fe deficiency where leaves are yellow instead of green (Mengel, 1994). Plant species that use the reduction-uptake strategy, such as soybean [Glycine max (L.)Merr.], tend to be more sensitive to alkaline soils than grasses that use the chelation-uptake strategy. Approximately 30% of the earth has alkaline soils (Chen and Barak, 1982), including parts of the North-Central region of the U.S. In this region IDC is an important factor that limits soybean productivity and leads to yield losses of $260 million annually (Hansen et al., 2004; Peiffer et al., 2012). Soil alkalinity is primarily due to bicarbonate (Bic) and carbonate ions. Alkaline pH and Bic decrease solubility, and in turn availability of Fe, and may also inhibit Fe uptake gene expression and/or function (Lucena et al., 2007; Hsieh and Waters, 2016). IDC can be induced in hydroponic studies by buffering the nutrient solution at alkaline pH using Bic (Chaney et al., 1992). IDC can be induced in Bic-containing alkaline solutions even with Fe sources that remain available [e.g., Fe(III)-EDDHA, Halvorson and Lindsay, 1972] at quantities of soluble Fe that would be adequate in the ideal pH range. Thus, the effects of alkalinity extend beyond simply decreasing Fe availability. Plants that are Fe deficient from low Fe supply at normal pH also have chlorotic leaves, but it is not clear that this chlorosis is equivalent to IDC under alkaline conditions. Most plant Fe nutrition studies have induced Fe deficiency by limiting Fe supply in the normal, mildly acidic pH range of 5–6. Many studies have cataloged genes that are upregulated in roots in response to Fe deficiency in Arabidopsis thaliana and other plant species (Colangelo and Guerinot, 2004; Dinneny et al., 2008; García et al., 2010; Yang et al., 2010; Ivanov et al., 2012; Stein and Waters, 2012). Only a few transcriptomic, proteomic, or metabolomic studies have included alkaline pH in combination with low Fe supply to induce IDC (Rellán-Álvarez et al., 2010; Rodríguez-Celma et al., 2011a; Rodríguez-Celma et al., 2013; Schmidt et al., 2014). However, these studies did not include all combinations of normal and alkaline pH with high and low Fe supply. Thus, it is not clear whether results of Fe deficiency studies can be applied to IDC studies aimed at improving IDC tolerance in alkaline soils. Our first objective was to treat Fe supply and nutrient solution pH as separate variables to determine whether alkaline IDC conditions and low Fe supply affect soybean root gene expression in the same manner.
As discussed above, IDC effects are likely to be more complicated than Fe deficiency from low Fe supply. Soybean lines overexpressing the A. thaliana FRO2 gene were constructed to improve Fe uptake (Vasconcelos et al., 2006). These lines had increased leaf and root Fe concentration, and increased FRO2 expression and FCR activity in hydroponic nutrient solution at pH 5.5 (Vasconcelos et al., 2006). However, in alkaline soil field trials these lines did not have improved IDC tolerance (Kocak, 2014), suggesting that some factor other than FCR activity is the rate limiting step for Fe uptake under alkaline conditions, and/or the overexpressed FCR activity was not maintained in the alkaline soil conditions. In other dicot species, high Bic concentrations inhibited induction of FCR activity (Romera et al., 1992) and expression of Fe uptake genes (Lucena et al., 2007) in Fe deficient plants. However, plants supplied with Fe responded to low concentrations of Bic as if they were Fe deficient, with increased FCR activity and FRO1 gene expression (Hsieh and Waters, 2016). Although Fe uptake responses were upregulated by Bic, Fe accumulation was impaired (Hsieh and Waters, 2016), again suggesting that the reduction-uptake components of this system are not the rate-limiting factors for Fe uptake in alkaline conditions. Thus, our second objective was to use soybean root gene expression data to improve our understanding of the physiological and biochemical responses to low Fe supply and alkaline conditions. One of the key findings in this work was upregulation of the phenylpropanoid pathway by Fe deficiency and alkalinity. In A. thaliana, this pathway produces phenolic root exudate compounds in the coumarins class that are involved in Fe uptake in alkaline conditions (Fourcroy et al., 2014; Schmid et al., 2014; Schmidt et al., 2014).
There is substantial variation for IDC tolerance in soybean (Rodriguez de Cianzio et al., 1979). IDC has been studied from plant breeding and genetics approaches, such as quantitative trait loci (QTL) mapping in biparental populations (Lin et al., 1997, 2000; Charlson et al., 2005) or introgression lines (Peiffer et al., 2012). Genome-wide association mapping studies (GWAS) were used to identify QTL associated with IDC (Wang et al., 2008; Mamidi et al., 2011, 2014). However, translating results from these genetic studies to specific genes for IDC tolerance has been challenging, since many QTL for IDC have small genetic effects, do not replicate robustly, or cover large genomic regions. Even without knowledge of specific genes that provide IDC tolerance, plant breeders can develop soybean lines with IDC tolerance (Prohaska and Fehr, 1981), however, a further understanding of the molecular aspects of IDC tolerance could accelerate breeding efforts by allowing breeders to target specific genes. Our third objective was to determine gene expression differences between IDC tolerant and susceptible varieties to aid in identifying specific factors that provide IDC tolerance.
The long-term goal of this research is to develop new IDC tolerant soybean varieties. A more complete understanding of IDC and Fe deficiency will increase our fundamental understanding of plant biology and lead to new strategies for improving crop production on Fe-deficiency prone, alkaline soils. This knowledge could also allow manipulation of these mechanisms to increase Fe concentrations in edible portions of plants for biofortification of foods.
Materials and Methods
Plant Materials and Growth Conditions
The IDC tolerant line, U06-105454, shows extreme resistance to IDC on high-pH soils in Nebraska (Figure 1A). This line was selected from an inter-mated population developed from 10 original soybean parental lines selected on yield and better-than-average response to IDC. The line U06-105454 was selected after 10 cycles of recurrent selection for improved IDC tolerance based on foliar symptoms at the V3 stage. The IDC susceptible line, U06-625083, is a high-yield breeding line. Details about the genetic background of these lines and their IDC scores can be found in Kocak (2014).
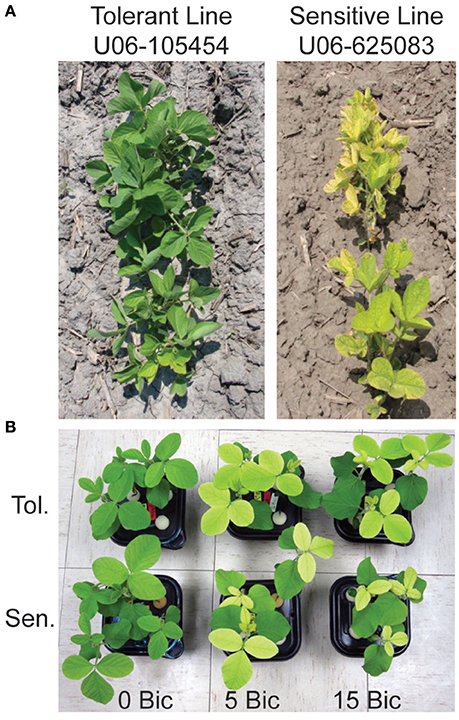
Figure 1. Soybean lines used in this study at the V3 stage. (A) IDC tolerant line U06-454 and IDC sensitive line UO6-625083 in an alkaline field in North Bend, Nebraska, USA. (B) The IDC tolerant and sensitive lines in hydroponic solution with low Fe supply [1 μM Fe(III)-EDDHA] and 0, 5, or 15 mM sodium bicarbonate at 12 d after planting.
A hydroponic system was developed to distinguish between IDC tolerant and sensitive lines to study root responses to iron deficiency and alkaline stress. Sodium bicarbonate (Bic) was added to the nutrient solution up to 15 mM, as indicated in each figure, to mimic high carbonate, alkaline soils, and to buffer the pH in the alkaline range. Seeds were germinated in germination paper soaked with 0.1 mM CaSO4 and incubated in the dark at 23°C for 7 days. Seedlings were transferred to black containers (two seedlings per tub) with 800 mL of nutrient solution made with 1.5 mM KNO3, 0.8 mM Ca(NO3)2, 0.3 mM (NH4)H2PO4, 0.2 mM MgSO4, 25 μM CaCl2, 25 μM H3BO3, 2 μM MnCl2, 2 μM ZnSO4, 0.5 μM Na2MoO4, 0.1 μM CuSO4, and 1 mM MES buffer (pH 5.5). For initial characterization, FCR activity, and RNA-seq gene expression, Fe was added as Fe(III)-EDDHA in experiments. The Fe(III)-EDDHA is stable at the mildly acidic and alkaline pH used in this study (Chaney et al., 1972; Halvorson and Lindsay, 1972). Plants were pretreated on either low (1 μM) or normal (25 μM) Fe for 6 d, then Bic was added to the solutions at the concentrations indicated for an additional 4 d. Final solution pH (at the end of each experiment) for each Bic concentration was: 0, 5.8 ± 0.59; 1.0 mM, 7.0 ± 0.34; 2.5 mM, 7.7 ± 0.16; 5 mM, 8.2 ± 0.15; 15 mM, 8.8 ± 0.19. For whole plant mineral analysis and fluorescent root exudate (FluRE) compound measurement experiments, plants were grown for 12 d in the solution as above, except that Fe was added as FeSO4, and Bic was included in the solutions for the entire 12 d.
Ferric Chelate Reductase Activity and Mineral Analysis
Ferric chelate reductase activity was measured using whole roots of individual 10 d old soybean plants after 4 d of Bic treatment. Roots were excised, rinsed in deionized water, and submerged in 50 ml assay solution (1 mM MES buffer, pH 5.5, 150 μM Fe(III)-EDTA, and 200 μM ferrozine [Sigma]) for 60 min. Ferrozine-Fe(II) was measured by absorbance at 562 nm and reduced Fe was calculated using the extinction coefficient of 28.16 mM cm−1. Chlorophyll of the first trifoliate leaf was determined using a SPAD-502 chlorophyll meter (Minolta) by taking the average of nine measurements per leaf (three per leaflet). Results are means from six individual runs of the same experiment. Significant differences between control and treatments, and between lines at each treatment were determined by t-test.
For leaf Fe concentration analysis, the first trifoliate leaf was collected from each of six plants grown in Fe(III)EDDHA with the final 4 d providing Bic treatments as above. For whole-plant Fe content analysis, four plants per treatment, grown as described for FluRE analysis, were dissected into roots, first trifoliate leaf, and the remainder of the plant (stem, unifoliate leaves, and cotyledons). Tissues were dried at 70°C in a drying oven. After measuring DW, tissue samples were digested in concentrated nitric acid/hydrogen peroxide with stepwise heating at 100, 125, 150, and 165°C to dryness, and then resuspended in 5 ml 1% HNO3 (Guttieri et al., 2015). Iron concentration was quantified in resuspended digests using an Agilent 7500cx ICP-MS (Agilent Technologies Inc., Santa Clara, CA) with Ar carrier and a He collision cell at the University of Nebraska Redox Biology Center Proteomics and Metabolomics Core Facility. Iron content of each plant part was calculated by multiplying tissue Fe concentration by sample DW, and total plant Fe content was calculated by the sum of all parts. Significant differences between control and treatments, and between lines at each treatment were determined by t-test.
RNA Sequencing
The IDC tolerant and susceptible lines were grown as described above with 1 μM or 25 μM Fe(III)EDHA for 10 d in solutions with 0, 2.5, or 5 mM NaH2CO3 for the last 4 d. RNA was isolated from roots using the Plant RNeasy kit (Qiagen). RNA-seq was performed at the University of Nebraska Medical Center Next Generation Sequencing Core Facility using an Illumina HiSeq 2000 instrument. Barcoded libraries were constructed from 3 μg of root total RNA, with three biological replicate libraries per treatment. Replicates were run in separate lanes, with a total of six samples from different treatments in each lane. In total, 414,801,800 RNA-seq paired end 50 bp sequencing reads were obtained from the sequencing facility following initial quality checks. Trimmomatic (Bolger et al., 2014) removed Illumina sequencing artifacts (ILLUMINACLIP) and poor quality reads using the following parameters LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36. Only 926 additional reads were removed, leaving an average of 11.8 million pairs of surviving reads per sample. The Williams 82 transcripts (Wm82.a2.v1) were downloaded from SoyBase (https://www.soybase.org/) and used as a reference for read mapping. The trimmed reads were mapped to the reference and differential gene expression was inferred between all pairwise sample-conditions using the Tuxedo pipeline (Trapnell et al., 2012). Venn diagrams were made using a tool found at http://bioinformatics.psb.ugent.be/webtools/Venn/. Pairwise comparisons were made within each genotype between RNA sequenced from roots of plants grown in control (25 μM Fe, 0 Bic) conditions and in complete solution (25 μM Fe) with Bic at 2.5 and 5 mM, and between control and low Fe (1 μM Fe) without (0 Bic) or with 2.5 or 5 mM Bic. Pairwise comparisons were made between genotypes in each combination of Fe and Bic supply. Genes were considered to be differentially expressed if they had a value in the treatments greater than or equal to the absolute value of the log2 control value. The full results of these pairwise comparisons are presented in Supplementary Tables as indicated in Results and Discussion. If a gene was differentially expressed in one treatment, we show the expression fold change values for all treatments and both genotypes in the tables, for completeness. The short reads are available to the soybean genomics community as NCBI BioProject: PRJNA389118.
Fluorescent Root Exudate and Extract Measurements
Soybean plants were grown in 800 ml nutrient solution as described above, with or without 2.5 mM Bic, at 1 or 20 μM FeSO4 for 14 d. Fluorescence of the nutrient solution was used to estimate the quantity of exuded fluorescent compounds. Nutrient solution fluorescence was determined in 200 μL aliquots in a BioTek Synergy 2 microplate reader with excitation at 360 nm and detection at 460 nm with 40 nm windows and a sensitivity setting of 50. Total fluorescence units per container were calculated by multiplying by solution volume, and total fluorescence units per plant were calculated by dividing fluorescence units per container by root FW. Significant differences between control and treatments, and between lines at each treatment were determined by t-test.
Results and Discussion
Previous studies of soybean root gene expression in response to Fe deficiency or alkalinity used different genetic backgrounds, conditions and/or gene expression technologies than we did in this study. The current study treated Fe supply and solution pH as separate variables to gain a better understanding of the specific effects of those treatments. The seedlings for RNA-seq were exposed to treatments in hydroponics for 4 d at moderate Bic concentrations (2.5 or 5 mM), which resulted in a more severe chlorosis in the IDC sensitive line than in the IDC tolerant line (Figure 1B). In other studies, RNAseq was used to study early responses at 12 and 24 h of Fe deficiency at pH 7.8 (Moran Lauter et al., 2014). Of the 489 differentially expressed genes in roots in the previous study, only 144 overlapped with the 5,288 differentially expressed genes in roots in our study, suggesting that gene expression at early time points in response to IDC conditions may differ significantly from seedlings with a longer-term exposure. Since IDC symptoms in the field develop over days to weeks, we used treatments with a long enough duration to begin to cause leaf chlorosis that would distinguish the IDC sensitive and tolerant lines. Previous molecular-scale IDC studies have compared two isogenic lines of soybean, Clark and Isoclark, with microarrays (O'Rourke et al., 2009) or RNAseq (Peiffer et al., 2012) in Fe-replete or Fe-limiting solutions that were buffered at pH 7.8. The lines compared in our study resulted from a recurrent selection strategy from 10 parental lines with selection for leaf chlorosis (or lack thereof) over 11 cycles, resulting in genetically diverged lines. Thus, the genetic underpinnings of the differences in IDC tolerance between the lines we used are likely due to multiple loci.
Physiological Characterization
Leaf chlorosis occurred only in plants treated with both low Fe supply and alkaline nutrient solution. In our hydroponic system at low Fe(III)-EDDHA supply without Bic, leaves of both the IDC sensitive and IDC tolerant lines remained green (Figure 2A). With normal Fe supply, addition of Bic at up to 15 mM did not result in leaf chlorosis, however, at low Fe supply, 5 mM Bic treatment resulted in chlorosis. Bic treatment resulted in greatly decreased leaf Fe concentration (Figure 2B) in both the IDC tolerant and sensitive lines and at both Fe supply levels. Although the leaves were green, leaf Fe concentration decreased from 200 to 300 μg/g without Bic to ~60 μg/g with Bic. Bic treatment decreased leaf Fe concentration more than low Fe supply alone, but a combination of Bic and low Fe resulted in the lowest Fe concentrations. This result suggests that Fe uptake was inhibited at alkaline pH, in agreement with previous soybean studies (Coulombe et al., 1984; Fleming et al., 1984), but it was not inhibited to the extent that leaves developed chlorosis.
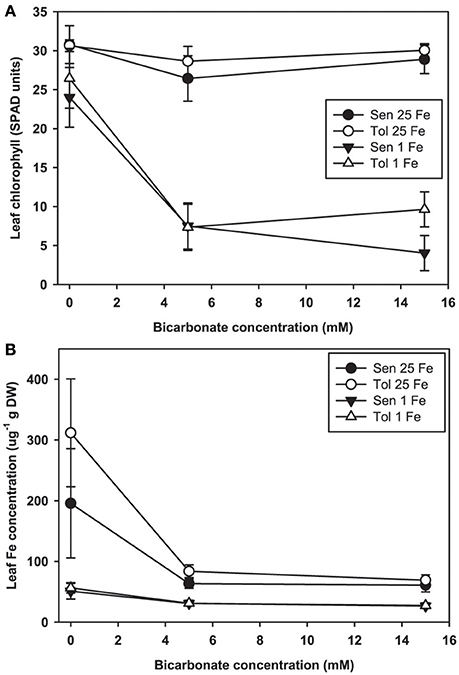
Figure 2. Leaf chlorophyll and iron (Fe) concentration in IDC tolerant and sensitive soybean plants. (A) chlorophyll (in SPAD units) and (B) Fe concentration in the first trifoliate leaf. Plants were pretreated with 1 or 25 μM Fe(III)-EDDHA for 6 d then treated with 1 or 25 μM Fe(III)-EDDHA and 0, 5, or 15 mM sodium bicarbonate for 4 d. At low Fe supply, Bic treatment resulted in statistically significantly lower leaf chlorophyll (p < 0.05), and the genotypes were significantly different at 15 mM Bic. At both Fe levels, addition of Bic resulted in statistically significantly lower leaf Fe concentration (p < 0.05), but there were no differences between genotypes.
To further explore effects of alkaline pH on Fe uptake processes, we measured leaf chlorophyll and tested FCR activity from plants grown at Bic concentrations up to 5 mM (Figure 3), since it was within this range that most of the chlorophyll and leaf Fe concentration decrease occurred (Figure 2). Over this range, again chlorosis only occurred in plants with both low Fe supply and Bic treatment. At 2.5 or 5 mM Bic and low Fe, the susceptible line had a greater decrease in chlorophyll than the tolerant line (Figures 3A,B), consistent with field results (Figure 1). For plants grown in solutions without Bic (pH 5.9), both lines had higher FCR activity at low Fe supply relative to normal Fe, reflecting the expected upregulation of Fe uptake responses (Kobayashi and Nishizawa, 2012) by this mild Fe deficiency. As Bic supply was increased to 2.5 or 5 mM, the susceptible line's FCR activity was no longer stimulated at low Fe supply relative to normal Fe supply (Figure 3A). However, the tolerant line maintained higher FCR activity at low Fe supply as Bic increased (Figure 3B). Thus, the IDC tolerant line's FCR activity was not as susceptible to alkaline stress as FCR activity in the sensitive line, although this activity was apparently not sufficient to maintain Fe uptake (Figure 2). This result may explain why AtFRO2 overexpressing soybean plants did not have improved IDC tolerance on alkaline soils (Kocak, 2014).
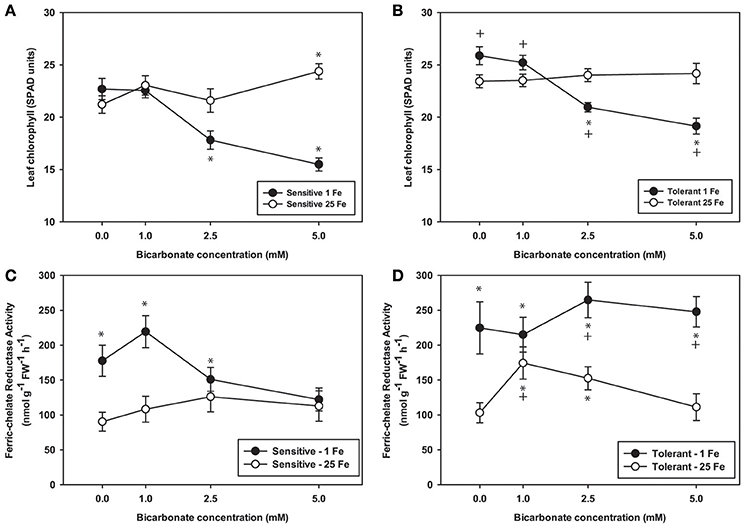
Figure 3. Leaf chlorophyll and root ferric-chelate reductase activity in IDC tolerant and sensitive soybean plants. Plants were pretreated with 1 or 25 μM Fe(III)-EDDHA for 6 d then treated with 1 or 25 μM Fe(III)-EDDHA and 0, 1, 2.5, or 5 mM sodium bicarbonate for 4 d. First trifoliate leaf chlorophyll (in SPAD units) in (A) IDC sensitive plants and (B) IDC tolerant plants. Root FCR activity in (C) IDC sensitive plants and (D) IDC tolerant plants. * indicates a significant difference (p < 0.05) between treatment and control (25 Fe, 0 Bic). + in (B) and (D) indicates a significant difference between the tolerant and sensitive lines in that treatment.
Gene Expression Profiling
Based on the results above, transcripts were quantified by RNA-seq from roots from IDC sensitive and tolerant plants grown at low Fe or normal Fe supply, with 0, 2.5, or 5 mM Bic. Our first goal was to determine whether, in soybean as a species, Fe deficiency and alkalinity are equivalent in terms of specific genes that are induced or repressed. As such, we combined results from both genotypes (Figure 4) to capture all DE genes in a larger set that was then observed in detail to determine each genotype's response. Of 5,288 differentially regulated genes (Supplementary Table 1), 474 were upregulated by both Fe deficiency and Bic treatment, while 175 were downregulated by both Fe deficiency and Bic treatment. A greater total number of genes were differentially regulated by only Bic or Fe deficiency than were regulated by both stresses. Many of the classical Fe responsive genes (FRO2, IRT1, OPT3, AHA2, and bHLH38) were upregulated by both Fe deficiency and alkaline stress (Figure 4, Supplementary Table 2). However, FIT and NRAMP6 were among the 365 genes that were upregulated by Fe deficiency and both upregulated and downregulated by alkaline stress, depending on genotype and treatment. Homologs of some genes that have been regulated by Fe deficiency in previous studies (Colangelo and Guerinot, 2004; García et al., 2010; Yang et al., 2010; Stein and Waters, 2012) were only upregulated by Bic treatment (FIT, bHLH38, OPT3, NRAMP3, ORG1). Some metal homeostasis genes were downregulated by Fe deficiency and upregulated by Bic treatment: FSD2, ZIP2, ZIP11, OPT7, and YSL3. Thus, while many of the Fe uptake genes were regulated similarly by Fe deficiency and alkaline stress, there were many genes that did not respond equivalently, suggesting that alkalinity stress stimulates expression of Fe uptake genes, but also has other effects.
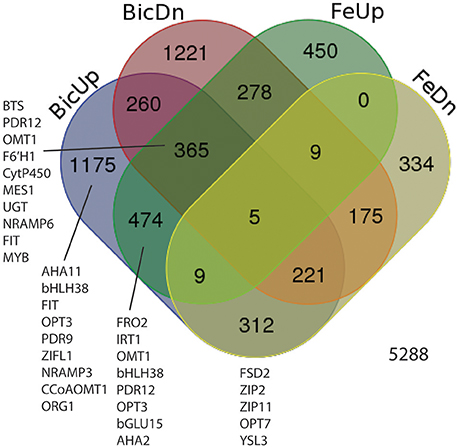
Figure 4. Venn diagram showing differentially expressed genes (DEGs) in roots of IDC sensitive or tolerant soybean lines (genotypes combined). BicUp, DEGs upregulated by 2.5 and/or 5 mM bicarbonate treatment relative to 0 bicarbonate; BicDn, DEGs downregulated by bicarbonate treatment; FeUp, DEGs upregulated by low Fe (1 μM) treatment relative to normal Fe (25 μM) treatment; FeDn, DEGs downregulated by low Fe (1 μM) treatment. Numbers represent number of elements (genes) in each set. Genes of interest in each set are listed near the set, and correspond to highlighted genes in Supplementary Table 2.
We compared differential expression of soybean homologs of Arabidopsis classical Fe deficiency response genes FRO2, IRT1, AHA2, and FIT across the soybean lines (Table 1). At low Fe supply the IDC sensitive line had significantly increased expression of the four Fe uptake genes, while the tolerant line had increased expression of only FRO2, suggesting that the IDC sensitive line was also more sensitive to Fe deficiency. However, as Bic increased and the pH became more alkaline, the tolerant line upregulated expression of FRO2, IRT1, and FIT, while their expression decreased in the sensitive line. While this result does not necessarily indicate that the proteins produced from these Fe uptake transcripts are functional in alkaline conditions, it does suggest that, like with FCR activity results (Figure 3), expression of Fe uptake genes in the sensitive line was inhibited by Bic to a greater extent than in the tolerant line. Iron uptake gene expression was also stimulated by alkaline stress at normal Fe supply, with significant upregulation of FRO2, IRT1, AHA2, and FIT in the tolerant line, and upregulation of FRO2 and IRT1 in the sensitive line. Since the Fe(III)-EDDHA is pH stable (Halvorson and Lindsay, 1972), this response is not due to decreased Fe availability to the roots. The response of classical Fe uptake genes indicates that both low Fe supply and alkaline pH stress induces Fe uptake responses at the molecular level. At this point it is unclear whether Fe uptake gene upregulation is due to the plant directly sensing alkalinity at the roots, or whether the upregulation results from a leaf-originated or local root signal that is stimulated by low Fe concentration in the plant tissues (Figure 2) resulting from alkalinity inhibited Fe uptake. Results from cucumber indicated that when alkaline stress is combined with Fe deficiency the normal Fe deficiency sensing in the leaf is blocked, potentially resulting in loss of shoot-to-root signal of plant Fe status (Hsieh and Waters, 2016).
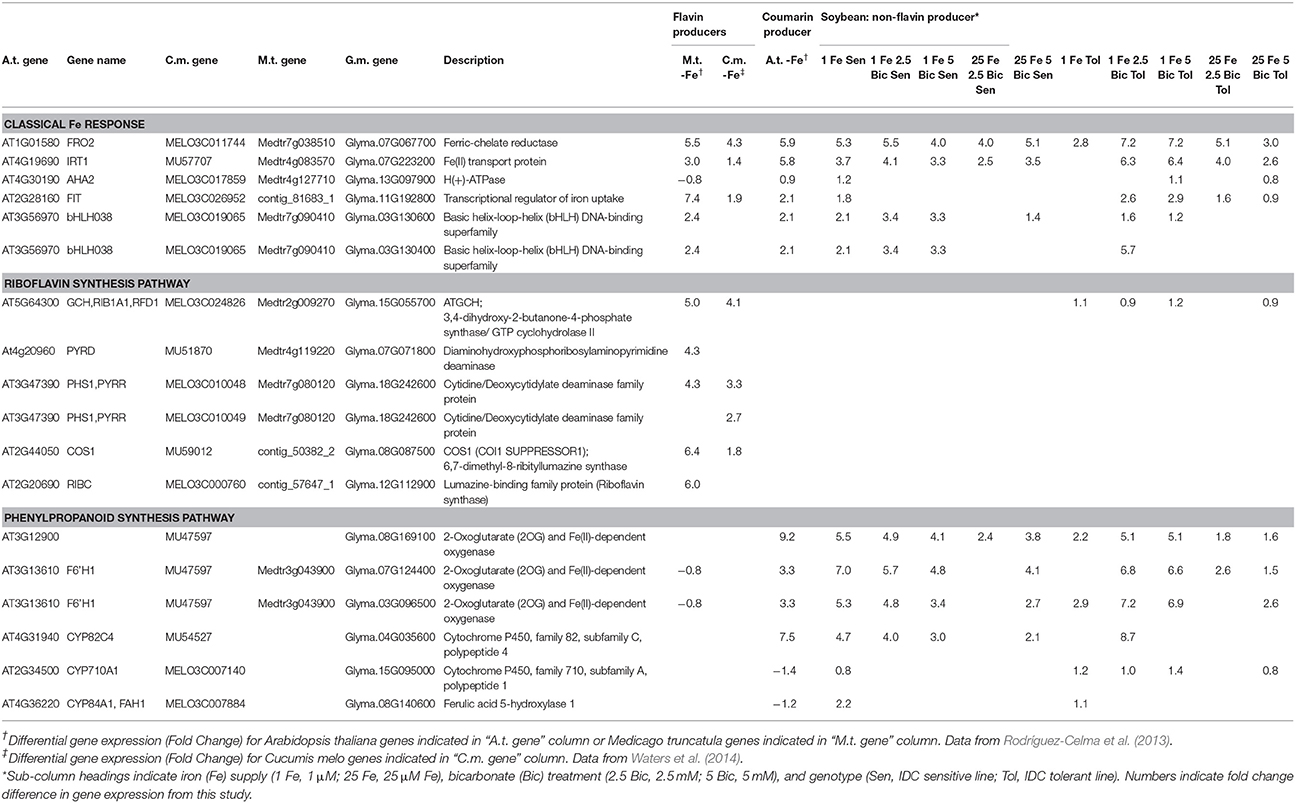
Table 1. Comparison of differentially expressed genes for classical iron deficiency response, riboflavin synthesis, and phenylpropanoid synthesis from Medicago truncatula (M.t.), Cucumis melo (C.m.), Arabidopsis thaliana (A.t.), and soybean, Glycine max (G.m.).
We also used our RNA-seq data to gain insight into key physiological responses to alkalinity and Fe deficiency. Some of the genes that were most highly responsive to both Fe deficiency and alkaline stress were homologs of phenylpropanoid synthesis gene AtF6'H1 (Supplementary Table 2), which are needed to synthesize FluRE such as scopoletin (Kai et al., 2008), that are important for Fe uptake under alkaline conditions in Arabidopsis (Fourcroy et al., 2014; Schmid et al., 2014; Schmidt et al., 2014). In addition to the F6'H1 genes, other putative phenylpropanoid genes were strongly upregulated by Fe deficiency and/or alkaline stress in soybean roots (e.g., CYP82C4, MES1, UGT73C1, OMT1, BGLU15, CCoAOMT1; Figure 4, Supplementary Table 2). We also noted upregulation of two putative phenylpropanoid transporters, most similar to AtPDR9 and AtPDR12. AtPDR9 and BGLU42 are necessary to secrete coumarins from roots under Fe deficiency (Fourcroy et al., 2014; Zamioudis et al., 2014).
Iron deficient dicots are known to increase production and efflux of either flavin compounds or fluorescent phenolic compounds, but not both within a single species (Cesco et al., 2010; Rodríguez-Celma and Schmidt, 2013). Iron deficient Arabidopsis increases production of phenolics of the coumarin class (Rodríguez-Celma et al., 2013; Schmid et al., 2014; Schmidt et al., 2014) by increasing expression of the phenylpropanoid pathway, and our results suggest that soybean has a similar response. To indicate whether soybean might increase flavin production under Fe deficiency or alkaline stress, we compared soybean riboflavin synthesis gene expression to expression from other plant species under Fe deficiency (Rodríguez-Celma et al., 2013; Waters et al., 2014). The riboflavin synthesis genes were upregulated by Fe deficiency in melon (Cucumis melo) and Medicago truncatula, known flavin producers (Welkie, 2000; Rodríguez-Celma et al., 2011b), but not in Arabidopsis or soybean. Both Arabidopsis and soybean had increased expression of phenylpropanoid synthesis genes in response to Fe deficiency, but melon and Medicago did not (Table 1). In soybean, these phenylpropanoid synthesis genes also responded to Bic treatment. A simplified diagram of the known and some presumed steps in coumarin synthesis and efflux is shown in Supplementary Figure 1, showing Arabidopsis genes and their soybean homologs. Many of these genes are upregulated by Fe deficiency at the protein and/or transcript level in Arabidopsis (Lan et al., 2010; Rodríguez-Celma et al., 2013), and soybean homologs for all of these genes were upregulated in one or more Fe deficiency or alkaline stress conditions (Supplementary Table 2). These results suggest that soybean upregulates synthesis of coumarins in response to Fe deficiency and alkaline stress, similar to Arabidopsis. It is worth noting that, like the classical Fe response genes, expression of potential phenylpropanoid synthesis genes tended to decrease in the IDC sensitive line as Bic supply increased, while expression increased or remained high in the IDC tolerant line (Supplementary Table 2), which is why some of the Fe upregulated genes were categorized as both Bic upregulated and Bic downregulated (Figure 4).
Fluorescent Root Exudates
Fe deficiency increases efflux of coumarins from Arabidopsis roots (Fourcroy et al., 2014; Schmid et al., 2014; Sisó-Terraza et al., 2016), and alkaline growth conditions result in greater coumarin production and exudation (Schmidt et al., 2014). Since coumarin compounds are fluorescent, fluorescence of the culture media is a good estimate of total coumarin root exudates (Schmid et al., 2014; Schmidt et al., 2014). We measured fluorescence of hydroponic solutions following growth of soybean plants in solutions with normal and low concentrations of FeSO4 and with a range of Bic. We used FeSO4 rather than Fe(III)EDDHA in these experiments because the non-chelated FeSO4 would have decreased availability at alkaline pH, and if the FluRE could assist with Fe availability they would be helpful in growth on this Fe source. Phenolic root exudates are important for Fe uptake in Strategy I species (Clemens and Weber, 2016). In both red clover and Arabidopsis, removing phenolics from recirculating nutrient solution resulted in increased severity of Fe deficiency (Jin et al., 2007; Fourcroy et al., 2014). Phenolic compounds are needed to access precipitated Fe in the apoplast of roots (Jin et al., 2007; Ishimaru et al., 2011) and have Fe chelating properties and Fe(III) reducing activity (Schmid et al., 2014; Schmidt et al., 2014). When plants were grown in hydroponics with Bic and FeSO4 as the Fe source, IDC tolerant lines had higher leaf chlorophyll (Figure 5A). In both the IDC sensitive and IDC tolerant lines, alkaline growth conditions resulted in greatly increased fluorescence (Figure 5B), and fluorescence was higher from the IDC tolerant line than for the IDC sensitive line, although the difference was not statistically significant.
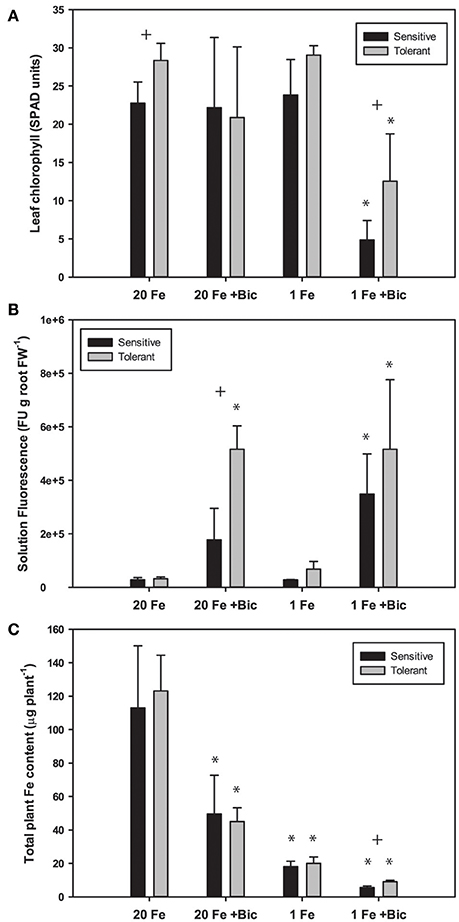
Figure 5. Leaf chlorophyll, nutrient solution fluorescence, and total plant Fe accumulation in IDC tolerant and sensitive soybean plants. Plants were treated with 1 or 20 μM FeSO4 and 0 or 2.5 mM sodium bicarbonate for 12 d. (A) leaf chlorophyll of first trifoliate leaf in SPAD units; (B) total fluorescence units of nutrient solution normalized to root FW; (C) Total plant Fe accumulation (content), in μg Fe per plant. * indicates a significant difference (p < 0.05) between treatment and control (20 Fe, 0 Bic). + indicates a significant difference between the tolerant and sensitive lines in that treatment.
Arabidopsis T-DNA lines with disruptions in genes required for synthesis or efflux of FluRE were more sensitive to Fe deficiency and had poor growth under alkaline conditions relative to wild-type plants; specifically for the F6'H1 gene (Schmid et al., 2014; Schmidt et al., 2014), a 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase needed for coumarin synthesis (Kai et al., 2008), and ABCG37/PDR9, a coumarin efflux transporter gene (Fourcroy et al., 2014). Homologs of these genes were significantly upregulated in soybean roots (Table 1). If these coumarin compounds are important for Fe uptake at alkaline pH, then the higher FluRE in the solution of the IDC tolerant line could explain why it can grow better in alkaline, IDC-prone conditions. To test whether the IDC tolerant line that made greater quantities of FluRE had higher overall Fe accumulation than the IDC sensitive line, we grew the plants in hydroponics for 12 d, changed the Fe source to FeSO4 to more closely mimic soil conditions without synthetic chelators, and also began Bic treatments upon planting. Total plant Fe content was not different between IDC sensitive and tolerant lines (Figure 5C), even though the sensitive line had more severe leaf chlorosis under the low Fe with Bic treatment. Alkaline growth solution decreased Fe accumulation in both lines, while low Fe supply decreased Fe accumulation further. This result suggests that the difference in IDC sensitivity between these lines is not attributable to differences in overall Fe uptake, at least in hydroponic growth conditions. This finding suggests that the IDC tolerant line has higher Fe use efficiency than the sensitive line. We have observed genetic differences in Fe use efficiency in cucumber seedlings (Waters and Troupe, 2012), but the basis for these differences are not well-understood. However, our mineral analysis method can only detect bulk Fe content, and cannot detect symplastic/apoplastic partitioning, subcellular Fe localization, or other fine-scale details. Further studies could determine whether these two lines partition Fe differently within the plant organs and indicate the basis for differences in Fe use efficiency.
There was a difference between IDC tolerant and sensitive lines for whole-plant Fe accumulation in our hydroponic system in the low Fe +Bic treatment (Figure 5C), where FluRE may be important for Fe uptake. There may be an even more important role for FluRE compounds in alkaline soil (Clemens and Weber, 2016), which is more chemically complex than hydroponic solution. It is also possible that FluRE are involved in processes in addition to Fe uptake. Certain microorganisms or volatile compounds they produce can stimulate expression of Fe uptake responses (Zhang et al., 2009; Zamioudis et al., 2015; Zhou et al., 2016), including FluRE production (Zamioudis et al., 2014), and many of the genes that are induced by these microbes in Arabidopsis have homologs that are induced by Fe deficiency and or alkalinity stress in this study (Supplementary Table 3). These results together suggest that a possible role for these fluorescent compounds is in modification of the rhizomicrobiome (Badri et al., 2013; Gu et al., 2016), which could in turn improve soybean immunity to pathogens (Zamioudis and Pieterse, 2011), or influence uptake or availability of Fe or other mineral nutrients (Cesco et al., 2010).
Phenylpropanoid pathway genes are regulated by certain MYB transcription factors, such as MYB58 and MYB63 in Arabidopsis (Zhou et al., 2009). The MYB72 gene is upregulated by Fe deficiency in Arabidopsis roots (Colangelo and Guerinot, 2004; García et al., 2010; Yang et al., 2010; Stein and Waters, 2012). Roots of a myb72 mutant did not increase production or efflux of FluRE at pH 7.0 relative to control conditions at pH 5.8, whereas WT roots did produce and secrete FluRE (Zamioudis et al., 2014). Overexpression of Arabidopsis MYB58 and MYB63 and sorghum MYB60 transcription factors resulted in increased expression of the entire phenylpropanoid pathway and increased lignin synthesis in Arabidopsis and sorghum (Zhou et al., 2009; Scully et al., 2016). Additionally, overexpression of the MYB72 gene in Arabidopsis led to upregulation of the entire phenylpropanoid pathway and increased expression of FluRE (Zamioudis et al., 2014). Several soybean MYB transcription factors were upregulated by Fe deficiency or alkaline stress in a manner consistent with upregulation of the phenylpropanoid synthesis genes. However, further experiments are required to determine which of these MYB genes are specifically required for regulation of FluRE in soybean.
Genotypic Differences and Correspondence to QTL
To gain insight into the genetic aspects of IDC tolerance inherent in the two lines in this study, we compared transcript abundance in each of the lines in each treatment. Two thousand fifty genes had at least 1.0-fold difference in abundance (log2 scale) between IDC tolerant and sensitive lines, and 1,626 of these genes were also differentially regulated by Fe or alkalinity treatments (Figure 6). These 1,626 genes are shown along with the differential expression by treatment, in Supplementary Table 4. Many of the putative phenylpropanoid synthesis genes had higher transcript levels in the IDC tolerant line than in the IDC sensitive line.
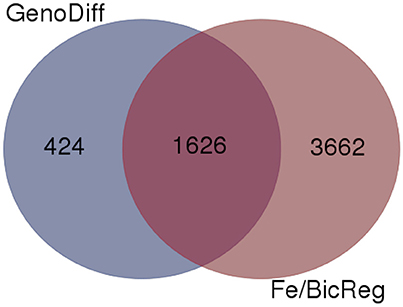
Figure 6. Venn diagram comparing genes with differential expression in a specific condition (low or normal Fe supply with 0, 2.5, or 5 mM bicarbonate) between IDC sensitive and tolerant lines (GenoDiff) to genes that responded to low Fe and/or bicarbonate in either line (Fe/BicReg). The 1,626 overlapping genes are featured in Supplementary Table 4.
To further compare our RNA-sequencing results with existing genetic IDC data, and determine whether phenylpropanoid genes are within previously mapped IDC QTL from biparental populations (Lin et al., 1997, 2000; Charlson et al., 2005; Wang et al., 2008; Mamidi et al., 2011, 2014; Kocak, 2014) and from GWAS studies (Wang et al., 2008; Mamidi et al., 2011, 2014) we aligned our RNA-seq results with QTL intervals. We used the information on Soybase to anchor the flanking genetic markers to the physical map, and genes within these intervals are shown in Supplementary Table 5. Notably, FRO2 corresponded with a QTL on chromosome 7. FRO2 was highly upregulated by Fe deficiency and Bic treatment, and had greater transcript abundance in one variety, depending on the treatment. Another Fe homeostasis gene, BTS, an E3 ligase that is involved in regulation of Fe deficiency responses (Long et al., 2010; Selote et al., 2015), corresponded with a QTL on chromosome 5. Genes for BTS-interacting transcription factors bHLH115 and bHLH105/IRL3 (Long et al., 2010) were upregulated under low Fe and low Fe +Bic treatments in both genotypes. One of the QTL intervals on Chr. 3 that explained 70% of the variation in an Anoka X A7 population (Lin et al., 1997) and that was introgressed in two IDC sensitive NILs (Severin et al., 2010; Peiffer et al., 2012) contained bHLH38 (Glyma.03G130600). This gene was highly upregulated by Fe deficiency and alkaline stress, and was more abundant in the IDC tolerant line than in the sensitive line at 25 μM Fe with 2.5 mM Bic (Table 1, Supplementary Tables 2, 5). A previous IDC study (Peiffer et al., 2012) suggested that a 12 bp deletion in the bHLH38 gene was the likely difference between the IDC tolerant (Clark) and sensitive (isoClark) isolines. That 12 bp variant in bHLH38 was not present in our IDC tolerant and sensitive germplasm (data not shown), suggesting that the IDC sensitivity differences between our lines is not due to this particular variant. However, since the bHLH38 protein interacts with the FIT protein to control most Fe uptake responses in Arabidopsis (Yuan et al., 2008), the bHLH38 gene may be a major factor for IDC tolerance.
Natural variation is present for coumarin synthesis in A. thaliana, with multiple QTL controlling this trait in plants grown without stress treatments (Siwinska et al., 2014). Variation for fluorescent exudate production in response to Fe deficiency and alkalinity stress conditions is also present in the soybean IDC sensitive and tolerant lines used in this study. Notably, the major chromosome 3 QTL region may also contain one of the F'6H1 homologs (Glyma.03g096500) that was strongly upregulated by Fe deficiency and Bic treatment and was more abundant in the IDC tolerant line. Several other previously mapped IDC QTL intervals contained potential phenylpropanoid synthesis genes that were upregulated by Fe deficiency or alkaline stress, including a cytochrome P450 family gene (Glyma.04g035600). There are also MYB genes, which may be involved in regulating the phenylpropanoid synthesis pathway, in QTL regions on chromosomes 2, 4, 11, 14, 16, and 19. These results indicate that differences in coumarin production may underlie some of the IDC QTL.
Conclusions
This study has indicated a correlation between FluRE quantity and IDC tolerance, and has indicated specific genes that may be involved in the regulation, synthesis, and efflux of these compounds. Further physiological studies to fully define the roles for FluREs in Fe deficiency and IDC tolerance will be helpful. A future direction will be to test whether FluRE quantities from other soybean varieties correlate with IDC tolerance. Our transcriptomic results for alkaline stress and Fe deficiency regulated genes can be a valuable resource for other researchers to cross-reference to genetic studies. It will be useful to extend the study of FluRE to linkage mapping populations to further understand genetic control of FluRE production, and to determine whether FluRE contribute to IDC tolerance. The IDC tolerant and susceptible parents from this study were used to develop a 320-line F6-derived F8 recombinant inbred line (RIL) population, which was phenotyped in the field for IDC (Kocak, 2014). Thus, we plan to use this population to identify the factors controlling IDC tolerance and FluRE production.
Author Contributions
BW designed and conducted experiments, analyzed data, and wrote the manuscript. KA designed RNA-seq protocols and performed transcriptomic analysis. GG provided soybean germplasm and analyzed data. All authors edited and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Nebraska Soybean Board for funding for this work. The authors thank Tony Delaney and Erin Kinley for technical assistance, and Dr. Javier Seravalli for mineral analysis assistance. This research was partially supported by the Nebraska Agricultural Experiment Station with funding from the Hatch Act (Accession Number 1007260) through the USDA National Institute of Food and Agriculture. The University of Nebraska DNA Sequencing Core receives partial support from the National Institute for General Medical Science (NIGMS) INBRE—P20GM103427-14 and COBRE—1P30GM110768-01 grants as well as The Fred & Pamela Buffett Cancer Center Support Grant—P30CA036727. This publication's contents are the sole responsibility of the authors and do not necessarily represent the official views of the NIH or NIGMS. Part of this work was presented at the “Legumes Workshop” at the Plant and Animal Genome conference in January, 2016.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00010/full#supplementary-material
Supplementary Figure 1. Diagram of phenylpropanoid synthesis pathway genes.
Supplementary Table 1. All genes that were differentially expressed by Fe or Bic treatments.
Supplementary Table 2. Selected differentially expressed genes.
Supplementary Table 3. Soybean homologs of Arabidopsis genes that are upregulated by microorganisms.
Supplementary Table 4. Thousand six hundred and twenty-six genes that were differentially expressed between genotypes and also were differentially expressed by Fe or Bic treatments.
Supplementary Table 5. Differentially expressed genes that correspond to previous QTL intervals.
Abbreviations
IDC, iron deficiency chlorosis; FluRE, fluorescent root exudates; Bic, bicarbonate; FCR, ferric-chelate reductase.
References
Badri, D. V., Chaparro, J. M., Zhang, R., Shen, Q., and Vivanco, J. M. (2013). Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 288, 4502–4512. doi: 10.1074/jbc.M112.433300
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Cesco, S., Neumann, G., Tomasi, N., Pinton, R., and Weisskopf, L. (2010). Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 329, 1–25. doi: 10.1007/s11104-009-0266-9
Chaney, R. L., Brown, J. C., and Tiffin, L. O. (1972). Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 50, 208–213.
Chaney, R. L., Coulombe, B. A., Bell, P. F., and Angle, J. S. (1992). Detailed method to screen dicot cultivars for resistance to Fe-chlorosis using FeDTPA and bicarbonate in nutrient solutions. J. Plant Nutr. 15, 2063–2083. doi: 10.1080/01904169209364459
Charlson, D. V., Bailey, T. B., Cianzio, S. R., and Shoemaker, R. C. (2005). Molecular marker Satt481 is associated with iron-deficiency chlorosis resistance in a soybean breeding population Crop Sci. 45, 2394–2399. doi: 10.2135/cropsci2004.0510
Chen, Y., and Barak, P. (1982). Iron nutrition of plants in calcareous soils. Adv. Agron. 35, 217–240. doi: 10.1016/S0065-2113(08)60326-0
Clemens, S., and Weber, M. (2016). The essential role of coumarin secretion for Fe acquisition from alkaline soil. Plant Signal. Behav. 11:e1114197. doi: 10.1080/15592324.2015.1114197
Colangelo, E. P., and Guerinot, M. L. (2004). The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16, 3400–3412. doi: 10.1105/tpc.104.024315
Coulombe, B. A., Chaney, R. L., and Wiebold, W. J. (1984). Bicarbonate directly induces iron chlorosis in susceptible soybean cultivars. Soil Sci. Soc. Am. J. 48, 1297–1301. doi: 10.2136/sssaj1984.03615995004800060019x
Dinneny, J. R., Long, T. A., Wang, J. Y., Jung, J. W., Mace, D., Pointer, S., et al. (2008). Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320, 942–945. doi: 10.1126/science.1153795
Eide, D., Broderius, M., Fett, J., and Guerinot, M. L. (1996). A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. U.S.A. 93, 5624–5628. doi: 10.1073/pnas.93.11.5624
Fleming, A., Chaney, R., and Coulombe, B. (1984). Bicarbonate inhibits Fe-stress response and Fe uptake-translocation of chlorosis-susceptible soybean cultivars. J. Plant Nutr. 7, 699–714. doi: 10.1080/01904168409363235
Fourcroy, P., Sisó-Terraza, P., Sudre, D., Savirón, M., Reyt, G., Gaymard, F., et al. (2014). Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytologist 201, 155–167. doi: 10.1111/nph.12471
García, M. J., Lucena, C., Romera, F. J., Alcántara, E., and Pérez-Vicente, R. (2010). Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J. Exp. Bot. 61, 3885–3899. doi: 10.1093/jxb/erq203
Gu, Y., Wei, Z., Wang, X., Friman, V.-P., Huang, J., Wang, X., et al. (2016). Pathogen invasion indirectly changes the composition of soil microbiome via shifts in root exudation profile. Biol. Fertil. Soils 52, 997–1005. doi: 10.1007/s00374-016-1136-2
Guttieri, M. J., Baenziger, P. S., Frels, K., Carver, B., Arnall, B., and Waters, B. M. (2015). Variation for grain mineral concentration in a diversity panel of current and historical great plains hard winter wheat germplasm. Crop Sci. 55, 1035–1052. doi: 10.2135/cropsci2014.07.0506
Halvorson, A., and Lindsay, W. (1972). Equilibrium relationships of metal chelates in hydroponic solutions. Soil Sci. Soc. Am. J. 36, 755–761. doi: 10.2136/sssaj1972.03615995003600050022x
Hansen, N. C., Jolley, V. D., Naeve, S. L., and Goos, R. J. (2004). Iron deficiency of soybean in the north central US and associated soil properties. Soil Sci. Plant Nutr. 50, 983–987. doi: 10.1080/00380768.2004.10408564
Hsieh, E. J., and Waters, B. M. (2016). Alkaline stress and iron deficiency regulate iron uptake and riboflavin synthesis gene expression differently in root and leaf tissue: implications for iron deficiency chlorosis. J. Exp. Bot. 67, 5671–5685. doi: 10.1093/jxb/erw328
Ishimaru, Y., Kakei, Y., Shimo, H., Bashir, K., Sato, Y., Sato, Y., et al. (2011). A rice phenolic efflux transporter is essential for solubilizing precipitated apoplasmic iron in the plant stele. J. Biol. Chem. 286, 24649–24655. doi: 10.1074/jbc.M111.221168
Ivanov, R., Brumbarova, T., and Bauer, P. (2012). Fitting into the Harsh reality: regulation of iron-deficiency responses in dicotyledonous plants. Mol. Plant 5, 27–42. doi: 10.1093/mp/ssr065
Jin, C. W., You, G. Y., He, Y. F., Tang, C., Wu, P., and Zheng, S. J. (2007). Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol. 144, 278–285. doi: 10.1104/pp.107.095794
Kai, K., Mizutani, M., Kawamura, N., Yamamoto, R., Tamai, M., Yamaguchi, H., et al. (2008). Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J. 55, 989–999. doi: 10.1111/j.1365-313X.2008.03568.x
Kobayashi, T., and Nishizawa, N. K. (2012). Iron Uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 63, 131–152. doi: 10.1146/annurev-arplant-042811-105522
Kocak, K. (2014). Evaluation of Iron Deficiency Chlorosis in Soybeans (Glycine max). Ph.D. dissertation, Univeristy of Nebraska.
Lan, P., Li, W. F., Wen, T. N., Shiau, J. Y., Wu, Y. C., Lin, W. D., et al. (2010). iTRAQ protein profile analysis of arabidopsis roots reveals new aspects critical for iron homeostasis. Plant Physiol. 155, 821–834. doi: 10.1104/pp.110.169508
Lin, S., Cianzio, S., and Shoemaker, R. (1997). Mapping genetic loci for iron deficiency chlorosis in soybean. Mol. Breed. 3, 219–229. doi: 10.1023/A:1009637320805
Lin, S. F., Grant, D., Cianzio, S., and Shoemaker, R. (2000). Molecular characterization of iron deficiency chlorosis in soybean. J. Plant Nutr. 23, 1929–1939. doi: 10.1080/01904160009382154
Long, T. A., Tsukagoshi, H., Busch, W., Lahner, B., Salt, D. E., and Benfey, P. N. (2010). The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. Plant Cell 22, 2219–2236. doi: 10.1105/tpc.110.074096
Lucena, C., Romera, F. J., Rojas, C. L., Garcia, M., Alcantara, E., and Perez-Vicente, R. (2007). Bicarbonate blocks the expression of several genes involved in the physiological responses to Fe deficiency of strategy I plants. Func. Plant Biol. 34, 1002–1009. doi: 10.1071/FP07136
Mamidi, S., Chikara, S., Goos, R. J., Hyten, D. L., Annam, D., Moghaddam, S. M., et al. (2011). Genome-wide association analysis identifies candidate genes associated with iron deficiency chlorosis in soybean. Plant Genome 4, 154–164. doi: 10.3835/plantgenome2011.04.0011
Mamidi, S., Lee, R. K., Goos, J. R., and McClean, P. E. (2014). Genome-wide association studies identifies seven major regions responsible for iron deficiency chlorosis in soybean (Glycine max). PLoS ONE 9:e107469. doi: 10.1371/journal.pone.0107469
Mengel, K. (1994). Iron availability in plant-tissues - iron chlorosis on calcareous soils. Plant Soil 165, 275–283. doi: 10.1007/BF00008070
Moran Lauter, A. N., Peiffer, G. A., Yin, T., Whitham, S. A., Cook, D., Shoemaker, R. C., et al. (2014). Identification of candidate genes involved in early iron deficiency chlorosis signaling in soybean (Glycine max) roots and leaves. BMC Genomics 15:702. doi: 10.1186/1471-2164-15-702
O'Rourke, J. A., Nelson, R. T., Grant, D., Schmutz, J., Grimwood, J., Cannon, S., et al. (2009). Integrating microarray analysis and the soybean genome to understand the soybeans iron deficiency response. BMC Genomics 10:376. doi: 10.1186/1471-2164-10-376
Peiffer, G. A., King, K. E., Severin, A. J., May, G. D., Cianzio, S. R., Lin, S. F., et al. (2012). Identification of candidate genes underlying an iron efficiency quantitative trait locus in soybean. Plant Physiol. 158, 1745–1754. doi: 10.1104/pp.111.189860
Prohaska, K. R., and Fehr, W. R. (1981). Recurrent selection for resistance to iron deficiency chlorosis in soybeans. Crop Sci. 21, 524–526. doi: 10.2135/cropsci1981.0011183X002100040011x
Rellán-Álvarez, R., Andaluz, S., Rodríguez-Celma, J., Wohlgemuth, G., Zocchi, G., Álvarez-Fernández, A., et al. (2010). Changes in the proteomic and metabolic profiles of Beta vulgaris root tips in response to iron deficiency and resupply. BMC Plant Biol. 10:120. doi: 10.1186/1471-2229-10-120
Robinson, N. J., Procter, C. M., Connolly, E. L., and Guerinot, M. L. (1999). A ferric-chelate reductase for iron uptake from soils. Nature 397, 694–697. doi: 10.1038/17800
Rodríguez-Celma, J., Lattanzio, G., Grusak, M. A., AbadíA, A., AbadíA, A. J., and LóPez-MilláN, A. F. (2011a). Root responses of Medicago truncatula plants grown in two different iron deficiency conditions: changes in root protein profile and riboflavin biosynthesis. J. Proteome Res. 10, 2590–2601. doi: 10.1021/pr2000623
Rodríguez-Celma, J., Lin, W. D., Fu, G. M., Abadía, J., López-Millán, A. F., and Schmidt, W. (2013). Mutually exclusive alterations in secondary metabolism are critical for the uptake of insoluble iron compounds by Arabidopsis and Medicago truncatula. Plant Physiol. 162, 1473–1485. doi: 10.1104/pp.113.220426
Rodríguez-Celma, J., and Schmidt, W. (2013). Reduction-based iron uptake revisited: on the role of secreted iron-binding compounds. Plant Signal. Behav. 8:e26116. doi: 10.4161/psb.26116
Rodríguez-Celma, J., Vázquez-Reina, S., Orduna, J., Abadía, A., Abadía, J., Álvarez-Fernández, A., et al. (2011b). Characterization of flavins in roots of Fe-deficient strategy I plants, with a focus on Medicago truncatula. Plant Cell Physiol. 52, 2173–2189. doi: 10.1093/pcp/pcr149
Rodriguez de Cianzio, S., Fehr, W. R., and Anderson, I. C. (1979). Genotypic evaluation for iron deficiency chlorosis in soybeans by visual scores and chlorophyll concentration. Crop Sci. 19, 644–646. doi: 10.2135/cropsci1979.0011183X001900050024x
Romera, F., Alcantera, E., and De La Guardia, M. (1992). Effects of bicarbonate, phosphate, and high pH on the reducing capacity of Fe-deficient sunflower and cucumber plants. J. Plant Nutr. 15, 1519–1530. doi: 10.1080/01904169209364418
Santi, S., and Schmidt, W. (2009). Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 183, 1072–1084. doi: 10.1111/j.1469-8137.2009.02908.x
Schmid, N. B., Giehl, R. F., Döll, S., Mock, H. P., Strehmel, N., Scheel, D., et al. (2014). Feruloyl-CoA 6′-hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol. 164, 160–172. doi: 10.1104/pp.113.228544
Schmidt, H., Günther, C., Weber, M., Spörlein, C., Loscher, S., Böttcher, C., et al. (2014). Metabolome analysis of Arabidopsis thaliana roots identifies a key metabolic pathway for iron acquisition. PLoS ONE 9:e102444. doi: 10.1371/journal.pone.0102444
Scully, E. D., Gries, T., Sarath, G., Palmer, N. A., Baird, L., Serapiglia, M. J., et al. (2016). Overexpression of SbMyb60 impacts phenylpropanoid biosynthesis and alters secondary cell wall composition in Sorghum bicolor. Plant J. 85, 378–395. doi: 10.1111/tpj.13112
Selote, D., Samira, R., Matthiadis, A., Gillikin, J. W., and Long, T. A. (2015). Iron-binding E3 ligase mediates iron response in plants by targeting basic helix-loop-helix transcription factors. Plant Physiol. 167, 273–286. doi: 10.1104/pp.114.250837
Severin, A. J., Peiffer, G. A., Xu, W. W., Hyten, D. L., Bucciarelli, B., O'rourke, J. A., et al. (2010). An integrative approach to genomic introgression mapping. Plant Physiol. 154, 3–12. doi: 10.1104/pp.110.158949
Sisó-Terraza, P., Luis-Villarroya, A., Fourcroy, P., Briat, J. F., Abadía, A., Gaymard, F., et al. (2016). Accumulation and secretion of coumarinolignans and other coumarins in Arabidopsis thaliana roots in response to iron deficiency at high pH. Front. Plant Sci. 7:1711. doi: 10.3389/fpls.2016.01711
Siwinska, J., Kadzinski, L., Banasiuk, R., Gwizdek-Wisniewska, A., Olry, A., Banecki, B., et al. (2014). Identification of QTL affecting scopolin and scopoletin biosynthesis in Arabidopsis thaliana. BMC Plant Biol. 14:280. doi: 10.1186/s12870-014-0280-9
Stein, R. J., and Waters, B. M. (2012). Use of natural variation reveals core genes in the transcriptome of iron-deficient Arabidopsis thaliana roots. J. Exp. Bot. 63, 1039–1055. doi: 10.1093/jxb/err343
Trapnell, C., Roberts, A., Goff, L., Pertea, G., Kim, D., Kelley, D. R., et al. (2012). Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578. doi: 10.1038/nprot.2012.016
Vasconcelos, M., Eckert, H., Arahana, V., Graef, G., Grusak, M. A., and Clemente, T. (2006). Molecular and phenotypic characterization of transgenic soybean expressing the Arabidopsis ferric chelate reductase gene, FRO2. Planta 224, 1116–1128. doi: 10.1007/s00425-006-0293-1
Wang, J., McClean, P. E., Lee, R., Goos, R. J., and Helms, T. (2008). Association mapping of iron deficiency chlorosis loci in soybean (Glycine max L. Merr.) advanced breeding lines. Theor. Appl. Genet. 116, 777–787. doi: 10.1007/s00122-008-0710-x
Waters, B. M., McInturf, S. A., and Amundsen, K. (2014). Transcriptomic and physiological characterization of the fefe mutant of melon (Cucumis melo) reveals new aspects of iron–copper crosstalk. New Phytol. 203, 1128–1145. doi: 10.1111/nph.12911
Waters, B. M., and Troupe, G. C. (2012). Natural variation in iron use efficiency and mineral remobilization in cucumber (Cucumis sativus). Plant Soil 352, 185–197. doi: 10.1007/s11104-011-0988-3
Welkie, G. W. (2000). Taxonomic distribution of dicotyledonous species capable of root excretion of riboflavin under iron deficiency. J. Plant Nutr. 23, 1819–1831. doi: 10.1080/01904160009382145
Yang, T. J., Lin, W. D., and Schmidt, W. (2010). Transcriptional profiling of the Arabidopsis iron deficiency response reveals conserved transition metal homeostasis networks. Plant Physiol. 152, 2130–2141. doi: 10.1104/pp.109.152728
Yuan, Y., Wu, H., Wang, N., Li, J., Zhao, W., Du, J., et al. (2008). FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 18, 385–397. doi: 10.1038/cr.2008.26
Zamioudis, C., Hanson, J., and Pieterse, C. M. J. (2014). β-Glucosidase BGLU42 is a MYB72-dependent key regulator of rhizobacteria-induced systemic resistance and modulates iron deficiency responses in Arabidopsis roots. New Phytol. 204, 368–379. doi: 10.1111/nph.12980
Zamioudis, C., Korteland, J., Van Pelt, J. A., van Hamersveld, M., Dombrowski, N., Bai, Y., et al. (2015). Rhizobacterial volatiles and photosynthesis-related signals coordinate MYB72 expression in Arabidopsis roots during onset of induced systemic resistance and iron-deficiency responses. Plant J. 84, 309–322. doi: 10.1111/tpj.12995
Zamioudis, C., and Pieterse, C. M. J. (2011). Modulation of host immunity by beneficial microbes. Mole. Plant Microbe Interact. 25, 139–150. doi: 10.1094/MPMI-06-11-0179
Zhang, H., Sun, Y., Xie, X., Kim, M. S., Dowd, S. E., and Paré, P. W. (2009). A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 58, 568–577. doi: 10.1111/j.1365-313X.2009.03803.x
Zhou, C., Guo, J., Zhu, L., Xiao, X., Xie, Y., Zhu, J., et al. (2016). Paenibacillus polymyxa BFKC01 enhances plant iron absorption via improved root systems and activated iron acquisition mechanisms. Plant Physiol. Biochem. 105, 162–173. doi: 10.1016/j.plaphy.2016.04.025
Keywords: soybean, iron deficiency chlorosis, IDC, phenylpropanoid, transcriptomics, alkalinity stress
Citation: Waters BM, Amundsen K and Graef G (2018) Gene Expression Profiling of Iron Deficiency Chlorosis Sensitive and Tolerant Soybean Indicates Key Roles for Phenylpropanoids under Alkalinity Stress. Front. Plant Sci. 9:10. doi: 10.3389/fpls.2018.00010
Received: 23 October 2017; Accepted: 03 January 2018;
Published: 19 January 2018.
Edited by:
Felipe Klein Ricachenevsky, Universidade Federal de Santa Maria, BrazilReviewed by:
Hannetz Roschzttardtz, Pontificia Universidad Católica de Chile, ChileHuei-Hsuan Tsai, Academia Sinica, Taiwan
Copyright © 2018 Waters, Amundsen and Graef. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian M. Waters, bwaters2@unl.edu
 Brian M. Waters
Brian M. Waters Keenan Amundsen
Keenan Amundsen George Graef
George Graef