- 1Research Institute of Resource Insects, Chinese Academy of Forestry, Kunming, China
- 2Research Institute of Forest Resource Information Techniques, Chinese Academy of Forestry, Beijing, China
Rhododendron longipedicellatum is a narrow endemic species and a subject of urgent demand in the domestic market and overseas. Its fascinating shapes, brilliantly gilvous flowers, and unusual flowering time endow this species with extremely high ornamental value. However, only five wild populations of R. longipedicellatum surviving in limestone habitat have been found through elaborate field investigation, and the number of the populations decreases further or is even confronted with risk of extinction due to the damage of human activities. To enhance the protection and utilization of R. longipedicellatum, this study systematically investigated several important aspects of reproductive biology, including floral syndrome, pollen viability and stigma receptivity, petal color reflectance, breeding system, and pollination biology. The results demonstrated that arched styles not only create obvious herkogamy that avoide self-pollination, but also effectively reduce rain damage to the intrinsic characteristics of the stigma surface secretions, promoting the female fitness of R. longipedicellatum in poor weather. Pollen viability maintained a high level over the flowering period. The reflectance spectrum of petals had two peaks at wavelengths of 360 and 580 nm. Tests of OCI, P/O and artificial pollination all indicated that R. longipedicellatum was self-compatible and that the breeding system was mixed mating. Geitonogamy mediated by Bombus braccatus was the primary pollination route in the natural environment, which suggested that the breeding system of R. longipedicellatum might be evolving from selfing to outcrossing. The pollination vector of R. longipedicellatum was very specific, in that only B. braccatus was confirmed to deliver pollen to the stigmas. Visitation frequency was influenced by the activity rhythms and resource requirements of the different castes (i.e., sex). B. braccatus workers were the most effective pollinators because of higher visitation frequency and more effective contribution to fruit production, whereas the presence of B. braccatus males might enhance pollen flow within the population to a certain extent. Finally, these findings not only provided a reliable theoretical basis for hybridization breeding of R. longipedicellatum as parents, but also laid a solid foundation for further molecular biology studies to more broadly reveal the mechanisms of its endangerment in the future.
Introduction
Reproduction is not only the most important and a relatively fragile step in the life cycle of plants but also the core of their evolutionary process. Therefore, study of the characteristics of reproductive biology of a species is indispensable in exploring the mechanisms by which it has become endangered. Numerous reports exist on the reproductive biology of Rhododendron L. (Escaravage et al., 1997; Mejias et al., 2002; Escaravage and Wagner, 2004; Stout, 2007; Ono et al., 2008; Epps et al., 2015; Kuswantoro, 2017). However, very few studies exist of the reproductive biology of Rhododendron species in the southwestern regions of China, where the diversity of Rhododendron is the highest; only the floral traits, flowering characteristics, pollinating insects, visitors behavior, and visitation frequency of R. excellens, R. cyanocarpum, R. siderophyllum, and R. floccigerum have been investigated (Tian, 2011; Bai et al., 2014; Ma et al., 2014b; Georgian et al., 2015).
Rhododendron L. is the largest genus in the family Ericaceae and one of the most widespread woody plants in the northern hemisphere. As the global distribution center of Rhododendron, China possesses approximately 6 subgenera and 581 species, of which 421 are endemic, and wild Rhododendron species are distributed in all provinces except Xinjiang and Ningxia (Chamberlain et al., 1996; Fang et al., 2005; Cai et al., 2016). As a celebrated dictum goes in European and American countries, “If it were not for the rhododendrons of China, there would be no garden in the western world.” Meanwhile, Rhododendron plants play important roles in maintaining biodiversity, preserving water and soil, and stabilizing the ecosystem. However, in the current century, the genetic resources of wild rhododendrons have been damaged severely due to the constant increase in human social and economic activities, and some species have become critically endangered (Ma et al., 2014a). Therefore, studies of conservation centered on reproductive biology in endangered species with high ornamental value, of which Rhododendron longipedicellatum is a representative, should be performed without delay.
Rhododendron longipedicellatum is a newly discovered and highly endangered species surviving in limestone habitat in Southeast Yunnan, during field investigations of Kunming Institute of Botany from Chinese Academy of Sciences in December 2014, is an evergreen shrub of subsect. Pseudovireya with a beautiful tree form, thick petal texture, and rare gilvous appearance without spots or blotch (Cai et al., 2016). Compared with other congeneric species, such as R. sulfureum, R. wardii, R. molle, and R. caruolinea growing in the United States, its appearance is more bright and pure. Surprisingly, unlike other wild rhododendrons whose flowering times occur between March and June, the natural flowering time of R. longipedicellatum lasts from the last 10-day period of November to the first 10-day period of February. Meanwhile, R. longipedicellatum is also an excellent breeding parent with important study and exploitation value. Since 2014, our study group has found five wild populations of 80–350 plants each surviving in limestone habitat in Babu Community, Malipo County, Yunnan Province, through multiple professional field investigations. The seedlings in each population are few and relatively poor in natural regeneration. The remaining partial populations have been seriously damaged by human activity; these wild resources urgently need to be protected, as their status is of grave concern. According to IUCN Red List Categories and Criteria (IUCN, 2001), R. longipedicellatum is a critically endangered [CR B1ab (i, iii, v)] species.
Flowers shape and color are long being thought as the result of co-evolution between flower and its pollinators (Fenster et al., 2004). Bees are considered to be pollinators of many Rhododendron species (Ono et al., 2008; Georgian et al., 2015), and the effective pollinating insects for Rhododendron species can be more specialized than previously thought, with specialization toward pollination by bumblebees (Stout, 2007). Meanwhile, some studies have indicated that yellow flowers were more preferred by bees (Robinson, 1991; Shi et al., 2008). R. longipedicellatum has pure yellow flowers, arched styles and unusual flowering time, we hypothesized that (1) insect visitors are various; (2) the visitation frequency of effective pollinators is higher; and (3) arched styles may be closely relate to the visit behavior of pollinating insects of this unusual plant. In order to validate the hypothesis and understand the mating system, pollination mechanism and possible strategies for reproductive assurance of R. longipedicellatum, this study investigates the floral syndrome, pollen viability, stigma receptivity, petal reflectance spectrum, and breeding system of R. longipedicellatum, as well as the types, pollination behavior and visitation frequencies of its pollinating insects. Based on the results, we further discuss the causes of its endangerment under the lens of reproductive biology; and evaluate its endangered status more comprehensively to provide a theoretical basis for its further protection and use.
Materials and Methods
Plant Materials and Study Sites
Rhododendron longipedicellatum is a multi-branched shrub 1–3.3 m tall. Its leaves are whorled, and the leaf blade is obovate to oblong-obovate, with an emarginate leaf apex. Each inflorescence possesses 1–12 flowers, with 3- to 5.5-cm-long pedicels, and 9–12 stamens uneven in length. The filaments are densely white-pubescent in the lower half but glabrous at the base and apex. The corolla is campanulate and brightly gilvous without any spots or blotch (Figure 2A). Most of the flowers have 5 petals, while a few have 6. R. longipedicellatum is distributed in limestone habitat with an altitude of approximately 1180–1320 m.
This study was conducted in the largest known population of R. longipedicellatum (approximately 350 mature plants) (WBL, ca. 1312 m a.s.l., 104°94′ E, 23°15′ N), while a controlled trial was conducted in the second largest population (approximately 210 mature plants) (ZWL, ca. 1270 m a.s.l., 104°93′ E, 23°16′ N) for artificial bagging pollination and observation of pollinating insects. The main accompanying plants in these habitats were limestone-loving vegetation, such as Cyclobalanopsis glaucoides, Pistacia weinmanniifolia, Buxus sinica, R. simsii, Goldfussia pentstemonoides, Ternstroemia gymnanthera, Pieris japonica, Paphiopedilum malipoense, and Paphiopedilum micranthum.
The Biological Characteristics of R. longipedicellatum Flowers
Observation of Flowering Phenology and Floral Traits
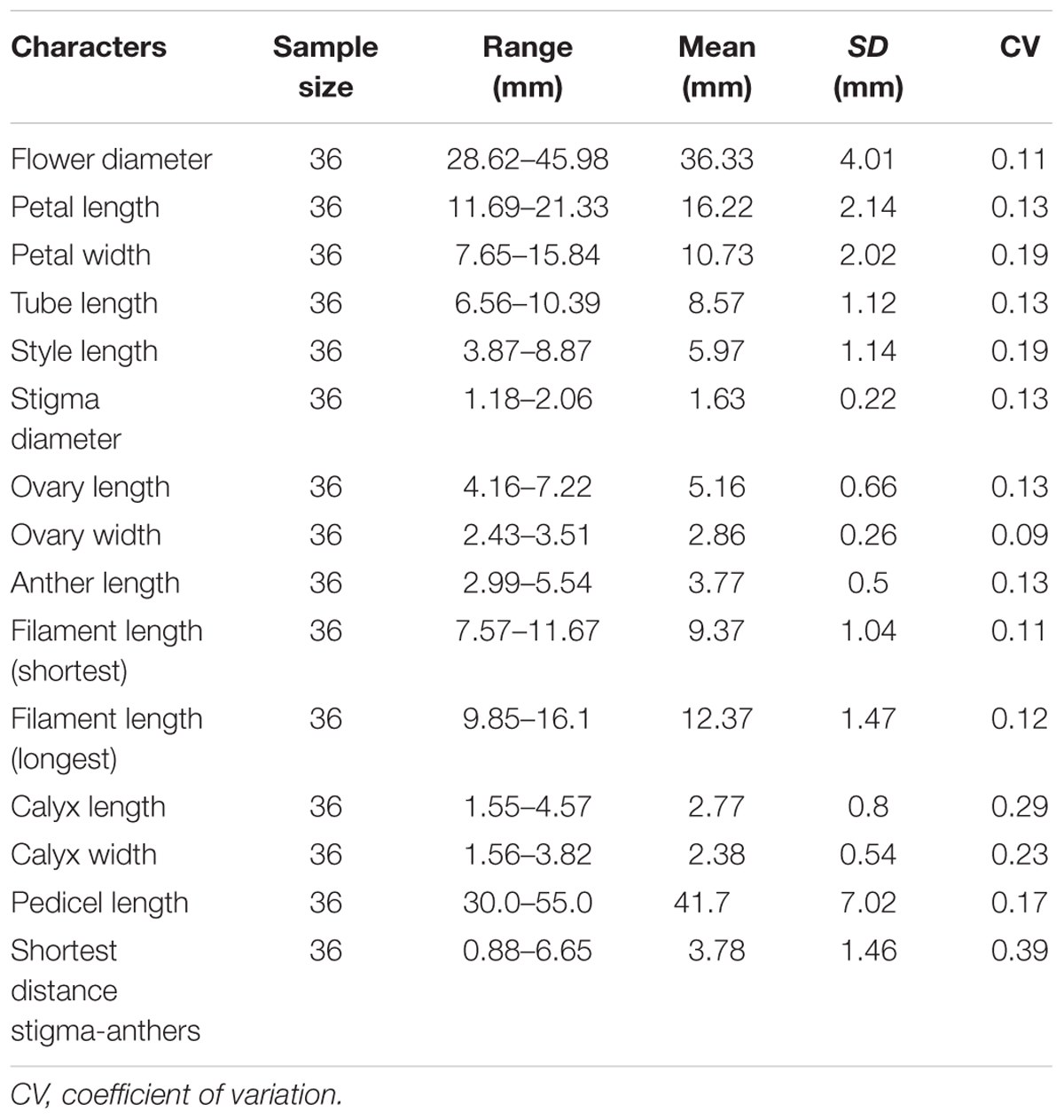
Three inflorescences from each of 15 individuals were randomly selected. The number of flowers in each inflorescence was recorded, and the time interval from the opening of the first flower up to the wilting of the last flower in each inflorescence was observed. One flower was selected from each inflorescence, with 45 flowers in total. Each flower was labeled during the sympetalous period and observed every day until opening. The flowers were observed every 2 h on the first day of anthesis and once daily after anthesis until the corolla wilted. The duration of pollen shed, stigma secretion, and stigma bending, as well as changes in floral traits such as corolla morphology and stigma color were recorded. Subsequently, 36 flowers (from 12 individuals, 3 flowers per individual) in full bloom were selected randomly to record flower diameter, the length and width of the petals, style length, stigma length, ovary length and width, anther length, longest and shortest filament lengths, calyx lobe length and width, pedicel length, and the closest distance between anthers and stigma.
Detection of Pollen Viability and Stigma Receptivity
Detection of pollen viability
(1) Dynamic changes in pollen viability on the first day of anthesis. A total of 10 flowers (from 5 individuals, 2 flowers per individual) on the first day of anthesis were selected, and pollen was collected every 2 h from 9:00 to 21:00. The pollen from one stamen was collected and painted evenly on a lattice glass slide (Matsunami Glass, Osaka, Japan). A 5% sucrose solution containing 0.5% triphenyl tetrazolium chloride (TTC) was added to the slide. The slide was placed away from light at room temperature for 2 h. Then, the ratio of red pollen grains to all observed pollen grains in 3–5 visual fields in the center of the slide was recorded under a microscope. (2) Changes in pollen viability over different days of flowering. A total of 45 flowers (from 9 individuals, 5 flowers per individual) during the sympetalous period (mainly refers to 1∼2 days before flowering) were selected randomly. Among them, five flowers were selected, and 3–5 stamens were selected from each flower at 9:00 every day to assess pollen viability until day 9 after anthesis. Pollen viability = the number of pollen grains stained red/the total number of observed pollen grains × 100%.
Detection of stigma receptivity
The above 45 flowers were used as materials. Among them, five flowers were selected randomly at 11:00 every day. The stigmas were collected and immersed in a depression slide containing benzidine-hydrogen peroxide reaction solution (1% benzidine: 3% hydrogen peroxide: ddH2O = 4:11:22, volume ratio). A magnifier was used to observe the staining site of the stigmas after 10 min, and the change of reaction solution was recorded. The stigmas were determined to have receptivity if the reaction solution showed blue color and bubbles. Stronger receptivity was indicated by a more dramatic reaction.
Flower Petal Reflectance
An S2000 miniature fiber-optic spectrometer with a PX-2 pulsed xenon lamp (Ocean Optics, Dunedin, FL, United States) was used to conduct spectral measurement of 36 mature petals (from 12 different individuals, 3 flowers per individual, one petal per flower), with a wavelength range of 250–750 nm. The increments, average detection frequency and smoothness were set as 0.38 nm, 3 and 9, respectively. As preliminary testing of the reflectance pattern at 10 randomly chosen locations along the same petal did not show any variation, only one measurement per petal was used.
The Breeding System of R. longipedicellatum
Estimation of Out-Crossing Index (OCI)
A total of 30 inflorescences (from 15 individuals, 2 inflorescences per individual) were selected randomly and their diameters were measured. Combined with the above mentioned study results of the floral syndrome, pollen viability and stigma receptivity, the hybridization index was evaluated according to the criteria of Dafni (1992).
Estimation of Pollen-to-Ovule (P/O) Ratio
A total of 30 flowers (from 10 individuals, 3 flowers per individual) that had bloomed but their anthers had not dehisced were selected randomly. The number of ovules in each flower was counted under a dissecting microscope. Approximately, 3–5 anthers were selected from each flower. The anthers were crushed, placed into an ethanol solution containing 0.5% methylene blue dye liquor with a metered volume of 5 ml, and then homogenized in an ultrasonic bath. Then, 10 μl of solution was extracted to count the number of pollen grains under a microscope, and this process was repeated five times to obtain the average number of pollen grains in each stamen. The total number of pollen grains in one flower = the average number of pollen grains in each stamen × the number of the stamens in the flower. The P/O ratio was estimated based on the criteria of Cruden (1977).
Hand Pollination Treatments
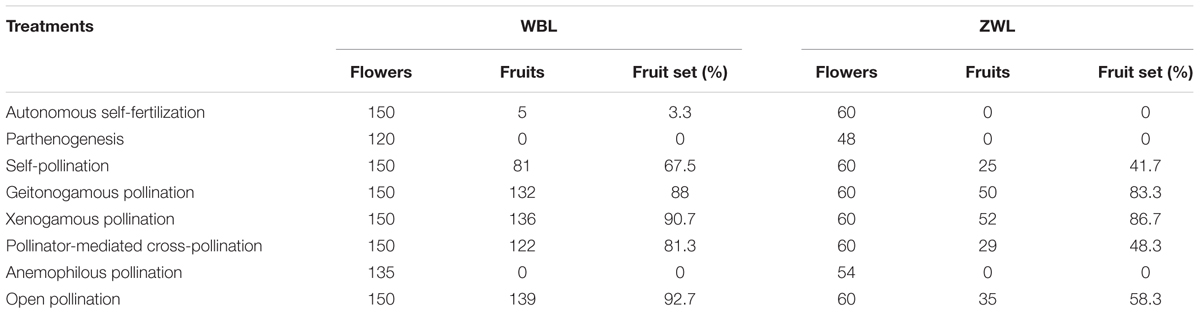
To further evaluate the breeding system of R. longipedicellatum scientifically, both the WBL and ZWL populations were tested with eight different types of treatments, as follows. For each treatment, 15 and 6 individuals with distances of >5 m between them were selected randomly in WBL and ZWL, respectively, and 8–10 flowers were selected from each individual at 9:00–10:00 am. The following tests were performed: (1) autonomous self-fertilization test, in which flower buds were bagged with waxed paper bags; (2) parthenogenesis test, in which flower buds were emasculated and bagged with waxed paper bags; (3) self-pollination test, in which flower buds were bagged with waxed paper bags and flowers were emasculated and artificially self-pollinated 4 days after anthesis before being rebagged; (4) geitonogamy test, in which flower buds were emasculated and bagged with waxed paper bags, and then flowers were pollinated using pollen from other flowers of the same individual 5 days after anthesis before being rebagged; (5) xenogamous pollination test, in which flower buds were emasculated and bagged with waxed paper bags, and then flowers were pollinated using pollen from different individuals at least 10 m apart 5 days after anthesis before being rebagged; (6) pollinator-mediated cross-pollination test, in which flower buds were emasculated; (7) anemophilous pollination test, in which flower buds were emasculated and bagged with mesh bags; and (8) open pollination, in which flowers were not manipulated. The flowers used in these tests were marked with tags, and fruit set was recorded at the end of February of the next year, when the seeds were mature but the fruits had not dehisced. The fruit set = the number of mature fruits/the number of flowers treated × 100%.
The Pollination Biology of R. longipedicellatum
Collection of Pollinating Insects and Observation of Their Visiting Behavior
The pollinating insects in both the WBL and ZWL populations were observed and collected for 5 days in total during the full-bloom period (in December 2016). According to the evaluation criteria of Stout (2007), effective pollinating insects were defined as insects that not only picked up pollen but also deposited it on a receptive stigma. Thus, the effective pollinators for R. longipedicellatum were determined, and their visiting behavior was pictured and recorded.
Detection of Visitation Frequency of Effective Pollinators
Two quadrats (5 m × 5 m) were set up in each population, with more than four individuals with 30–160 flowers present in each quadrat. The visitation frequency of effective pollinators in each population was observed at 8:00–19:00 each day for 12 days in total (WBL from December 19, 2016, to December 30, 2016; ZWL from December 31, 2016, to January 11, 2017). The number of pollinators entering the quadrat to visit the flowers, the number of flowers visited and the visit time, and the number of flowers in bloom per individual were recorded daily for each quadrat. The visitation frequency was calculated as visits per flower per hour (number of flowers visited in 1 h/number of flowers in the quadrat).
Data Analysis
The one-sample Kolmogorov–Smirnov statistic was used to detect whether the data conformed to the normal distribution (Qu et al., 2007; Ma et al., 2012). Data with a normal distribution were subjected to pairwise comparisons using Student’s t-test. Significance of differences between fruit set in the hand pollination treatments was tested using a simple χ2-test. Two-way ANOVA was used to analyze the influence of different populations and weather conditions on visitation frequency. Both SPSS 18.0 (SPSS, Chicago, IL, United States) and SigmaPlot 12.5 (SYSTAT, Chicago, IL, United States) were used for the analysis and presentation of all data.
Results
Flowering Phenology and Floral Traits of R. longipedicellatum
The flowering time of R. longipedicellatum lasted from the last 10-day period of November to the first 10-day period of February in the next year, and the period from the middle 10 days of December to the middle 10 days of January was the full-bloom period. The life spans of an individual flower and inflorescence were 7.88 ± 2.09 days and 11.88 ± 2.26 days, respectively. Inflorescences were typically umbelliform or racemose and contained 1–12 flowers, with average of six (6.85 ± 2.86, n = 45). The stamens were 9–12 in number and uneven in length. The length of the shortest filament was 7.57–11.67 mm, significantly shorter than the 9.85–16.1 mm of the longest filament (Student’s t-test, t = 12.11; p < 0.001). The minimum distance between anther and stigma was 3.78 ± 1.46 mm. The primary floral morphological indexes are shown in Table 1.
The stamens of R. longipedicellatum matured first, and some anthers were mature and could release pollen during the sympetalous period. The anthers dehisced from apical pores, and adhesive silks ran through the pollen tetrads. Although the stigmas were nearly equal to the anthers in height at this stage, they were light green in color without receptivity. With the opening of the petals, the stigmas and styles bent, becoming arched in shape, which not only effectively avoided contact with the stamens but also altered the behavior of the pollinating insects. As flowering progressed, the morphology of the stigma changed from a crack-free sphere to five light cracks and then five deep cracks, while the color changed from light green to brownish red. At the end of flowering, the color of both stigmas and styles changed simultaneously to blood red (Figure 3G).
Pollen Viability and Stigma Receptivity of R. longipedicellatum
As shown in Figure 1A, pollen viability decreased gradually from 9:00 to 17:00 on the first day of anthesis, with the highest viability (92.18%) at 9:00 and the lowest (42.85%) at 17:00. However, the viability showed a slow increase after 17:00. As shown in Figure 1B, pollen viability was higher (78.20%) during the sympetalous period, but this early viability was evidently lower than that on the first day of anthesis (92.18%, Student’s t-test, t = 22.54; p < 0.001). Pollen viability decreased gradually as pollen grains were released from the anthers but remained relatively high (48.5%) until the end of flowering (on day 9 after anthesis).
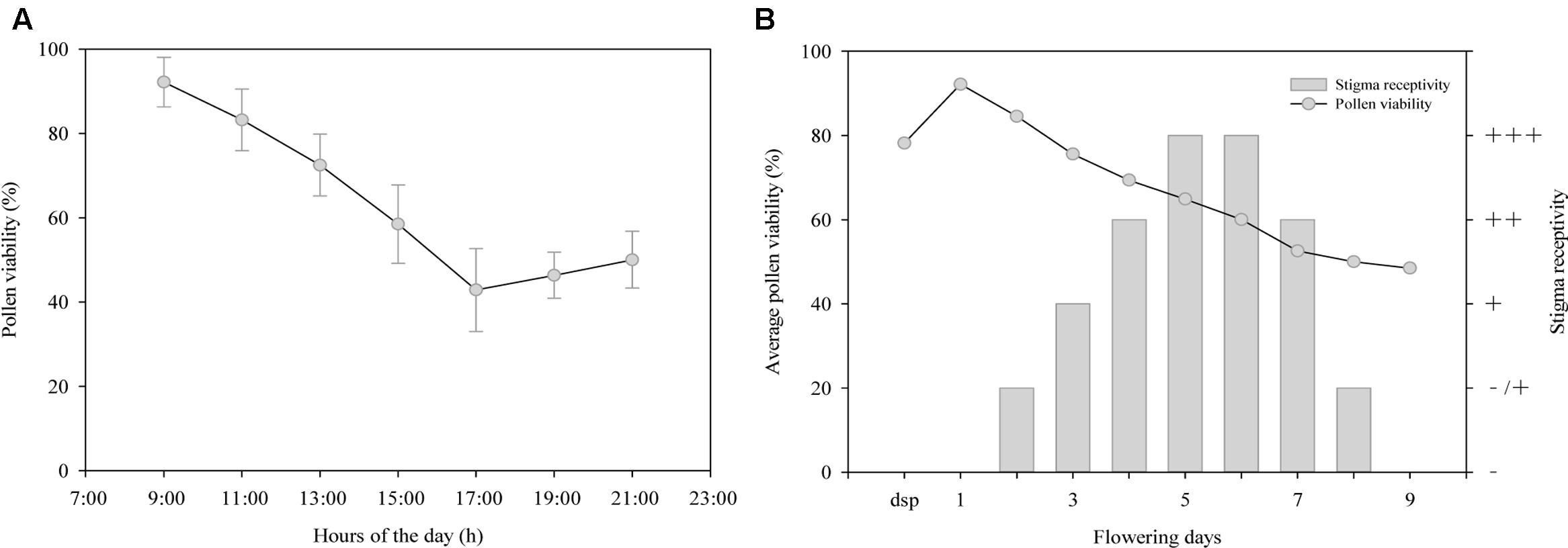
FIGURE 1. Changes of (A,B) pollen viability and (B) stigma receptivity during anthesis of Rhododendron longipedicellatum. dsp (during the sympetalous period, mainly refers to 1∼2 days before flowering), - stigmas have no receptivity, -/+ some stigmas have receptivity and some do not, + stigmas have receptivity, ++ stigmas have higher receptivity, +++ stigmas have the highest receptivity.
The results of the stigma receptivity test (Figure 1B) showed that the stigmas were not receptive during the sympetalous period or on the first day of anthesis, as they showed no secretions or staining reaction. On days 2–6 after anthesis, stigma secretions increased and reacted more dramatically with benzidine-hydrogen peroxide reaction solution, i.e., bubbles and blue stigma staining became more obvious. On days 5–6, receptivity was the highest, during which time the stigmas were transparent with copious secretions. After that point, receptivity decreased rapidly. On day 9 after anthesis, the stigmas began to lose receptivity and wither, and then the corollas and stamens were shed.
Petal Color Reflection of R. longipedicellatum
The reflectance spectrums of the 36 petals measured showed a consistent distribution mode and extremely low variation between plants (gray area in Figure 2B). All petals had marked peaks at wavelengths of 360 and 580 nm and an obvious trough at 450 nm. Meanwhile, the gilvous flowers of R. longipedicellatum had a high reflection rate with a variation range of 1.80–48.6% (Figure 2B).
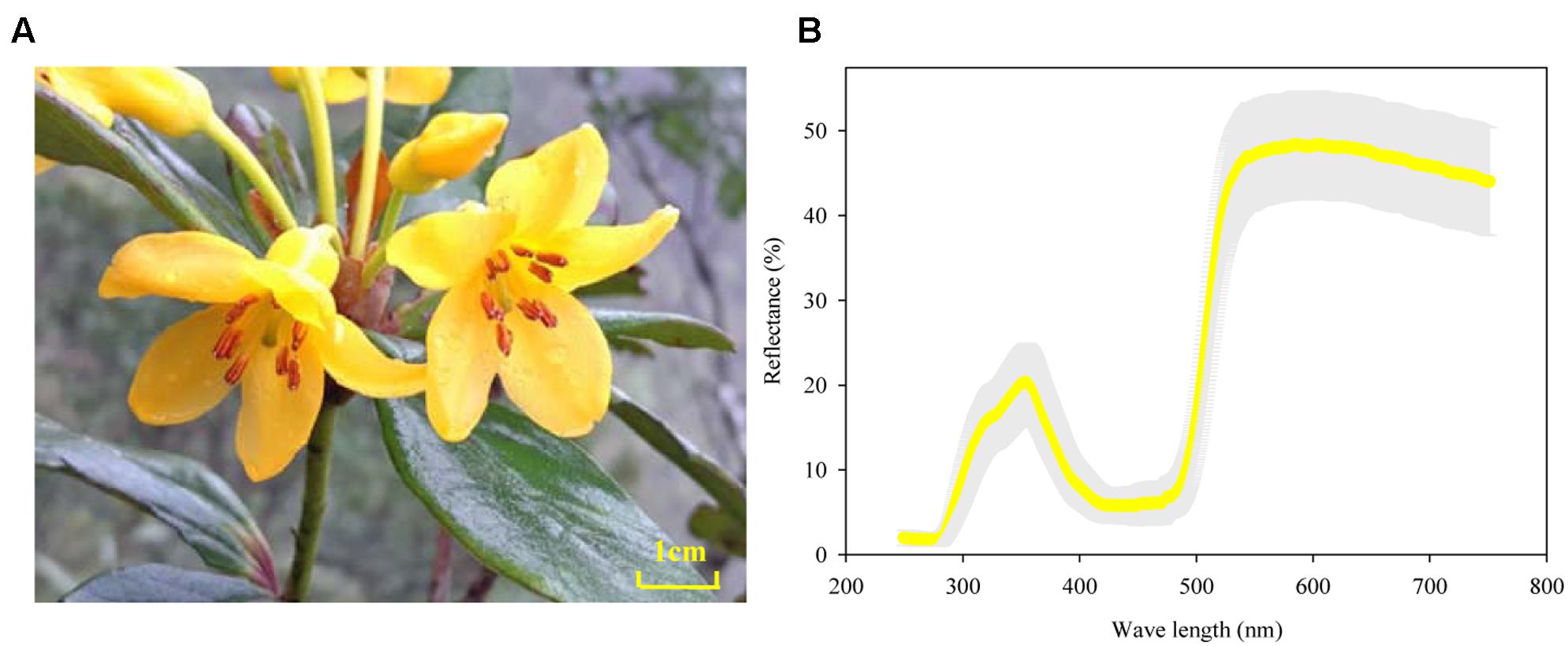
FIGURE 2. Flowers and floral reflectance spectrum of Rhododendron longipedicellatum. (A) The flower; (B) reflectance spectrum obtained by measuring 36 petals, with the standard deviation shown as a gray area.
OCI and P/O Ratio of R. longipedicellatum
The diameter of the inflorescences of R. longipedicellatum was 93.07 ± 18.53 mm (the diameter of each flower was 36.33 ± 4.01 mm), which was >6 mm and thus was scored as 3. The stamens matured first (Figure 1B), which was scored as 1. The arching of the style during flowering resulted in spatial isolation of stigmas and anthers, which was scored as 1. Therefore, the OCI of R. longipedicellatum was 5, indicating that its breeding system was partially self-compatible and outcrossing and that pollinators were needed.
The number of pollen grains, the number of ovules and the P/O ratio of R. longipedicellatum were 537486 ± 29414.2, 937.5 ± 77.10, and 573.3 ± 19.9, respectively. Therefore, the breeding system was facultative xenogamy.
Hand Pollination of R. longipedicellatum
Both the emasculated flowers bagged with waxed paper bags and those covered with mesh bags showed no fruit set at all (Table 2), demonstrating that anemophilous pollination and parthenogenesis did not occur in R. longipedicellatum. In the flower buds bagged with waxed paper bags, the fruit set was 0 in the ZWL population and only 3.3% in the WBL population. Therefore, the possibility of autonomous self-pollination was excluded. (Five flowers in WBL population produced fruits, which might be because a small amount of pollen fell on the stigmas. However, the possibility was relatively low in the wild because the arched stigmas stick close to the base of the petals, and most mature pollen grains fell on the upper half of the petals and on the leaves.) Both sites with self-pollination had relatively higher fruit set, suggesting that R. longipedicellatum was generally self-compatible. The fruit set of flower buds in the emasculation and open pollination treatments were 81.3 and 92.7% in the WBL population (χ2 = 0.828, p = 0.363) and 48.3 and 58.3% in the ZWL population (χ2 = 0.943, p = 0.331), respectively, illustrating that, under natural conditions, pollinator-mediated cross-pollination was the primary pollination method for R. longipedicellatum. Open pollination had relatively higher fruit set than the emasculation treatment, which further indicated that the orange pollen grains of R. longipedicellatum might have a certain effect on attracting pollinators. The fruit set of both geitonogamous and xenogamous pollination treatments were relatively high, 88 and 90.7% in the WBL population and 83.3 and 86.7% in ZWL, respectively, which further suggested that R. longipedicellatum was self-compatible. Meanwhile, the fruit set in both the geitonogamous and xenogamous pollination treatments were significantly higher than that of the natural control in the ZWL population (χ2 = 4.433, p = 0.035 for geitonogamy vs. control; χ2 = 5.800, p = 0.016 for xenogamy vs. control), demonstrating that pollen limitation existed at this site.
Pollinating Insects and Their Visiting Behavior
A total of six kinds of pollinating insects from three orders and four families were found (Table 3). The visitation frequencies of Bombus braccatus (males and workers) and Syrphus ribesii were the highest, while those of Aphid sp. and Apis cerana were rare. The pollinators were similar in both populations except that no B. braccatus males or A. cerana were found in the ZWL population. The pollinators of R. longipedicellatum could be classified into two types according to their visiting behavior. One was disoperative insects that gnawed flower organs such as filaments, petals and stigmas, such as ants and Aphid sp., but this kind of insect had no contribution to pollination. The other was foraging insects that were rewarded by pollen and nectar. However, only one insect was identified as an effective pollinator, as defined by Stout (2007). Our observations suggest that pollination of R. longipedicellatum is nearly exclusively effected by B. braccatus (males and workers). Although syrphids visited at a higher frequency, they mainly picked up pollen grains from pedicels, styles and leaves, while only a few of them alighted on anthers to collect pollen directly; none of their body parts carried pollen or touched stigmas. In the WBL population, A. cerana visited R. longipedicellatum four times, but these insects only collected pollen without touching the stigmas with their bodies. Therefore, A. cerana was not an effective pollinator.
The style of R. longipedicellatum was bent into an arch, which led to herkogamy. In addition, this unusual shape was mutually adapted to the pollination habit of B. braccatus. The range of activity of B. braccatus was the space between adjacent flowers or inflorescences on one plant; B. braccatus moved among different plants infrequently. When collecting pollen, the whole body of B. braccatus alighted on one petal, with two mesopedes and one of the metapedes fixed on this petal to balance its body, whereas the pretarsi and claws of the other metapede were fixed on the edge of an adjacent petal to collect the pollen delivered from the corresponding propede (Figure 3A). One of the propedes was used to reel linear pollen silks from the anther pores and attach the bundled pollen to the digitus and tibias of the corresponding metapedes, while the other propede was fixed on the filament to balance the body (Figure 3B). When collecting nectar, B. braccatus fixed all feet on the petals and filaments, placed its head into the middle part of the filament, and sucked in the nectar using its long rostrum of approximately 7.3–8.7 mm (Figure 3H). The nectary pore selected by B. braccatus to collect nectar was located in the base of the second petal of the stigma in a counter-clockwise direction (Figure 3I). After pollen and nectar were collected, B. braccatus remained on the corolla to manage the pollen (Figure 3C). A pollen mass of approximately half the size of the stigma was managed and placed on the claws of the metapedes, while the rest of the pollen was swept into the pollen collets on the metapedes. Then, B. braccatus flew to the next flower (Figure 3D), where the sticky pollen mass was brought into light contact with the stigma by the metapedes (Figures 3E,F), and pollen or nectar were collected by the same method as above. During the period of maximum B. braccatus visitation frequency, most flowers were pollinated 1–3 times.

FIGURE 3. The pollination process of Bombus braccatus, the only effective pollinator of Rhododendron longipedicellatum. (A) B. braccatus alighting on the corolla. (B) B. braccatus collecting pollen and reeling linear pollen silks. (C) B. braccatus managing the pollen on the corolla. (D) B. braccatus delivering the managed pollen mass to the next flower. (E,F) B. braccatus pollinating by claws and pretarsi of metapedes. (G) Fruitlet after successful pollination (expanded ovary and blood red stigmas and styles). (H) B. braccatus sucking nectar on the first day of anthesis. (I) Punctured nectary pore. The B. braccatus individuals in (A–G) were workers, among which the one in (F) had one body color while the rest had another color; the B. braccatus in (H) was a male.
Visitation Frequency of Effective Pollinators
During the observational period, sunny days, cloudy days, and rainy days occurred at rates of 46, 29 and 25%, respectively. B. braccatus was the sole effective pollinator in both populations, but differences in the castes (i.e., sex) were present. In the WBL population, both B. braccatus workers of two different colors and males (28% of males and 72% of workers) occurred, whereas in the ZWL population, only B. braccatus workers of one color were present. The foraging time of one B. braccatus on the same flower and inflorescence was 11.71 ± 5.54 s (range: 4–28 s) and 33.45 ± 11.93 s (range: 9–62 s). The average visitation frequency was 3.37 ± 1.05 times flower-1 h-1 in the WBL population, which was evidently higher than the 0.95 ± 0.34 times flower-1 h-1 in the ZWL population (Figures 4A–C). Two-way ANOVA indicated that different populations and weather conditions had both a significant effect on the visitation frequency of B. braccatus (populations: F = 11.39, p = 0.002; weather conditions: F = 3.54, p = 0.042), with the interaction term between the two factors being not significant (F = 1.23; p = 0.307). In the WBL population, the visitation frequency of B. braccatus workers was markedly higher than that of B. braccatus males (Student’s t-test, t = 6.0203; p < 0.001), which, combined with the fact that only B. braccatus workers were found in the ZWL population, suggested that B. braccatus workers were the most effective pollinators of R. longipedicellatum. On sunny days, the average change tendencies in total visitation frequency of B. braccatus (workers and males) in both populations were almost consistent; all were in a double-peak curve with maximum visitation frequencies occurring from 10:00–11:00 and 16:00–17:00 and minimum at 13:00–14:00 due to high temperature. After 19:30, very few B. braccatus appeared (Figure 4D).
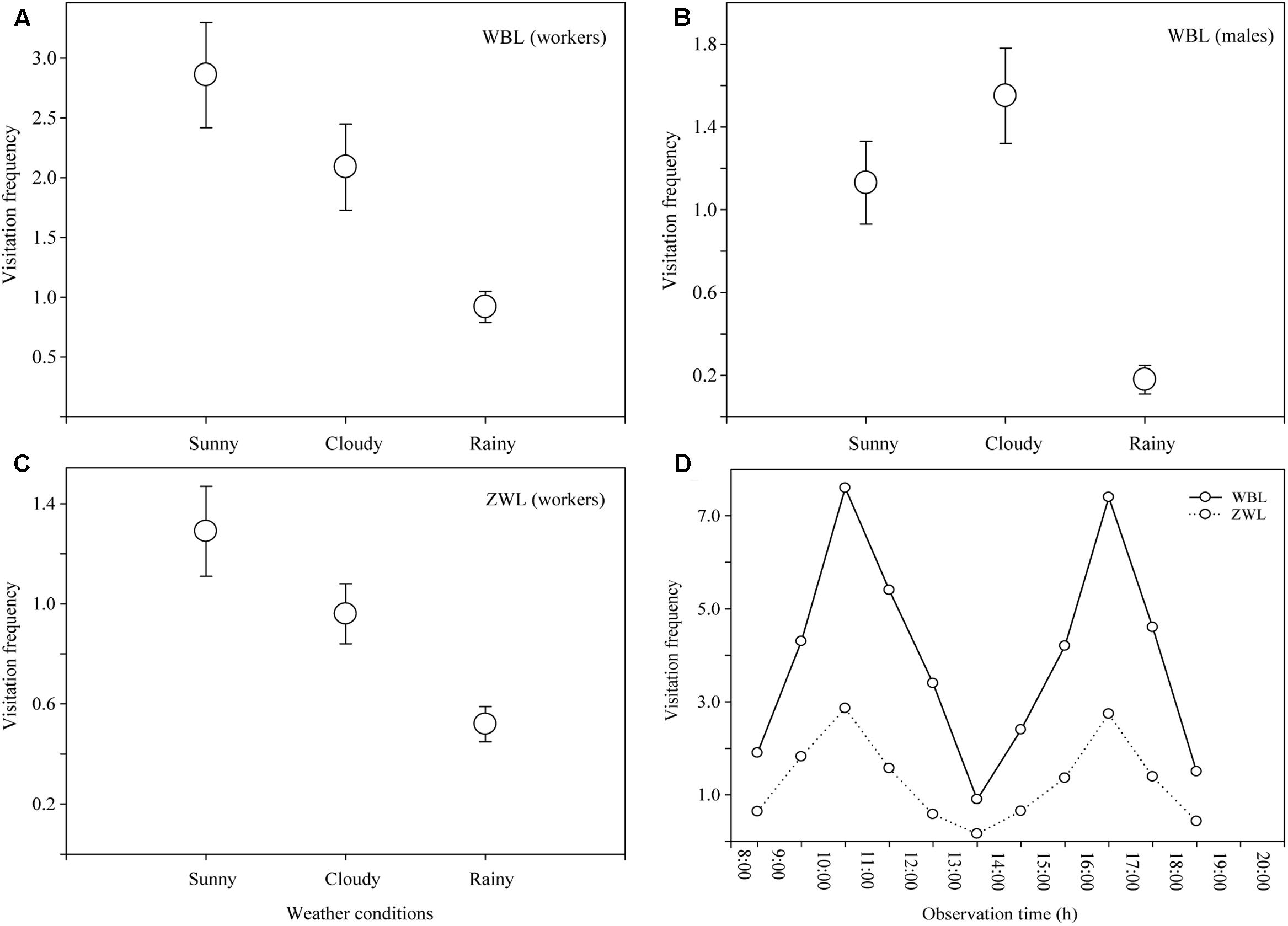
FIGURE 4. Visitation frequency (visits per flower per hour) of Bombus braccatus under different weather conditions in different populations. (A) B. braccatus workers (of two colors) in the WBL population. (B) B. braccatus males in the WBL population. (C) B. braccatus workers (of one color) in the ZWL population. (D) Curve showing the average change tendency in total visitation frequency in both populations on sunny days.
Discussion
Floral Traits, Pollen Viability, and Stigma Receptivity
Flower color is one of the most important ornamental characteristics for rhododendrons. At present, the breeding of flower color in rhododendrons tends to favor pure-colored flowers, especially gilvous rhododendrons. The flowering time of most wild rhododendrons falls in the period from March to June; therefore, the cultivation of Rhododendron varieties with different flowering times is also an important goal for the reproduction of rhododendrons (Li et al., 2008; Lan et al., 2012; Xiao et al., 2016). Rare gilvous flowers and distinctive flowering time (from the last 10-day period of November to the first 10-day period of February) will make R. longipedicellatum popular among rhododendrons enthusiasts.
Rhododendron longipedicellatum is a typical entomophilous flower. Its corolla is campanulate and marked by slight zygomorphy, and its thick petals provide a useful landing platform for pollinating insects. Many entomophilous flowers tend to adapt to insect visitation in morphological structure or floral variation, especially plants with specialized pollinating insects (Huang and Guo, 2000). In R. longipedicellatum, the arched styles are in close association with its specialized pollinator, B. braccatus. The styles bend as the petals open on the first day of anthesis, which effectively avoids body contact with the pollinator, since only the pollen-carrying feet of B. braccatus can deliver pollen to the stigma successfully. The arched styles can also effectively reduce rain damage to the intrinsic characteristics of the stigma surface secretions, promoting the female fitness of R. longipedicellatum in poor weather.
The stamens and pistils of R. longipedicellatum have a degree of dichogamy. When the stigma receptivity is the strongest, the pollen viability decreases, though it remains higher than 50%. Although a transient overlap period exists between male and female fertility, the arched styles visibly promote herkogamy by effectively avoiding the possibility of self-pollination. The results of a test using single, unemasculated flowers covered with waxed paper bags have also verified the above conclusion. Henceforth, when using R. longipedicellatum for crossbreeding, the pollen at 9:00 on the first day of anthesis are ideal if it is used as the male parent, and artificial pollination conducted on days 5–6 after anthesis will be more beneficial to seed set if it is used as the female parent.
Flower Petal Reflectance
The reflected light of petals could also act as a signal for attracting potential pollinators (Dyer et al., 2012). The study of Ma et al. (2015) on flower color polymorphism in R. cyanocarpum showed that the reflectance spectrum of pink corollas had two peaks at wavelengths of 430 and 650 nm, while the white flowers had only one peak at 430 nm, and their shared pollinators (bumblebees) had a notably higher frequency of visits to pink flowers than to white flowers. The flowers of R. longipedicellatum are gilvous in color, which leads to high visitation frequency by B. braccatus on sunny days and some visits on cloudy and rainy days. The reflectance spectrum of its corollas also has two peaks, at wavelengths of 360 and 580 nm with a trough at 450 nm. The wavelengths of these peaks and troughs differ from those of R. delavayi and R. cyanocarpum (Ma et al., 2015, 2016). B. braccatus has three types of photoreceptor cells to distinguish substances at close range, namely, ultraviolet (short wavelength), blue (medium wavelength), and green (long wavelength) (Giurfa et al., 1996, 1997; Frey et al., 2011; Ma et al., 2014b), which have their highest sensitivities near 340, 430, and 540 nm, respectively (Peitsch et al., 1992). The spectrum data of R. longipedicellatum suggests that the bright yellow flowers attract B. braccatus to collect pollen and nectar mainly by stimulating the ultraviolet and green photoreceptors. Therefore, yellow flowers in Rhododendron species are markedly different from pink, red and white flowers in their reflectance spectrum, which may be one of the reasons why yellow flowers are more preferred by bees (Robinson, 1991; Shi et al., 2008).
Breeding System
Breeding system is a manifestation of the interaction between the internal genetic mechanisms of plants and the external environment, which plays an important role in the process of evolution and feature variation in plants (Grant, 1971). Knowledge about the breeding system of a species is beneficial in making clear the characteristics of evolution and life history caused by different genetic and ecological factors, which influence allogamy and autogamy (Escaravage et al., 1997). The OCI and P/O ratio as well as the results of hand pollination treatments in R. longipedicellatum revealed that R. longipedicellatum was self-compatible and cross-fertile, and its breeding system was mixed mating. However, its arched styles effectively counteract self-pollination, indicating that the breeding system of R. longipedicellatum may be in the process of evolving from selfing to outcrossing. Combining these results with the visiting behavior of B. braccatus, an effective pollinator for R. longipedicellatum, this study concluded that B. braccatus-mediated geitonogamy might be the primary pollination method for R. longipedicellatum under natural conditions. There are two reasons, as follows: (1) B. braccatus often visits adjacent flowers and moves frequently within one inflorescence or different inflorescences on the same plant, concentrating on an area of high flower density in order to save autologous energy and substance; and (2) R. longipedicellatum in wild populations tends to reproduce by transverse stems, and thus, most adjacent plants are the vegetatively propagated offspring of one original plant, which further promotes the occurrence of geitonogamy. Geitonogamy is also found in multiple congeneric plants such as R. ferrugineum and R. arboretum (Escaravage and Wagner, 2004; Wheelwright et al., 2006; Stout, 2007; Ono et al., 2008).
Inbreeding is relatively common in species with small numbers of populations and individuals, narrow habitats, or small founding populations (Lee et al., 2004; Qiao et al., 2010; Tian, 2011). Inbreeding or selfing is of great significance for the preservation and reproduction of these species, but it also leads to inbreeding depression. The level of inbreeding depression can be evaluated primarily by comparing the fruit sets obtained by self-pollination and cross-pollination (Martínez-García et al., 2012). Nevertheless, the influence of inbreeding depression on offspring fitness can manifest at any stage of the life history, such as fruit set, number and germination rate of seeds, and even subsequent growth and development, as well as morphogenesis. Whether R. longipedicellatum has inbreeding depression and the stages at which it might manifest remain unknown. Additionally, the seedlings of R. longipedicellatum are rarely seen under natural conditions, which may indicate a low germination rate due to inbreeding depression, decreased seedling fitness, or an inappropriate germination environment for seeds. These questions must be further studied in the future.
Specialized Pollinators
Bumblebees can carry large amounts of pollen securely due to their large bodies. These insects are among the most important pollinators of diverse temperate plants with poricidally dehiscent anthers, including the plants of the Ericaceae (Free, 1970; Reader, 1977; Dorr, 1981; Escaravage and Wagner, 2004). The pollinators of Rhododendron species are diverse, including 4 orders and 16 families (Tian, 2011). However, most studies worldwide have indicated that bumblebees were the primary effective pollinators (Mejias et al., 2002; Fenster et al., 2004; Stout, 2007; Ono et al., 2008). Of the Rhododendron species reported domestically, some have specialized pollinators: the effective pollinating insects are B. festivces and B. richardsi for endangered R. cyanocarpum (Ma et al., 2014a) and A. cerana for R. excellens (Tian, 2011). Our study demonstrated that B. braccatus was the only insect that not only foraged and carried pollen but also positively delivered pollen to the stigmas when visiting R. longipedicellatum, which supported the point of Stout (2007). In addition, compared with other Rhododendron species, the effective pollinating insects for R. longipedicellatum were more specialized.
On cloudy and rainy days when the temperature decreases to 5–14°C, B. braccatus still maintains a high visitation frequency (WBL, cloud: 3.63 ± 0.15 times flower-1h-1; rain: 1.09 ± 0.15; ZWL, cloud: 0.96 ± 0.12; rain: 0.52 ± 0.07), which can be attributed to its strong adaptation to an environment with low temperature and high humidity. Compared with most other pollinating insects, B. braccatus can forage in harsher environments (Ng and Corlett, 2000; Escaravage and Wagner, 2004).
Visitation Frequency
During the observation of pollinating insects, no significant difference was found between the two populations based on weather conditions, but the visitation frequency of B. braccatus in the WBL population was notably higher than that in the ZWL population, which might be the primary cause of the high fruit set (92.7%) and lack of pollen limitation in this population under natural conditions. The difference in visitation frequency between the two populations is mainly caused by artificial factors: the ZWL population is closer to the living area of local residents where human activities are more frequent, thus leading to the persistent shrinking of the ZWL population, whereas the WBL population is more remote from the living area and free from human interference. Moreover, the difference may also be closely associated with differences in the local bumblebee fauna and forms of selection for plant reproductive traits among populations (Medel et al., 2007; Ono et al., 2008). Only a small number of B. braccatus workers of one body color have been found near the ZWL population, whereas a large number of B. braccatus males and workers of two body colors have been found near the WBL population.
On sunny days, the average visitation frequency of B. braccatus was 3.96 ± 0.20 times flower-1h-1 (7.63 maximum) in the WBL population, markedly higher than that of bumblebees in most other congeneric plants (Escaravage and Wagner, 2004; Ono et al., 2008). There are two primary reasons: (1) Only one plant in the area, P. malipoense, has an overlapping flowering time, and a microscopic examination of samples of B. braccatus captured in the wild has found that all pollen carried on the whole body of B. braccatus is the tetrad pollen characteristic of R. longipedicellatum. The flowers of R. longipedicellatum are the primary food source for B. braccatus in this area from the last 10-day period of November to the first 10-day period of February. (2) The success rate of pollination depends on the visiting preference of pollinating insects. Nectariferous plants or those with striking colors such as yellow or blue–violet greatly increase their attractiveness to bumblebees (Shi et al., 2008). The flowers of R. longipedicellatum are bright gilvous in color, which greatly increases their attraction to B. braccatus.
In the WBL population, the visitation frequency of B. braccatus workers is considerably higher than that of B. braccatus males, which may be related to their varied visiting habits and resource requirements. The daily routine of males consists of patrolling flights to seek unmated queens, which results in large fluctuations in their searching and foraging behaviors over the day, whereas workers constantly and intensively collect pollen and nectar for the larvae (Alcock and Alcock, 1983; Jennersten et al., 1991; Ono et al., 2008). Males are termed “vagabonds” because they have large foraging range (Williams and Dodson, 1972). In the trial, the flying distance of B. braccatus males was observed to be notably longer than that of B. braccatus workers, which increased the opportunity for xenogamy. This large range might act as a compensation mechanism for geitonogamy, decreasing the influence of inbreeding depression in R. longipedicellatum to some extent.
Conservation Implications
In southwestern regions of China many species of Rhododendron especially those grow at low altitude face increasing threats due to anthropogenic activities and habitat degradation (Ma et al., 2014a). R. longipedicellatum is a newly discovered and critically endangered species, has a very limited distribution range with an altitude lower than 1400 m and only five extant limestone populations of less than 2000 individual plants in the southeast part of Yunnan province in China. Based on this situation, we propose that it should be protected for Wild Plants with Extremely Small Population program in China. Comprehensive studies on the reproductive biology are very important for small population species effective conservation and management planning. Our study of the reproductive biology of R. longipedicellatum indicates that the major factors that threaten the persistence of its population are ecological factors (e.g., habitat fragmentation) rather than sexual reproduction early hindered, because of the presence of high fruit set under natural conditions. Considering its habitat fragmentation and continued human disturbance, we suggest that priority for in situ alongside the remaining populations should be given to maintain the appropriate effective population size, especially the most severely damaged of the ZWL population, it should be regarded as a priority protection of ESU (evolutionary significant unit). Meanwhile, the local government and forest police should actively publicize to the surrounding masses to protect R. longipedicellatum, improve the protection awareness and participation enthusiasm of the local residents. Ex situ conservation and seed collection should then be carried out to provide provenance for its future recovery. Furthermore, considering the isolation of the residual populations of R. longipedicellatum in the wild and the limited pollination range of B. braccatus, corresponding measures should be undertaken to protect R. longipedicellatum, such as artificial enhancement of pollen flow among populations, reintroduction of plants in different populations, and artificial long-distance pollination to relieve the genetic pressure of geitonogamy on R. longipedicellatum. Additionally, further investigations are required to characterize the genetic diversity and structural patterns of this species within and between the extant five natural populations to provide genetic data assistant current and future conservation activities.
Conclusion
Rhododendron longipedicellatum can attract pollinating insects to achieve successful reproduction with its gilvous flowers, unusual flowering time, distinctive light reflection patterns, strong pollen viability, and high output of pollen and nectar. Pollination treatments indicated that R. longipedicellatum was self-compatible and, cross-fertile and that the breeding system was mixed mating. The floral trait of arched styles not only leads to herkogamy and avoids the occurrence of autonomous self-fertilization at the single-flower level but also represents an adaptation to the pollination behavior of B. braccatus, its only effective pollinator, B. braccatus-mediated geitonogamy is the primary pollination mode for R. longipedicellatum. The interference of artificial factors greatly impacts the visitation frequency and caste distribution of B. braccatus, thus further influencing fruit set in R. longipedicellatum. Therefore, as to the protection of R. longipedicellatum, the protection of B. braccatus, is also of particular concern. Our findings support the hypothesis that the arched styles are in close association with the visit behavior of pollinating insects, and specialized pollinators (B. braccatus) can play a driver of evolution in R. longipedicellatum. These information about the reproductive biology of R. longipedicellatum not only has important implications for conservation and management of this special threatened plant but also is helpful for breeding system and pollination ecology studies in the multiple endangered Rhododendron species.
Author Contributions
HM designed the study; ZL, HM, and LF collected the materials; TL, LF, XXL, and YW performed the experiments; TL, HM, LF, and XFL analyzed and interpreted the data; TL, XFL, and HM wrote the manuscript. All authors agree to be accountable for the final manuscript.
Funding
This work was supported by Technology Innovation Talent Project of Yunnan Province (2016HB007).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors sincerely thank Mr. Chengyun Zheng, Mr. Yongan Wang, Dr. Guohai Qi, and Mr. Fukuan Deng for assistance in the field. They are very grateful to Dr. Zhenghua Xie from the Research Institute of Resource Insects, Chinese Academy of Forestry for insect identification.
References
Alcock, J., and Alcock, J. P. (1983). Male behaviour in two bumblebees, Bombus nevudensis auricomus and B. griseicollis (Hymenoptera: Apidae). J. Zool. 200, 561–570. doi: 10.1111/j.1469-7998.1983.tb02816.x
Bai, T., Guan, W., Song, J., Xie, W., and Li, S. (2014). Flowering characteristics and breeding system of Rhododendron siderophyllum. J. West China For. Sci. 43, 47–53.
Cai, L., Neilsen, J., Dao, Z. L., and Ma, Y. P. (2016). Rhododendron longipedicellatum (Ericaceae), a new species from Southeastern Yunnan, China. Phytotaxa 282, 296–300. doi: 10.11646/phytotaxa.282.4.7
Chamberlain, D., Hyam, R., Argent, G., Fairweather, G., and Walter, K. S. (1996). The Genus Rhododendron: Its Classification and Synonymy. Edinburgh: Royal Botanic Garden Edinburgh, 181.
Cruden, R. W. (1977). Pollen-ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31, 32–46. doi: 10.1111/j.1558-5646.1977.tb00979.x
Dafni, A. (1992). Pollination Ecology: A Practical Approach. New York, NY: Oxford University Press, 59–89.
Dyer, A. G., Boyd-Gerny, S., McLoughlin, S., Rosa, M. G. P., Simonov, V., and Wong, B. B. M. (2012). Parallel evolution of angiosperm colour signals: common evolutionary pressures linked to hymenopteran vision. Proc. R. Soc. B Biol. Sci. 279, 3606–3615. doi: 10.1098/rspb.2012.0827
Epps, M. J., Allison, S. E., and Wolfe, L. M. (2015). Reproduction in flame azalea (Rhododendron calendulaceum, Ericaceae): a rare case of insect wing pollination. Am. Nat. 186, 294–301. doi: 10.1086/682006
Escaravage, N., Pornon, A., Doche, B., and Till-Bottraud, I. (1997). Breeding system in an alpine species: Rhododendron ferrugineum L. (Ericaceae) in the French northern Alps. Can. J. Bot. 75, 736–743. doi: 10.1139/b97-084
Escaravage, N., and Wagner, J. (2004). Pollination effectiveness and pollen dispersal in a Rhododendron ferrugineum (Ericaceae) population. Plant Biol. 6, 606–615. doi: 10.1055/s-2004-821143
Fang, M. Y., Fang, R. Z., He, M. Y., Hu, L. Z., Yang, H. B., Qin, H. N., et al. (2005). “Ericaceae,” in Flora of China, eds Z. K. Wu and P. H. Raven (Beijing: Science Press), 260–445.
Fenster, C. B., Armbruster, W. S., Wilson, P., Dudash, M. R., and Thomson, J. D. (2004). Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 35, 375–403. doi: 10.1146/annurev.ecolsys.34.011802.132347
Frey, F. M., Dunton, J., and Garland, K. (2011). Floral color variation and associations with fitness-related traits in Malva moschata (Malvaceae). Plant Species Biol. 26, 235–243. doi: 10.1111/j.1442-1984.2011.00325.x
Georgian, E., Fang, Z., Emshwiller, E., and Pidgeon, A. (2015). The pollination ecology of Rhododendron floccigerum Franchet (Ericaceae) in Weixi, Yunnan Province, China. J. Poll. Ecol. 16, 72–81.
Giurfa, M., Vorobyev, M., Brandt, R., Posner, B., and Menzel, R. (1997). Discrimination of coloured stimuli by honeybees: alternative use of achromatic and chromatic signals. J. Comp. Physiol. A 180, 235–243. doi: 10.1007/s003590050044
Giurfa, M., Vorobyev, M., and Kevan, P. (1996). Detection of coloured stimuli by honeybees: minimum visual angles and receptor specific contrasts. J. Comp. Physiol. A 178, 699–709. doi: 10.1007/BF00227381
Huang, S. Q., and Guo, Y. H. (2000). Advances in pollination biology. Chin. Sci. Bull. 45, 225–237. doi: 10.1007/BF02898884
IUCN (2001). IUCN Red List Categories and Criteria: Version3.1. Gland: IUCN Species Survival Commission, 30.
Jennersten, O., Morse, D. H., and O’Neil, P. (1991). Movements of male and worker bumblebees on and between flowers. Oikos 62, 319–324. doi: 10.2307/3545496
Kuswantoro, F. (2017). Flower-insect visitor interaction: case study on Rhododendron inundatum Sleumer in Bali Botanic Garden. J. Trop. Biodivers. Biotechnol. 2, 35–38. doi: 10.22146/jtbb.25443
Lan, X., Zhang, L. H., Zhang, J. Z., Cui, H. X., Jiang, C. D., and Shi, L. (2012). Research progress of Rhododendron breeding. Acta Hortic. Sin. 39, 1829–1838.
Lee, P. L. M., Patel, R. M., Conlan, R. S., and Hipkin, C. R. (2004). Comparison of genetic diversities in native and alien populations of hoary mustard (Hirschfeldia incana (L.) Lagreze-Fossat). Int. J. Plant Sci. 165, 833–843. doi: 10.1086/422043
Li, C. H., Wang, L. S., Shu, Q. Y., Xu, Y. J., and Zhang, J. (2008). Pigments composition of petals and floral color change during the blooming period in Rhododendron mucronulatum. Acta Hortic. Sin. 35, 1023–1030.
Ma, Y. P., Nielsen, J., Chamberlain, D. F., Li, X. Y., and Sun, W. B. (2014a). The conservation of Rhododendrons is of greater urgency than has been previously acknowledged in China. Biodivers. Conserv. 23, 3149–3154. doi: 10.1007/s10531-014-0764-9
Ma, Y. P., Wu, Z. K., Dong, K., Sun, W. B., and Marczewski, T. (2014b). Pollination biology of Rhododendron cyanocarpum (Ericaceae): an alpine species endemic to NW Yunnan, China. J. Syst. Evol. 53, 63–71. doi: 10.1111/jse.12114
Ma, Y. P., Wu, Z. K., Tian, X. L., Zhang, C. Q., and Sun, W. B. (2012). Growth discrepancy between filament and style facilitates autonomous self-fertilization in Hedychium yunnanense (Zingiberaceae). Plant Ecol. Evol. 145, 185–189. doi: 10.5091/plecevo.2012.652
Ma, Y. P., Wu, Z. K., Zhang, C. Q., and Sun, W. B. (2015). Flower color polymorphism in Rhododendron cyanocarpum (Ericaceae), an endangered Alpine species endemic to NW Yunnan, China. Plant Divers. Resour. 37, 21–28.
Ma, Y. P., Xie, W. J., Sun, W. B., and Marczewski, T. (2016). Strong reproductive isolation despite occasional hybridization between a widely distributed and a narrow endemic Rhododendron species. Sci. Rep. 6:19146. doi: 10.1038/srep19146
Martínez-García, P. J., Dicenta, F., and Ortega, E. (2012). Anomalous embryo sac development and fruit abortion caused by inbreeding depression in almond (Prunus dulcis). Sci. Hortic. 133, 23–30. doi: 10.1016/j.scienta.2011.10.001
Medel, R., Valiente, A., Botto-Mahan, C., Carvallo, G., Pérez, F., Pohl, N., et al. (2007). The influence of insects and hummingbirds on the geographical variation of the flower phenotype in Mimulus luteus. Ecography 30, 812–818. doi: 10.1111/j.2007.0906-7590.05175.x
Mejias, J. A., Arroyo, J., and Ojeda, F. (2002). Reproductive ecology of Rhododendron ponticum (Ericaceae) in relict Mediterranean populations. Bot. J. Linn. Soc. 140, 297–311. doi: 10.1046/j.1095-8339.2002.00103.x
Ng, S. C., and Corlett, R. T. (2000). Comparative reproductive biology of the six species of Rhododendron (Ericaceae) in Hong Kong, South China. Can. J. Bot. 78, 221–229. doi: 10.1139/b99-181
Ono, A., Dohzono, I., and Sugawara, T. (2008). Bumblebee pollination and reproductive biology of Rhododendron semibarbatum (Ericaceae). J. Plant Res. 121, 319–327. doi: 10.1007/s10265-008-0155-y
Peitsch, D., Fietz, A., Hertel, H., Souza, J. D., Ventura, D. F., and Menzel, R. (1992). The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A 170, 23–40. doi: 10.1007/BF00190398
Qiao, Q., Zhang, C. Q., and Milne, R. I. (2010). Population genetics and breeding system of Tupistra pingbianensis (Liliaceae), a naturally rare plant endemic to SW China. J. Syst. Evol. 48, 47–57. doi: 10.1111/j.1759-6831.2009.00064.x
Qu, R. M., Li, X. J., Luo, Y. B., Dong, M., Xu, H. L., Chen, X., et al. (2007). Wind-dragged corolla enhances self-pollination: a new mechanism of delayed self-pollination. Ann. Bot. 100, 1155–1164. doi: 10.1093/aob/mcm209
Reader, R. J. (1977). Bog ericad flowers: self-compatibility and relative attractiveness to bees. Can. J. Bot. 55, 2279–2287. doi: 10.1139/b77-259
Shi, H. Y., Wu, J., Li, J. L., and An, J. D. (2008). Foraging preference of the bumblebee Bombus hypocrita (Hymenoptera: Apidae). Acta Entomol. Sin. 51, 946–952.
Stout, J. C. (2007). Pollination of invasive Rhododendron ponticum (Ericaceae) in Ireland. Apidologie 38, 198–206. doi: 10.1051/apido:2006071
Wheelwright, N. T., Dukeshire, E. E., Fontaine, J. B., Gutow, S. H., Moeller, D. A., Schuetz, J. G., et al. (2006). Pollinator limitation, autogamy and minimal inbreeding depression in insect-pollinated plants on a Boreal island. Am. Midl. Nat. 155, 19–38. doi: 10.1674/0003-0031(2006)155[0019:PLAAMI]2.0.CO;2
Williams, N. H., and Dodson, C. H. (1972). Selective attraction of male euglossine bees to orchid floral fragrances and its importance in long distance pollen flow. Evolution 26, 84–95. doi: 10.1111/j.1558-5646.1972.tb00176.x
Keywords: limestone habitat, special threatened plant, flowering biology, reflectance spectrum, breeding system, pollination biology, Rhododendron
Citation: Li T, Liu X, Li Z, Ma H, Wan Y, Liu X and Fu L (2018) Study on Reproductive Biology of Rhododendron longipedicellatum: A Newly Discovered and Special Threatened Plant Surviving in Limestone Habitat in Southeast Yunnan, China. Front. Plant Sci. 9:33. doi: 10.3389/fpls.2018.00033
Received: 29 August 2017; Accepted: 09 January 2018;
Published: 31 January 2018.
Edited by:
Tian Tang, Sun Yat-sen University, ChinaReviewed by:
Yongpeng Ma, Kunming Institute of Botany (CAS), ChinaWenyan Du, Alforex Seeds LLC, United States
Copyright © 2018 Li, Liu, Li, Ma, Wan, Liu and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Ma, hortscience@163.com Liyong Fu, fuly@ifrit.ac.cn
 Taiqiang Li
Taiqiang Li Xiongfang Liu1
Xiongfang Liu1 Hong Ma
Hong Ma