- 1State Key Laboratory of Rice Biology, China National Rice Research Institute, Hangzhou, China
- 2State Key Laboratory of Soil and Sustainable Agriculture, China Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China
- 3Nuclear Technology Biotechnology Research Institute, Xinjiang Academy of Agricultural Sciences, Ürümqi, China
- 4Zhejiang Academy of Agricultural Sciences, Hangzhou, China
Hydrogen sulfide (H2S) plays a vital role in Al3+ stress resistance in plants, but the underlying mechanism is unclear. In the present study, pretreatment with 2 μM of the H2S donor NaHS significantly alleviated the inhibition of root elongation caused by Al toxicity in rice roots, which was accompanied by a decrease in Al contents in root tips under 50 μM Al3+ treatment. NaHS pretreatment decreased the negative charge in cell walls by reducing the activity of pectin methylesterase and decreasing the pectin and hemicellulose contents in rice roots. This treatment also masked Al-binding sites in the cell wall by upregulating the expression of OsSATR1 and OsSTAR2 in roots and reduced Al binding in the cell wall by stimulating the expression of the citrate acid exudation gene OsFRDL4 and increasing the secretion of citrate acid. In addition, NaHS pretreatment decreased the symplasmic Al content by downregulating the expression of OsNRAT1, and increasing the translocation of cytoplasmic Al to the vacuole via upregulating the expression of OsALS1. The increment of antioxidant enzyme [superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), and peroxidase (POD)] activity with NaHS pretreatment significantly decreased the MDA and H2O2 content in rice roots, thereby reducing the damage of Al3+ toxicity on membrane integrity in rice. H2S exhibits crosstalk with nitric oxide (NO) in response to Al toxicity, and through reducing NO content in root tips to alleviate Al toxicity. Together, this study establishes that H2S alleviates Al toxicity by decreasing the Al content in the apoplast and symplast of rice roots.
Introduction
Al toxicity is one of the most widespread factors limiting crop production, especially in acid soils. Approximately 30–50% of arable lands worldwide (and ∼21% in China) are acidic (He et al., 2012). The most important symptom of Al toxicity is the inhibition of root growth. Once Al toxicity disturbs roots, the uptake of water and nutrient elements is impaired, thereby constraining crop growth and yields (Blamey et al., 2004; Ma, 2005). The formation of an excess of reactive oxygen species (ROS) such as H2O2 and O2- in plant cells is another primary response to Al toxicity, as detected in soybean root tips (Horst et al., 1992), Cassia tora L. (Wang and Yang, 2005), Melaleuca trees (Tahara et al., 2008), rice leaves (Kuo and Kao, 2003), and cultured tobacco (Nicotiana tabacum L.) cells (Devi et al., 2003). The increased ROS levels severely damage the integrity of lipid membranes and limit plant growth. Other phytotoxic effects of Al have also been observed, such as disrupted Ca2+ homeostasis in the cytoplasm (Rengel, 2004), callose deposits at the plasmodesmata in roots (Sivaguru et al., 2000), and damaged respiration in the mitochondria (Yamamoto et al., 2002).
Plants have developed various strategies to cope with Al toxicity, including detoxifying Al inside the cell and inhibiting the deposition of Al in the apoplast or reducing Al transfer from the outside environment to the cell. Increasing the activities of the antioxidant enzymes superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), and catalase (CAT) to alleviate damage from ROS is a common way in which Al toxicity is alleviated in plants (Wang and Yang, 2005; Zhang et al., 2010b). Wang et al. (2017) recently found that the activity of cytosolic glucose-6-phosphate dehydrogenase is also involved in resistance to Al through mediating ROS levels in soybean (Wang et al., 2017). In addition, plants alleviate Al toxicity by combining Al with organic compounds to detoxify it in the cytoplasm, for example, combining Al with citrate in buckwheat or with delphinidin-3-glucoside and 3-caffeolylquinic acid in Hydrangea macrophylla (Ma et al., 1998; Ma and Hiradate, 2000; Schreiber et al., 2011). Excluding Al via the exudation of carboxylates from roots is a widespread mechanism found in plants that are resistant to Al toxicity, including the exudation of citrate in rice, maize, and soybean (Silva et al., 2001; Ma et al., 2002; Pineros et al., 2002), of malate in wheat and radish (Zheng et al., 1998a; Papernik et al., 2001), and of oxalate in taro and buckwheat (Ma et al., 1997; Ma and Miyasaka, 1998). Compartmentalizing Al in the vacuole is another important way to detoxify Al inside the plant (Shen et al., 2002). Finally, increasing the pH in the rhizosphere efficiently alleviates Al toxicity in Arabidopsis (Degenhardt et al., 1998).
The cell wall is the first cellular component that directly encounters Al. Most Al is fixed in the cell walls of plant. For example, approximately 85–90% and 99.9% of total Al is bound in the root cell wall of barley and in the cell wall of the giant alga Chara corallina, respectively (Clarkson, 1967; Rengel and Reid, 1997). Decreasing cell wall Al content through modifying the cell wall structure and polysaccharide content can significantly alleviate Al toxicity. Wang et al. (2015) demonstrated that the application of NH4+ decreased the Al3+ content in rice roots through decreasing the pectin content in rice roots (Wang et al., 2015). The degree of pectin methylation and the pectin content in roots determine the capacity for Al resistance in rice, and the hemicellulose content in Arabidopsis roots similarly determines its Al resistance because the Al is mainly deposited in hemicellulose in Arabidopsis (Yang et al., 2008, 2011). Thus, reducing the cell wall Al content is an efficient strategy to decrease Al toxicity in plants.
Hydrogen sulfide (H2S) has long been considered a phytotoxin due to its toxic effects on plant growth and development (García-Mata and Lamattina, 2013). For example, exposing plants to high H2S levels damages their leaves and inhibits plant growth (Nakamura et al., 2009). However, in recent years, the role of H2S has been re-evaluated because of its positive effect on plant growth and its mediation of abiotic and biotic stress resistance in plants at low concentrations. In low doses, H2S functions as a signaling molecule in various physiological processes, such as improving wheat seed germination under osmotic stress (Zhang et al., 2010c), regulating the opening and closing of stomata in Arabidopsis (Liu et al., 2011; Jin et al., 2013), and improving resistance to salt stress in alfalfa (Wang et al., 2012), heavy metals such as Al in barley (Chen et al., 2013), and oxidative stress in wheat under water-stress conditions (Shan et al., 2011). However, the role of H2S in alleviating Al toxicity in rice and the underlying mechanism remain elusive.
The rice cultivar Nipponbare was selected in the current study due to its high tolerance to Al. The rice seedlings were pretreated with the H2S donor NaHS prior to exposure to Al to study the effects of H2S on the alleviation of Al toxicity. The results of this study indicate that H2S alleviates Al toxicity via reducing the cell wall and symplast Al contents when rice is exposed to Al.
Materials and Methods
The japonica rice Nipponbare seeds were sterilized in 1% (v/v) sodium hypochlorite solution (NaClO). After 10 min, the seeds were washed three times with deionized water, followed by soaking in deionized water. After 1 day, the seeds were germinated on moist filter paper for 24 h. The germinated seeds were transferred to a net floating on 0.5 mM CaCl2 (pH 5.5) solution in a 5-liter plastic container. When the roots were approximately 1 cm long, various concentrations of NaHS (to a final concentration of 0, 1, 2, 5, and 10 μM) were added to fresh CaCl2 (pH 4.5) solution. After 8 h, the CaCl2 solution was discarded and the seedlings were separated into two groups: one group was transferred to fresh 0.5 mM CaCl2 (pH 4.5), and the other group was transferred to 0.5 mM CaCl2 (pH 4.5) containing different concentrations (30, 50, and 100 μM) of AlCl3. Root length was measured before and after Al treatment with a ruler. All experiments were performed in an environmentally controlled growth room at 30°C and a relative humidity of 60%.
For the NO and hypotaurine addition experiment, eight different treatments were performed as follows: CK, +Al, +Al+NaHS, +Al+HP, +Al+SNP, and +Al+c-PTIO. The final concentrations of the treatments were 2 μM NaHS, 100 μM hypotaurine (HP), 2.5 μM sodium nitroprusside (SNP), 10 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO), and 50 μM Al. NaHS and SNP were added to the CaCl2 solution, and 8 h later, the seedlings were transferred to 0.5 μM AlCl3 solution; HP and c-PTIO were applied together with 0.5 μM AlCl3. The pH of the solution was adjusted to 4.5.
Extraction of the Root Cell Wall and Its Components
Fresh rice roots were ground with liquid nitrogen, followed by extraction with (in order) 75% alcohol, acetone, a mixture of methyl alcohol and chloroform (1:1, v/v), and methyl alcohol. The resulting crude cell wall fraction was freeze dried, and pectin was extracted from the cell walls three times with 1 mL of distilled water at 100°C. The resulting pellet (CW-pectin) was subsequently extracted twice by incubation in 24% KOH (containing 0.02% KBH4) for 12 h each time. The supernatant was collected after centrifugation at 16,000 × g for 5 min and pooled to yield the hemicellulose fraction (Zhong and Lauchli, 1993; Yang et al., 2011; Zhu et al., 2012b).
Measurement of Cell Wall Components
The pectin content was measured based on uronic acid content, and the hemicellulose content was measured based on total sugar content, as described previously (Dubois et al., 1956; Blumenkrantz and Asboe-Hansen, 1973).
Determination of Al Contents in Different Fractions
Al Content in Root Tips
The root tips (0–1 cm) were collected with a razor blade, and samples comprising 10 root tips were soaked in 1 mL of 2 M HCl solution to extract Al. After 24 h, the supernatant was collected and diluted with 3 mL of ultrapure water, and the Al content was measured by inductively coupled plasma mass spectrometry (ICP-MS).
Al Content in Cell Sap
The root tips (0–1 cm) were collected with a razor blade, and samples comprising 10 root tips were placed on a centrifugal filter (0.45 μM) and frozen at 80°C overnight. Then, the frozen samples in the centrifugal filters were thawed at 25°C to release cell sap. After centrifugation at 20,600 × g for 10 min, the volume of cell sap was recorded, and the sap was diluted with 2 mL of ultrapure water (Xia et al., 2010). The Al content was measured by ICP-MS.
Al Content in Cell Walls
Approximately 2 mg of cell wall material was mixed with 1 mL of HCl (2 M) and placed in a vortex vibration meter. After 1 day, the supernatant was collected by centrifugation at 20,600 × g for 10 min and diluted in 2 mL of ultrapure water. The Al content was measured by ICP-MS.
Eriochrome Cyanine R Staining
Roots were washed in 0.5 mM CaCl2 three times and stained with 5 mL of 0.1% eriochrome cyanine R (v/w) for 10 min. The stained roots were washed with distilled water and observed with a MicroPublisher 3.3 RTV equipped with a digital microscope camera (QImaging, Canada). The pink color on the root surface indicates Al accumulation.
Pectin Methylesterase Activity Assay
The enzyme pectin methylesterase (PME) was extracted with 10 mM Tris buffer (pH 7.7) containing 1 mol/L NaCl. After 20 min incubation on ice, the samples were centrifuged at 18,500 × g at 4°C for 10 min, and the supernatant was collected and used to measure PME activity as described previously (Anthon and Barrett, 2004; Zhu et al., 2012a).
Measurement of Citrate Acid Efflux From Roots
The root exudates from plants under various treatments were collected using a cation and anion exchange column successively according to Zheng et al. (1998b). Fresh roots were weighed, and the citrate acid content in roots was measured via ion chromatography after the eluent was concentrated in a rotary evaporator at 40°C (Zhu et al., 2014).
Measurement of Enzyme Activities
Fresh rice roots were collected and weighed, then ground with 2 mL of extraction buffer (50 mM PBS, pH7.8, 1 mM EDTA-Na2 and 1 mM ascorbic acid) in an ice bath. The supernatant was regarded as the crude enzyme solution and collected by centrifugation at 15,000 × g for 15 min at 4°C.
The activity of SOD was detected by determining its ability to inhibit nitroblue tetrazolium (NBT) photochemical reduction. A 3-mL reaction mixture containing 1.5 mL 50 mM PBS (pH7.8), 0.3 mL 20 μM riboflavin, 0.3 mL 150 mM L-methionine, 0.3 mL 750 μM NBT, 0.3 mL 100 μM EDTA-Na2, 0.25 mL H2O, and 50 μL crude enzyme solution was incubated for 20 min under a white fluorescent lamp. A reaction without enzymes was used as a negative control. The absorbance of the solution was measured at 560 nm (Beauchamp and Fridovich, 1971).
Catalase activity was assayed based on the decrease of H2O2 content. A reaction mixture containing 1.9 mL 50 mM PBS (pH 7.0), 1 mL 0.1% H2O2 and 0.1 mL crude enzyme solution was prepared. The absorbance value at 240 nm was recorded at the beginning and 1 min after the reaction began (Dhindsa et al., 1981).
Ascorbate peroxidase activity was measured according to the decrease of ascorbate content. The 3-mL reaction mixture contained 1.5 mL 50 mM potassium phosphate buffer (pH 7.0), 0.5 mL 0.3 mM sodium ascorbate, 0.5 mL 0.1 mM EDTA-Na2, 0.4 mL 0.06 mM H2O2, and 0.1 mL of crude enzyme solution. The absorbance was recorded at 290 nm at 10 s and 30 s after the reaction began (Nakano and Asada, 1981).
Peroxidase activity was assayed according to the tetraguaiacol production rate and the 3-mL reaction mixture contained 1 mL 50 mM potassium phosphate buffer (pH 5.5), 0.95 mL 0.2% guaiacol, 1 mL 0.3% H2O2, and 50 μL of crude enzyme solution. The absorbance was recorded at 470 nm at 30 s and 90 s after the reaction began (Chen et al., 2013).
Measurement of Lipid Peroxidation
Lipid peroxidation was estimated via determining the content of malondialdehyde (MDA) according to Wang et al. (2017). Fresh rice roots (about 0.2 g) were ground and extracted in 2 mL of 10% (w/v) trichloroacetic acid; then the supernatant was collected by centrifuging at 8,000 × g for 10 min at 4°C. A 1.5-mL aliquot of the supernatant was used to determine the MDA content by addition of 1.5 mL reaction mixture (containing 0.5% thiobarbituric acid and 10% trichloroacetic acid) and incubation at 95°C for 30 min. After cooling on an ice bath, the reaction mixtures were centrifuged at 10,000 × g for 10 min at 25°C, and the supernatant were collected and measured at 440, 532, and 600 nm, respectively.
Assay of H2O2 Content
The fresh rice roots (about 0.2 g) were collected and ground with 2 mL of cooled acetone. Then, the supernatants were collected after centrifugation at 8,000 × g for 10 min at 4°C. Next, 0.1 mL of concentrated ammonia and 0.1 mL of 5% TiSO4 were applied to 1 mL supernatant and mixed. The reaction mixture was centrifuged at 4,000 × g for 10 min at 25°C and the supernatant discarded. The residue was dissolved in 4 mL of 2 M H2SO4. After 5 min of incubation at 25°C, the absorbance was recorded at 415 nm (Wang et al., 2017).
Determination of NO Contents in Roots
Root tips (0–1 cm) were collected and washed with 20 mM HEPES-KOH (pH 7.4) for 15 min at room temperature. Then, the collected roots were incubated with 0.5 mL of 4-amino-5-methylamino-2,7-difluorofluorescein diacetate (DAF-FM DA) probe (10 μM) in complete darkness. After 30 min, about 10 mL of 20 mM HEPES-KOH (pH 7.4) was used to wash the stained root tips three times, for 20 min each time. Micrographs were obtained with a Nikon Eclipse 80i fluorescence microscope, Nikon, EX 460–500, DM 505, BA 510–560. The intensity of fluorescence was calculated using PhotoShop 7.0 software (Adobe Systems) (Besson-Bard et al., 2009).
Assay of H2S Content
Hydrogen sulfide and dimethyl-p-phenylenediamine in H2SO4 can form methylene blue, which absorbance can be measured at 667 nm (Li et al., 2013). Fresh rice roots (0.5 g) were ground in 2 mL phosphate buffer (pH 6.8, 50 mM) containing 200 mM ascorbic acid and 0.1 mM EDTA-Na2. The supernatant was collected by centrifugation at 20,600 × g for 10 min and transferred into a test tube. Next, 2 mL of reaction solution containing 100 mM PBS (pH 7.4), 2 mM phosphopyridoxal and 10 mM L-cysteine, and 0.2 mL of zinc acetate (0.1%, w/v) was added into the tube. The mixture solution was incubated at room temperature for 30 min, and 0.15 mL of 5 mM dimethyl-p-phenylenediamine (dissolved in 3.5 mM H2SO4) was added to the tube, followed by 0.15 mL of 50 mM ferric ammonium sulfate (dissolved in 100 mM H2SO4). After 15 min at 25°C, the absorbance of the solution was monitored at 667 nm. A reaction without zinc acetate addition served as a blank.
Gene Expression Analysis
Rice roots were harvested, immediately frozen in liquid nitrogen, and ground for total RNA extraction. Total RNA extraction, reverse transcription, and PCR were performed according to Zhu et al. (2016). The sequences of the gene-specific primers are shown in Table 1 (Xia et al., 2010, 2013; Kengo et al., 2011; Huang et al., 2012). Each cDNA sample was run in triplicate. Relative gene expression levels were calculated via the 2-ΔΔCT method.
Statistical Analysis
Data were analyzed by one-way ANOVA, and the mean values were compared by Tukey’s post-test using SPSS (version 21.0.0; IBM, Armonk, NY, United States). Different letters on the graphs indicate that the mean values were statistically different at the P < 0.05 level.
Results
Pretreatment With NaHS Significantly Alleviates Al Toxicity
The japonica rice variety Nipponbare was used in the present study to gain insight into the role of H2S in Al resistance. Seedlings with roots of approximately 3 cm in length were treated with 0, 30, 50, and 100 μM Al3+ in 0.5 M CaCl2 (pH 4.5) solution for 24 h, then root length, Al3+ content in root apices and root H2S content were measured. As shown in Figures 1A–C, there was a significant decrease in root length and an increase of Al3+ content in root tips with increasing Al3+ level in the solution. Root length was reduced by approximately 50% in the presence of 50 μM Al3+ vs. the control, and therefore, 50 μM Al3+ was used in subsequent experiments. With increasing Al3+ concentrations in the solution, the H2S content in the rice roots also significantly increased (Figure 1D), indicating that H2S may be involved in the rice response to Al toxicity.
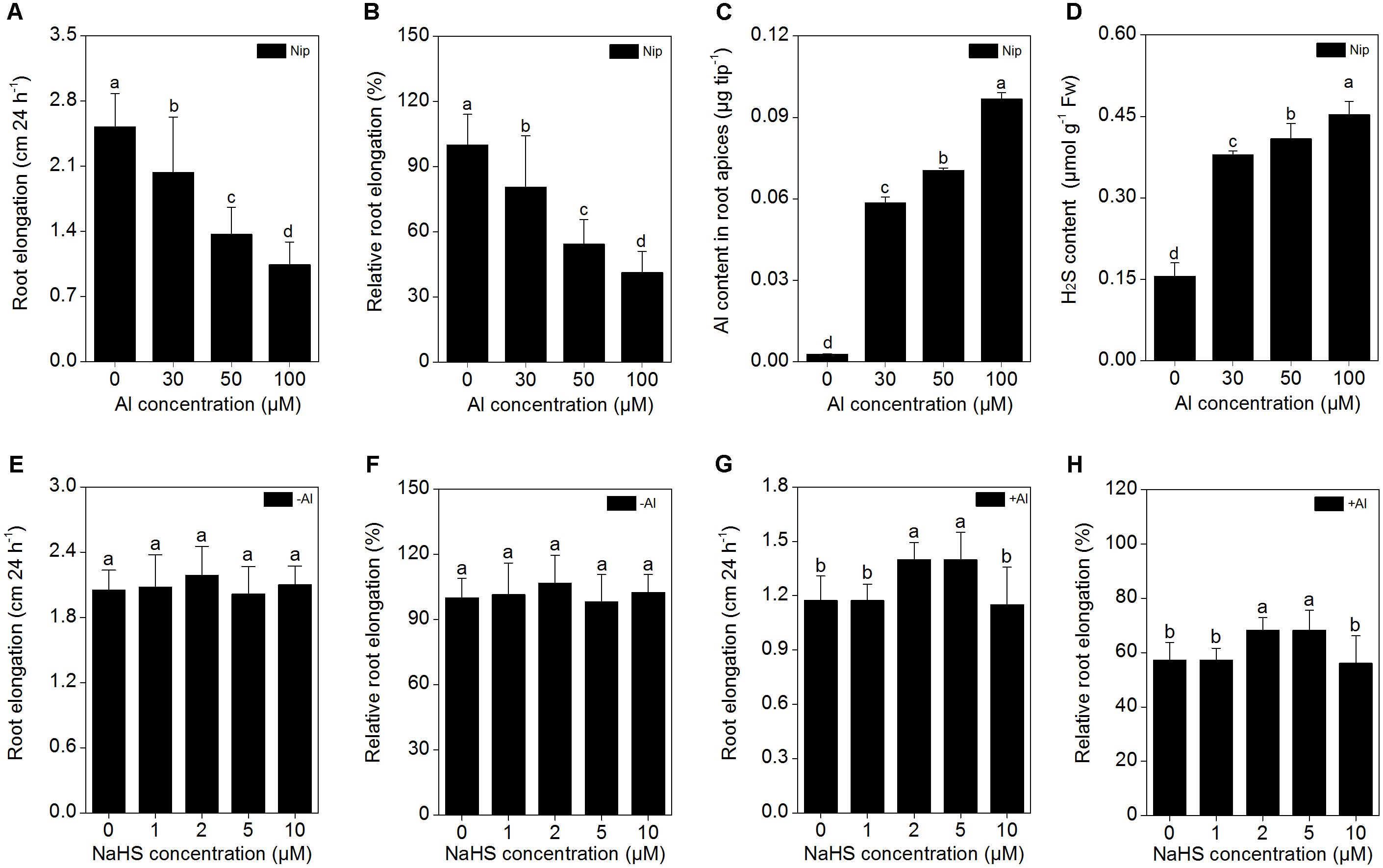
FIGURE 1. Effects of different Al3+ concentrations on root elongation (A), relative root elongation (B), Al content in root apices (C), H2S content in root (D), and effects of different NaHS concentrations on root elongation (E), relative root elongation (F) under Al-absent conditions and root elongation (G), relative root elongation (H) under 50 μM Al3+ conditions. Data are means ± SD (n = 10). Columns with different letters are significantly different at P < 0.05.
Next, the rice seedlings were pretreated with different concentrations (0, 1, 2, 5, 10 μM) of NaHS. After 8 h, the seedlings were transferred to 0.5 mM CaCl2 (pH 4.5) solution with or without 50 μM Al3+, and root length and Al3+ content in the root tips were measured 24 h later. As shown in Figures 1E,F, NaHS had no significant effect on root length under Al3+-absent conditions. However, root length significantly increased in seedlings pretreated with 2 and 5 μM NaHS under 50 μM Al3+ treatment (Figures 1G,H), indicating that NaHS significantly alleviated the inhibition of root growth under Al3+ conditions. The 2 μM NaHS concentration was selected for further analysis; the Al3+ content in root tips significantly decreased under 2 μM NaHS pretreatment (Figure 2A), indicating that the H2S decreased the Al content in rice roots, thereby alleviating the inhibited root growth induced by Al toxicity.
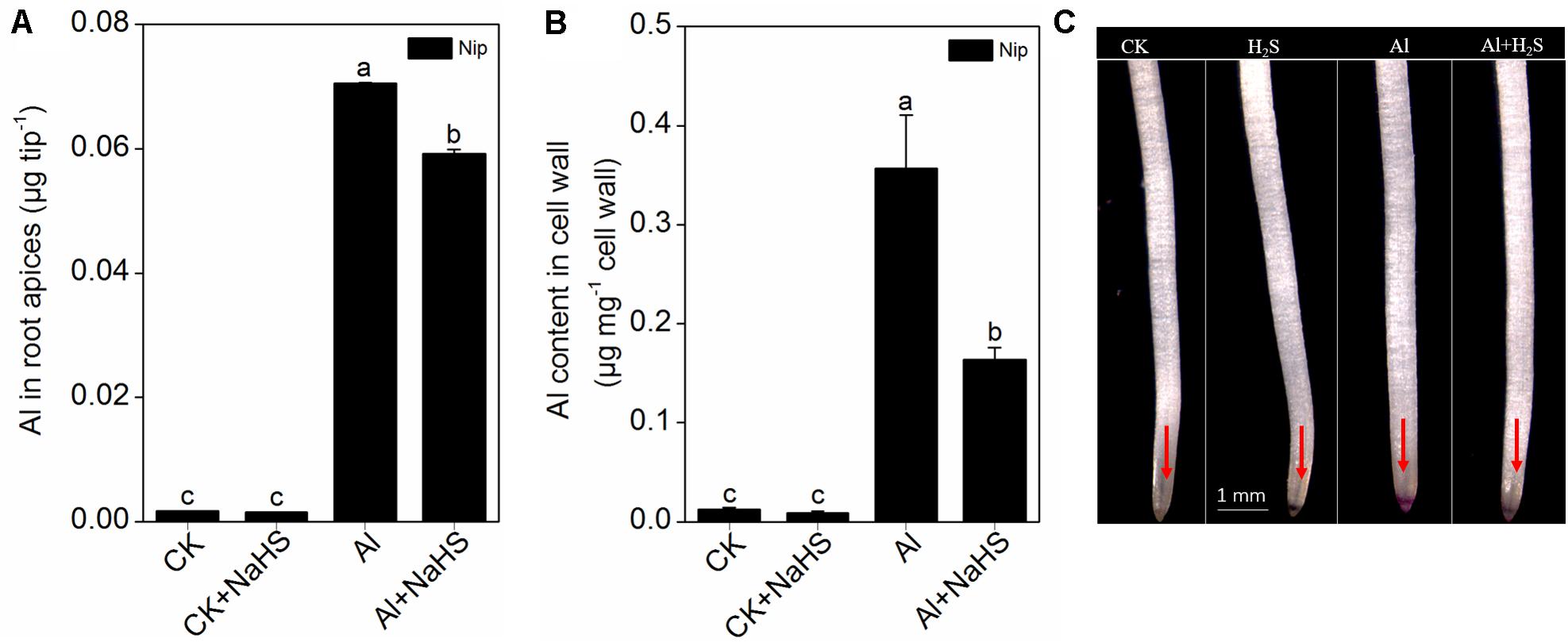
FIGURE 2. Effect of 2 μM NaHS on Al contents in root apices (A), root cell wall (B) and the Al deposited at the root surface (C). The root tips (0–1 cm) were stained with 0.1% eriochrome cyanine R (v/w) and the pink color on the root surface indicates Al accumulation (as the red arrows point out). Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
H2S Reduces the Al Content in Rice Roots by Modifying Cell Wall Components and Increasing Citrate Secretion
Most Al in the root is contained in the cell wall. Under high Al conditions, Al can be released from the cell wall through a change in the degree of methylation of pectin in the cell wall and in the content of hemicellulose, thereby alleviating Al toxicity (Clarkson, 1967; Yang et al., 2008, 2011). Therefore, the rice root cell walls and the components in the cell wall were extracted and analyzed in the present study. As shown in Figure 2B, pretreatment with 2 μM of the H2S donor NaHS significantly reduced the Al content in the cell walls of rice roots compared to the control under 50 μM Al3+ conditions. To visualize Al deposits at the root surface, the root segments (0–1 cm) were further stained with eriochrome cyanine R, demonstrating that the Al levels (stained pink) were significantly decreased by pretreatment with NaHS under 50 μM Al3+ conditions (Figure 2C), indicating that H2S decreased the binding of Al in the root surface. As pectin and hemicellulose are the two major polysaccharides in rice root cell walls that contribute to the release of Al from this structure (Yang et al., 2008, 2011), their contents in rice root cell walls were also measured. As shown in Figure 3, the pectin and hemicellulose contents in root cell walls both decreased in response to 2 μM NaHS pretreatment in the presence of 50 μM Al3+, which was accompanied by a decrease in PME activity in roots, suggesting that H2S treatment may alleviate Al toxicity in rice roots under high-Al conditions by decreasing the contents of cell wall polysaccharose and altering its structure. In addition, the expressions of OsSTAR1 and OsSTAR2, which modify the cell wall to reduce cell wall Al contents (Delhaize et al., 2012; Huang et al., 2012), were also significantly increased in the presence of NaHS under high-Al conditions (Figure 4).
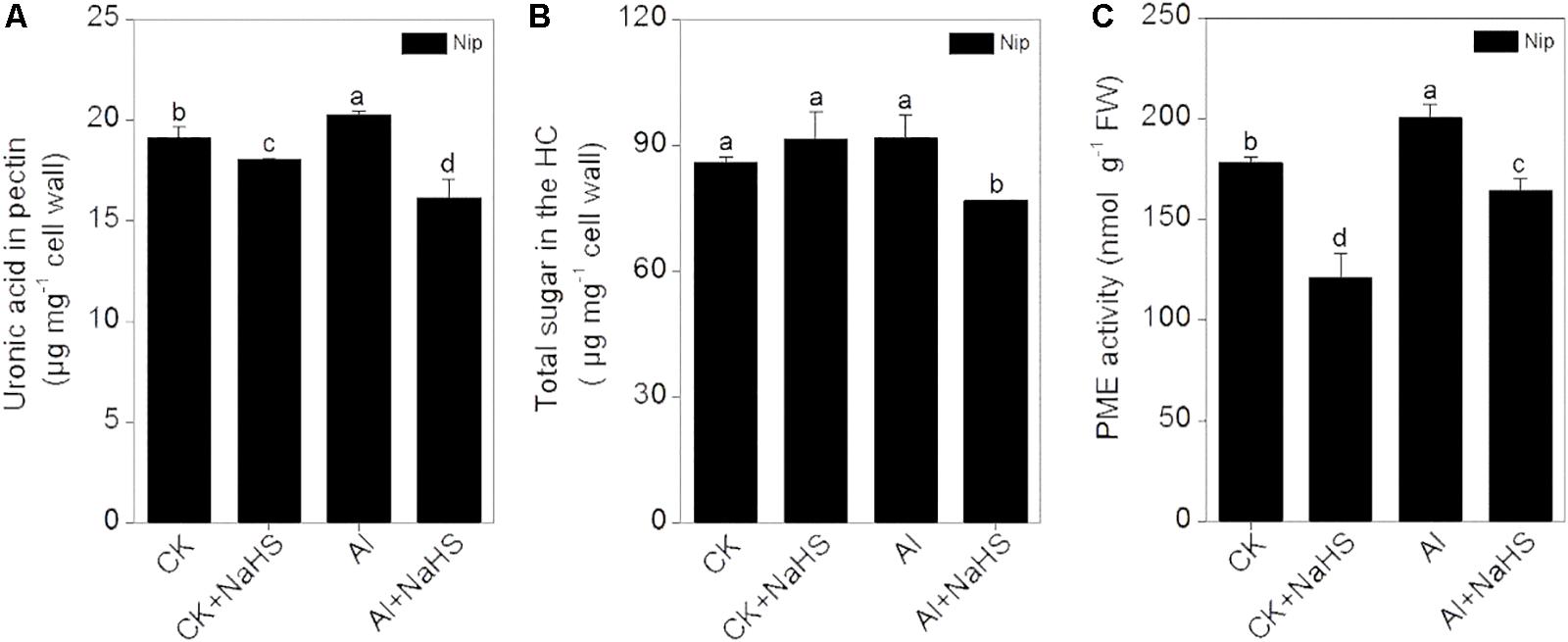
FIGURE 3. Effect of 2 μM NaHS on pectin content (A) and hemicellulose content (B) in the root cell wall and the activity of pectin methylesterase (PME) in rice roots (C). Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
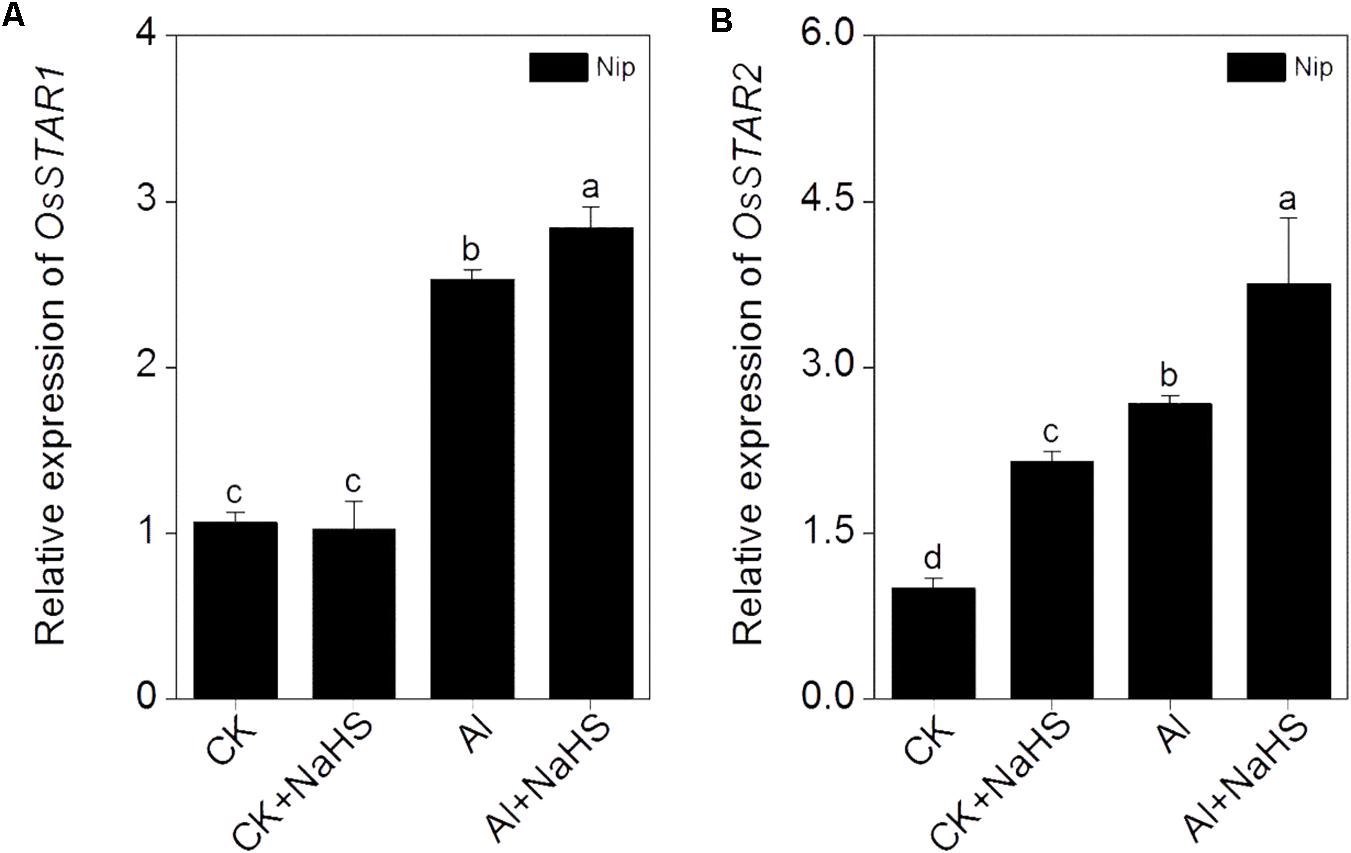
FIGURE 4. Effect of 2 μM NaHS on the expression of OsSTAR1 (A) and OsSTAR2 (B). Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
Increasing the secretion of organic acids from the root to decrease Al deposition at the root surface is another efficient mechanism used to alleviate Al toxicity (Ma et al., 2001), and OsFRDL4 is responsible for the efflux of citrate acid from rice roots (Yokosho et al., 2011). The expression of OsFRDL4 was significantly increased in response to NaHS pretreatment under 50 μM Al3+ conditions, accompanied with a significant increase in the secretion of citrate from roots (Figure 5), indicating that H2S also increases the secretion of citrate from the roots to decrease Al deposition, thereby alleviating Al toxicity.
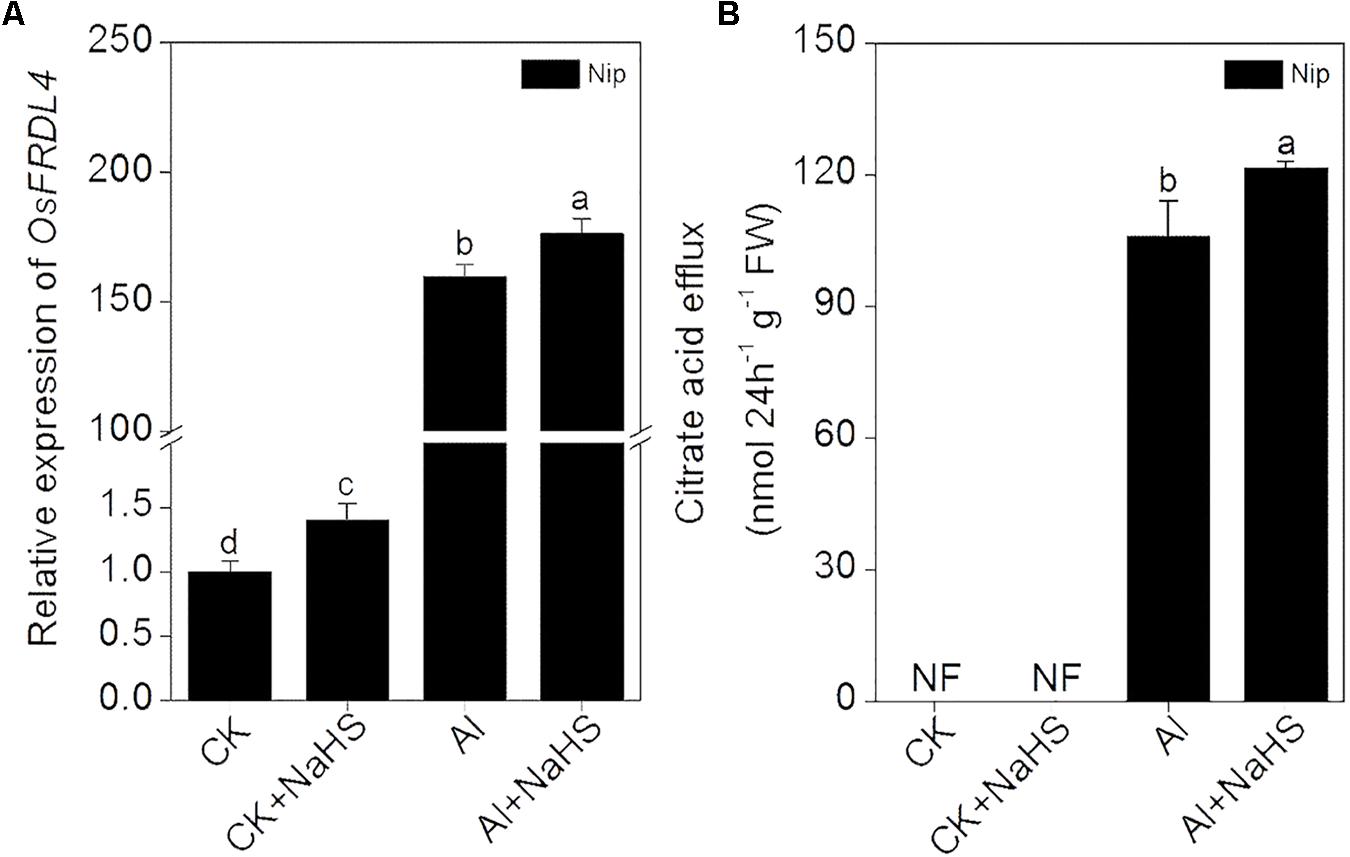
FIGURE 5. Effect of 2 μM NaHS on the expression of OsFRDL4 in rice roots (A) and the secretion of citrate acid from rice roots (B). Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
H2S Reduces Al3+ Transport to the Cell Interior and Increases Translocation of Al to the Vacuole
OsNRAT1 is responsible for the transport of Al3+ from the environment to the interior of the cell (Xia et al., 2010). After pretreatment with NaHS, the expression of OsNRAT1 significantly decreased in roots, which was accompanied by a decrease in Al levels in cell sap under Al toxicity conditions (Figure 6), suggesting that H2S reduces the transfer of Al3+ from the outside environment to the inside of the cell to alleviate its inhibitory effect on root elongation. Once Al enters the plant cell, it must be detoxified. OsALS1 is involved in translocating Al from the cytoplasm to the vacuole, thereby alleviating Al toxicity in plants (Huang et al., 2012). In the current work, the expression of OsALS1 in rice roots significantly increased in response to H2S treatment under 50 μM Al3+ conditions (Figure 6), indicating that H2S stimulates the transport of Al to the vacuole via OsALS1.
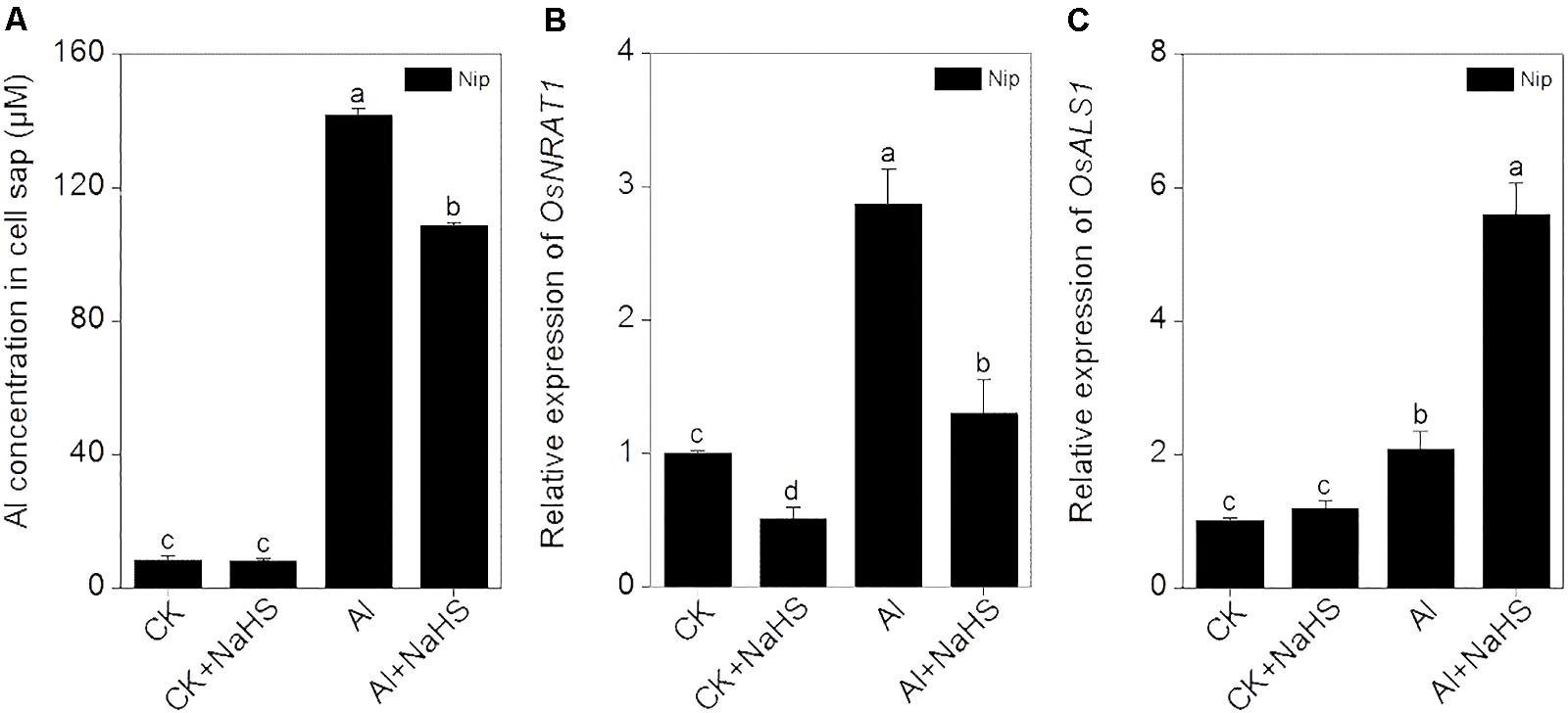
FIGURE 6. Effect of 2 μM NaHS on Al concentration in cell sap (A) and the expression of OsNRAT1 (B) and OsALS1 (C) in rice roots. Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
H2S Stimulates the Activity of Antioxidant Enzymes to Reduce ROS Damage in Rice Roots
The elimination of ROS through antioxidant enzymes is the most common strategy for alleviating Al toxicity (Ezaki et al., 2000; Mittler, 2002). In the present study, the activity of the antioxidant enzymes SOD, CAT, and POD were all significantly increased in response to Al, and further increased by the pretreatment with NaHS under 50 μM Al3+ conditions (Figure 7). However, the simple application of 50 μM Al3+ within 24 h failed to stimulate the activity of APX; only pretreatment with 2 μM NaHS and growth under 50 μM Al3+ conditions dramatically increased the activity of APX, indicating that APX may plays an important role in H2S-mediated alleviation of Al toxicity via increasing the antioxidative system. In addition, the content of MDA (which can be used to estimate the levels of lipid peroxidation) and H2O2 in rice roots exhibited similar changes as APX activity (Figure 7). These results indicate that H2S increases the activities of antioxidant enzymes, likely with APX having a key function, to alleviate Al toxicity in rice.
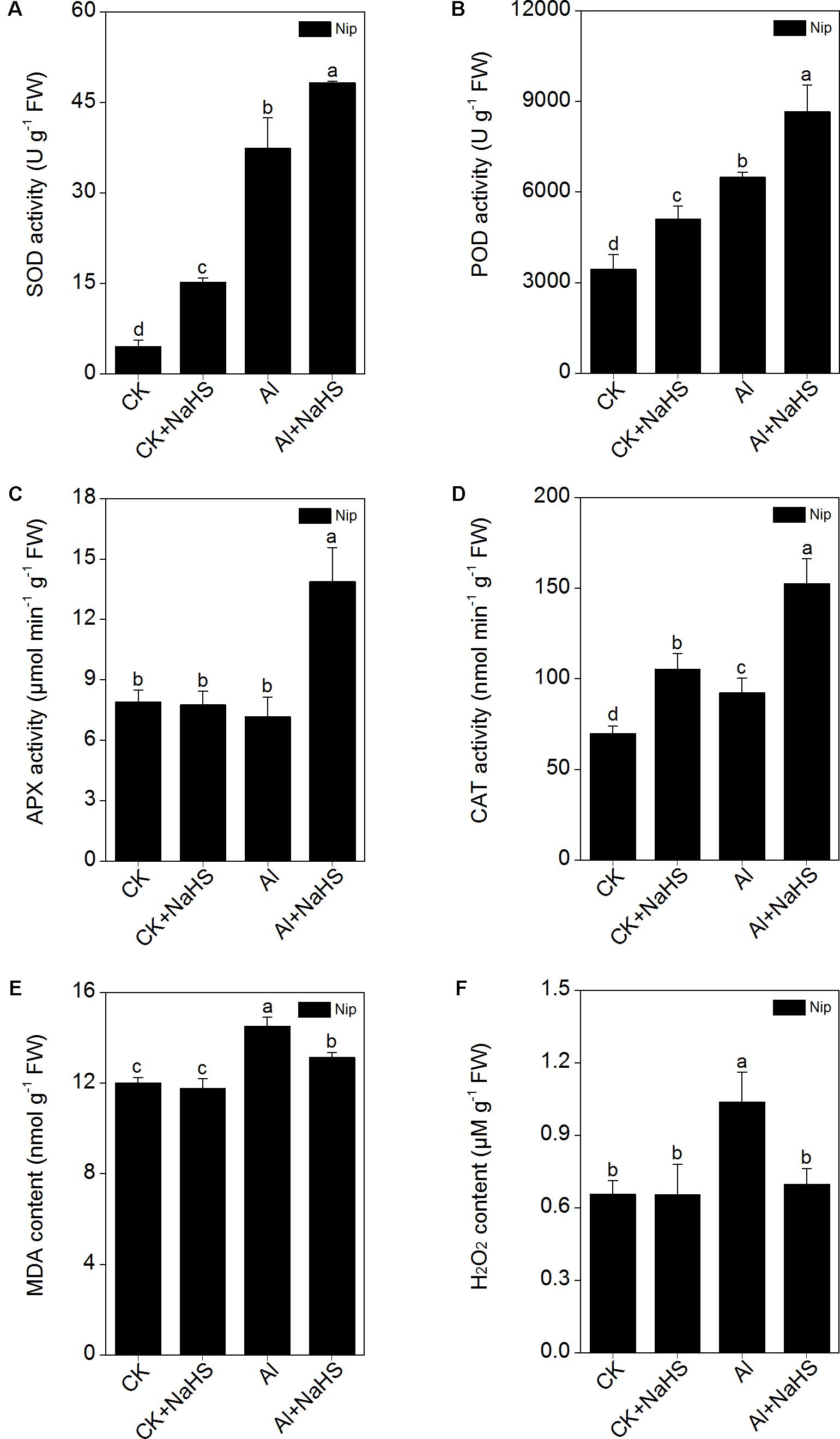
FIGURE 7. Effect of 2 μM NaHS on SOD (A), POD (B), APX (C), CAT (D) activity, MDA (E) and H2O2 (F) content in rice roots. Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
H2S Modulates the NO Content to Alleviate Al Toxicity
The signaling molecule nitric oxide (NO) plays a pivotal role in the plant response to Al toxicity, although the results are contradictory. For example, the addition of the NO donor SNP significantly alleviates Al toxicity in red kidney bean (Phaseolus vulgaris), wheat (Triticum aestivum L.) and Cassia tora L (Wang and Yang, 2005; Zhang et al., 2008b; Wang et al., 2010); however, the application of SNP dramatically aggravates Al toxicity in rice bean [Vigna umbellata (Thunb.) Ohwi and Ohashi ‘Jiangnan,’ Fabaceae] and rice (Oryza sativa) (Zhou et al., 2012; Zhu et al., 2017), and elimination of NO via adding the NO scavenger c-PTIO alleviates Al toxicity in wheat (Triticum aestivum L. cv. Yang-5) (Sun et al., 2016). In the present study, the NO content in rice roots significantly decreased in plants pretreated with NaHS compared to the control (Figure 8), suggesting that H2S might alleviate Al toxicity by decreasing NO content. To explore this notion, the NO donor SNP and the NO scavenger c-PTIO were used to treat rice seedlings under 50 μM Al3+ conditions. As shown in Figure 9, the addition of SNP significantly decreased root elongation and increased the Al content in root tips under 50 μM Al conditions, whereas c-PTIO had the opposite effect, indicating that NO plays a negative role in the alleviation of Al toxicity. In addition, the presence of the H2S scavenger hypotaurine significantly reduced root elongation (Figure 9), which was accompanied by an increase in Al contents in roots under high-Al conditions, further confirming the role of H2S in alleviating Al toxicity in rice.
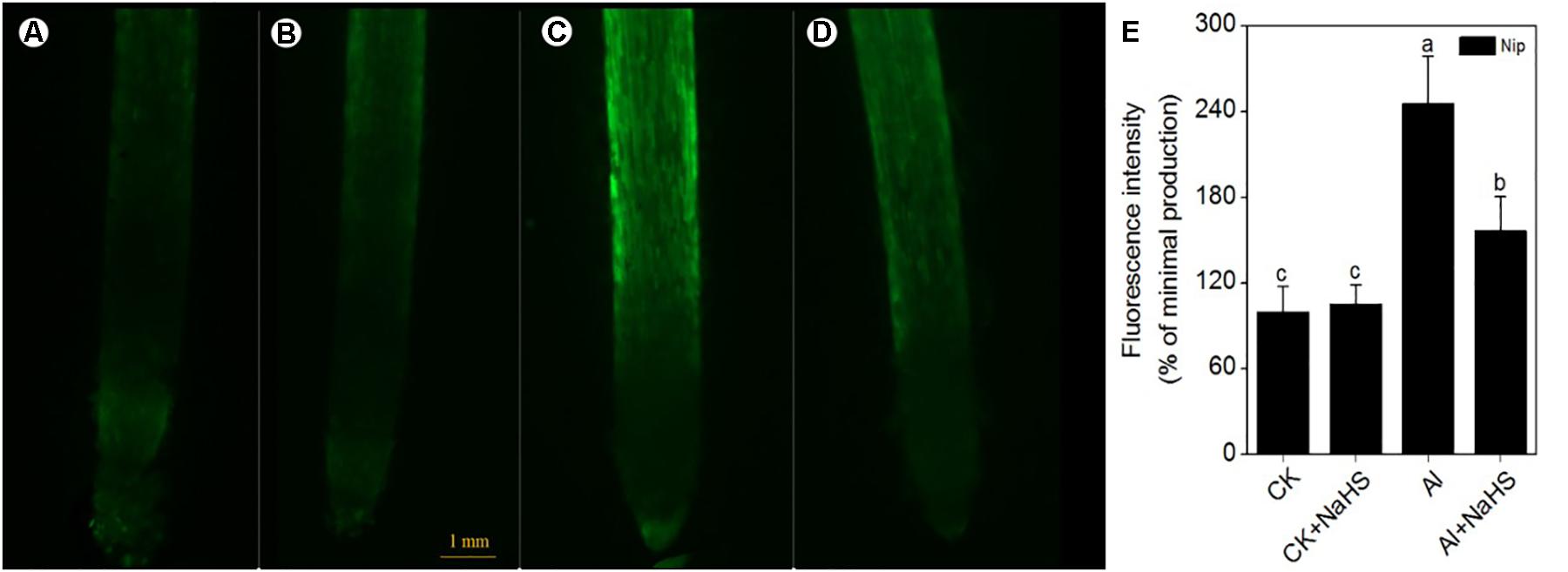
FIGURE 8. The fluorescence intensity of nitric oxide (NO) under CK (A), CK+NaHS (B), Al (C), Al+NaHS (D) conditions, and the relative fluorescence intensity of NO under different treatments (E) in rice root tips. The concentration of NaHS is 2 μM, and the concentration of Al is 50 μM. Data are means ± SD (n = 10). Scale bar = 1 mm. Columns with different letters are significantly different at P < 0.05.
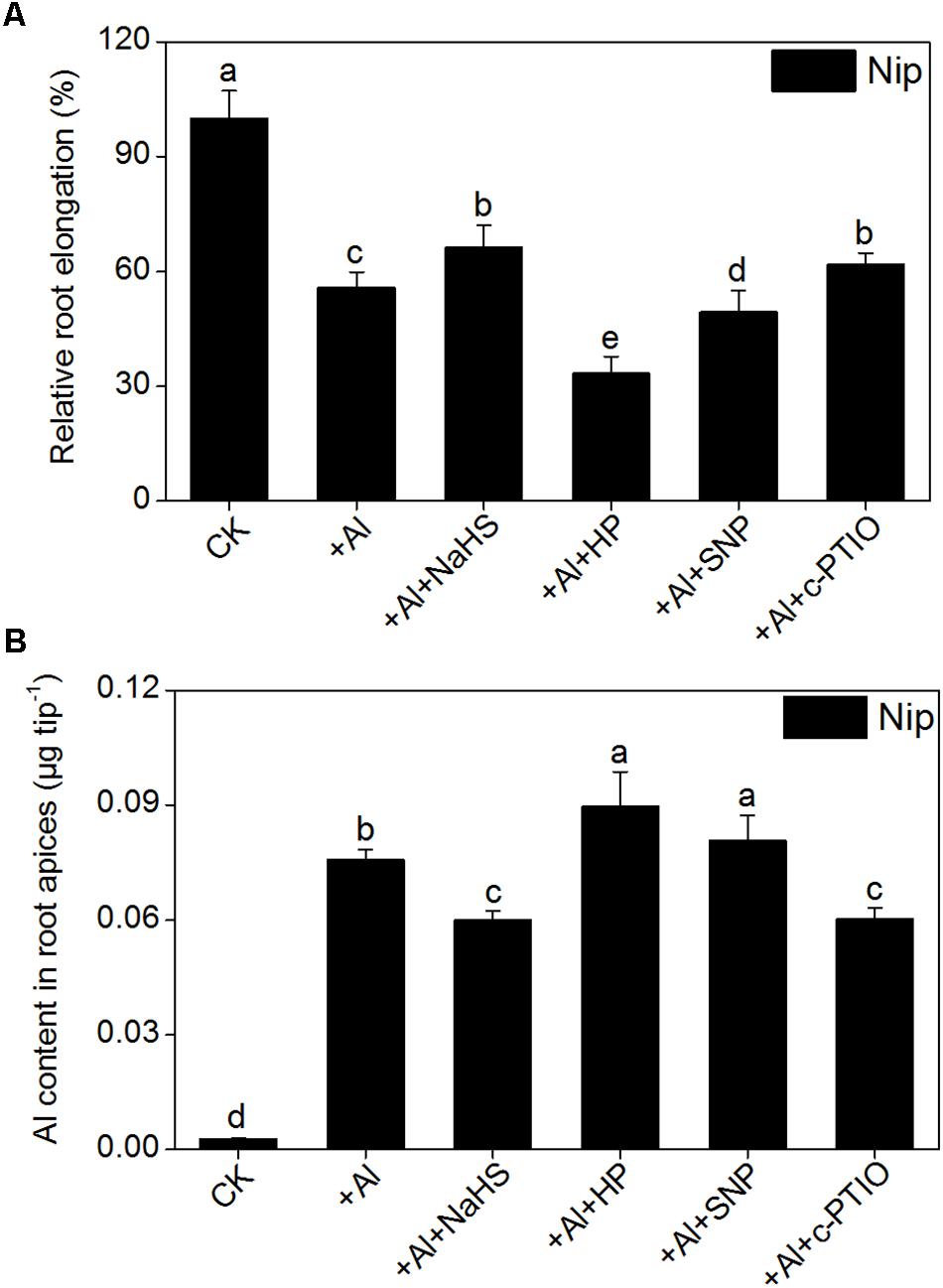
FIGURE 9. Effects of different treatments on relative root elongation (A) and Al content in root apices (B). Data are means ± SD (n = 10). Columns with different letters are significantly different at P < 0.05.
Discussion
In addition to NO and carbon monoxide (CO), H2S is another gas that functions as a signaling molecule in plants (Li and Gong, 2011). Accumulating evidence indicates that low doses of H2S have a positive effect on seed germination and plant growth, development, and abiotic/biotic stress resistance (Zhang et al., 2009; Yong et al., 2010; Li et al., 2011, 2012; Lisjak et al., 2013). H2S has been shown to increase plant resistance to heavy metals. For example, pretreating wheat seeds with NaHS (a H2S donor) significantly improves seed germination and seedling growth under Cr, Cu, and Al stress conditions by reducing lipid membrane damage and the deposition of these metals via increasing the activities of amylases, esterases, and antioxidant enzymes (Zhang et al., 2008a, 2010a,b). Pretreating barley seedlings with H2S significantly enhances the secretion of citrate, decreases lipid peroxidation and the ROS burst, and increases the amount of the plasma membrane H+-ATPase, which in turn improves root growth under Al toxicity conditions (Dawood et al., 2012; Chen et al., 2013). In the current study, the H2S content in the rice roots was significantly increased with increasing Al3+ content (Figure 1D) and 8 h of pretreatment with 2 μM NaHS significantly increased root elongation (Figures 1G,H) and decreased Al contents in rice root tips (Figure 1A) under 50 μM Al3+ conditions. The addition of H2S scavenger hypotaurine significantly aggravated the defects in root growth and increased the Al contents in root tips under 50 μM Al3+ stress (Figure 9), further confirming that H2S is involved in alleviation of Al3+ toxicity in rice.
Al mainly accumulates in the plant cell wall, and desorption of Al from the cell wall significantly alleviated the Al toxicity for plants. For example, 99.99% of total cellular Al is deposited in the cell wall of the giant alga Chara corallina (Rengel and Reid, 1997), and more than 77% of total Al is deposited in the root cell wall in the Al-sensitive wheat cultivar Scout 66 under 10 μM Al3+ conditions within 9 h (Ma et al., 2004). Pectin was the first polysaccharide in the cell wall shown to be involved in Al3+ tolerance in plants. Chang et al. (1999) demonstrated that nearly 89% of Al in tobacco is present in the cell wall, and approximately 76% is deposited in the pectin fraction (Chang et al., 1999). The difference in Al tolerance between Nipponbare (Al-tolerant cultivar) and Zhefu802 (Al-sensitive cultivar) rice is due to the different pectin contents in the root cell wall and the different levels of methylation in root cell wall pectin (Yang et al., 2008). In Arabidopsis roots, Al is mainly deposited in hemicellulose in the cell wall (Yang et al., 2011), indicating that both pectin and hemicellulose play pivotal roles in mediating Al toxicity in plants. In the current study, rice seedlings pretreated with NaHS had significantly reduced cell wall Al contents due to decreased levels of pectin and hemicellulose in the root cell wall (Figures 2, 3), indicating that H2S releases Al bound in the root cell wall by regulating the cell wall polysaccharide content. The enzyme PME catalyzes the demethylation of pectin, leading to the exposure of free carboxylic groups, which have a strong affinity for Al (Blamey and Dowling, 1995; Micheli, 2001); thus, high PME activity leads to a higher Al binding capacity in plant roots, and it has been demonstrated to be negatively associated with Al tolerance in rice (Yang et al., 2013). In the current study, pretreatment with NaHS significantly reduced the activity of PME in rice roots (Figure 3), indicating that H2S decreases PME activity in roots, thereby decreasing the availability of Al binding sites, which alleviates the inhibition of root elongation under Al-stress conditions.
The expression of OsSTAR1 and OsSTAR2 also significantly increased in response to NaHS pretreatment under high Al3+ conditions (Figure 4). OsSTAR1 and OsSTAR2 function in Al tolerance in rice, and the disruption of either gene severely increases Al toxicity. The STAR1-STAR2 complex (encoded by OsSTAR1 and OsSTAR2) functions as an ATP binding cassette (ABC) transporter and transports UDP-glucose from the cytoplasm to the cell wall, resulting in reduced Al deposition, ultimately alleviating Al toxicity (Huang et al., 2009). Pretreatment with H2S significantly induced the expression of OsSTAR1 and OsSTAR2 under Al3+ conditions in the current study (Figure 4), further confirming that H2S increases Al resistance in rice by reducing Al contents in the cell wall.
Plants often secrete organic acids to alleviate Al toxicity. For example, the addition of Al3+ significantly increased oxalic acid efflux from buckwheat. Oxalic acid in the rhizosphere binds with Al3+ to form an Al-oxalate complex, which is not phytotoxic to buckwheat (Ma et al., 1997). Citrate acid is the main organic acid present in exudate from rice roots under Al3+ conditions, and the secretion of citrate acid is a useful strategy for alleviating Al toxicity in rice (Ishikawa et al., 2000; Ma et al., 2002). OsFRDL4 encodes a citrate efflux transporter involved in Al-induced secretion of citrate from the roots and is involved in external Al detoxification in rice (Yokosho et al., 2011, 2016). In the present study, the expression of OsFRDL4 in rice roots was significantly increased under 50 μM Al3+ conditions and was further stimulated by pretreatment with H2S (Figure 5). In addition, pretreatment with H2S further increased the citrate acid content in rice root exudates under 50 μM Al3+ conditions (Figure 5), suggesting that the signaling molecule H2S also regulates the secretion of citrate to improve resistance to Al toxicity in rice.
Toxic Al rapidly enters the root cell and inhibits root growth. The trivalent Al ion is transported into root cells by NRAMP ALUMINIUM TRANSPORTER1(NRAT1). In the present study, the expression of OSNRAT1 significantly decreased in response to NaHS pretreatment, which was accompanied by a decrease in Al levels in cell sap from the root tip (0–1 cm) (Figure 6), implying that H2S decreases the amount of Al that enters root cells. Once Al enters the cell, the movement of cytoplasmic Al to the vacuole can decrease Al toxicity. OsALS1 is localized to the tonoplast and is a half-size ABC transporter. OsALS1 is responsible for sequestering cytoplasmic Al into the vacuole, and knockout of OsALS1 leads to an increase in cytosolic and nuclear Al content and increased sensitivity to Al toxicity (Huang et al., 2011). In the current study, the expression of OsALS1 was significantly induced by Al treatment and was further stimulated by H2S pretreatment under Al conditions (Figure 6), indicating that H2S is also involved in internal Al detoxification by increasing the expression of OsALS1 in rice roots.
Apart from decreasing the cell wall Al content and compartmentalizing Al in the vacuole, plants also increase antioxidant enzyme activity to resist Al toxicity. The main antioxidant enzymes in plants are SOD, CAT, APX, and POD, all of which are involved in reducing the ROS content (Yamamoto et al., 2002; Kobayashi et al., 2003) and inhibition of lipid peroxidation (Mittler, 2002) in plants under Al-present conditions, thus ameliorating Al toxicity in plants. Dawood et al. (2012) demonstrated that pretreatment with NaHS before Al treatment in barley significantly inhibited the lipid peroxidation via increasing the activity of CAT and POD, thus alleviating the oxidative damage and promoting root growth under Al stress. In the present study, the activities of SOD, POD, and CAT were significantly increased under Al conditions, and pretreatment with NaHS further increased the activities of these three antioxidant enzymes (Figure 7), with the activity of APX stimulated only under NaHS pretreatment and Al-present conditions, suggesting that the signaling molecule H2S also activates antioxidant systems to reduce the negative effects of Al toxicity on plant growth. As the activity of SOD, POD, and CAT were all similarly increased by NaHS pretreatment under Al-absent conditions, we hypothesize that these three enzymes may be specifically responsive to the NaHS treatment and play an indirect role in detoxification of Al-induced ROS. By contrast, the response of the APX enzyme to NaHS pretreatment only in the presence of Al, accompanied by the similar pattern of change in MDA and H2O2 contents in roots (Figure 7), implies that APX may be the main enzyme responsible for the H2S-mediated improved ROS detoxification during Al stress. The APX enzyme is the first line of defense against oxidative stress, using ascorbic acid as specific electron donor to catalyze H2O2 reduction to water, and is known to be involved in Al detoxication in rice (Sharma and Dubey, 2007). According to a previous study, the content of H2O2 in rice roots plays an important role in stimulating the synthesis of cell wall polysaccharide, especially in promoting the synthesis and demethylesterification of pectin in rice (Xiong et al., 2015). In the present study, the pectin content and the activity of PME (which is responsible for the pectin demethylesterification) were significantly decreased after pretreatment with NaHS under 50 μM Al3+ conditions, just as the activity of APX significantly increased and the H2O2 content dramatically decreased under NaHS pretreatment and 50 μM Al3+ conditions. These corresponding results indicate that the main functions of APX in H2S-mediated alleviation of Al toxicity may decrease not only the peroxidation damage from H2O2, but also pectin synthesis and the activity of PME to diminish the demethylesterification levels via decreasing the H2O2 content, to ultimately decrease the cell wall Al content in rice roots.
Other signaling molecules might also be involved in alleviating Al toxicity via H2S. NO plays a pivotal role in plant responses to Al toxicity and exhibits different effects in different plant cultivars and different culture conditions. The addition of the NO donor SNP significantly enhances the antioxidant capacity to decrease Al toxicity in wheat, soybean and Cassia tora L (Wang and Yang, 2005; Zhang et al., 2008b; Wang et al., 2016); however, other studies have demonstrated that NO aggravates Al toxicity to root elongation via regulating cell wall polysaccharose content and structure (Zhou et al., 2012; Sun et al., 2016). For example, when wheat roots were treated with the NO scavenger c-PTIO, PME activity decreased, leading to a higher degree of methylation of pectin in the root cell wall and decreasing the binding capacity between Al and pectin, which in turn promoted root growth and reduced Al levels in roots (Sun et al., 2016). Since both H2S and NO have been implicated in the regulation of Al-induced inhibition of root elongation, H2S might alleviate Al-induced root inhibition, and subsequently Al toxicity, by modulating NO accumulation. Pretreatment with NaHS significantly decreased the NO content in rice roots under high-Al conditions (Figure 8). The addition of the NO donor SNP dramatically increased the inhibitory effect of Al on root elongation and increased the Al content in root tips, whereas the addition of c-PTIO had the opposite effect (Figure 9), indicating that the signaling molecule H2S reduces NO content to alleviate Al toxicity in rice.
Conclusion
This is first report demonstrating that the signaling molecule H2S alleviates the inhibition of root growth in rice induced by Al toxicity and decreases Al accumulation in root tips. As show in Figure 10, H2S functions by decreasing the pectin and hemicellulose contents in the root cell wall, modifying the root cell wall, and decreasing PME activity in roots. Furthermore, H2S increases citrate acid secretion from roots, compartmentalizes Al into the vacuole, and increases antioxidant enzyme activity. In addition, H2S-mediated alleviation of Al toxicity in rice may involve decreasing the NO accumulation in roots.
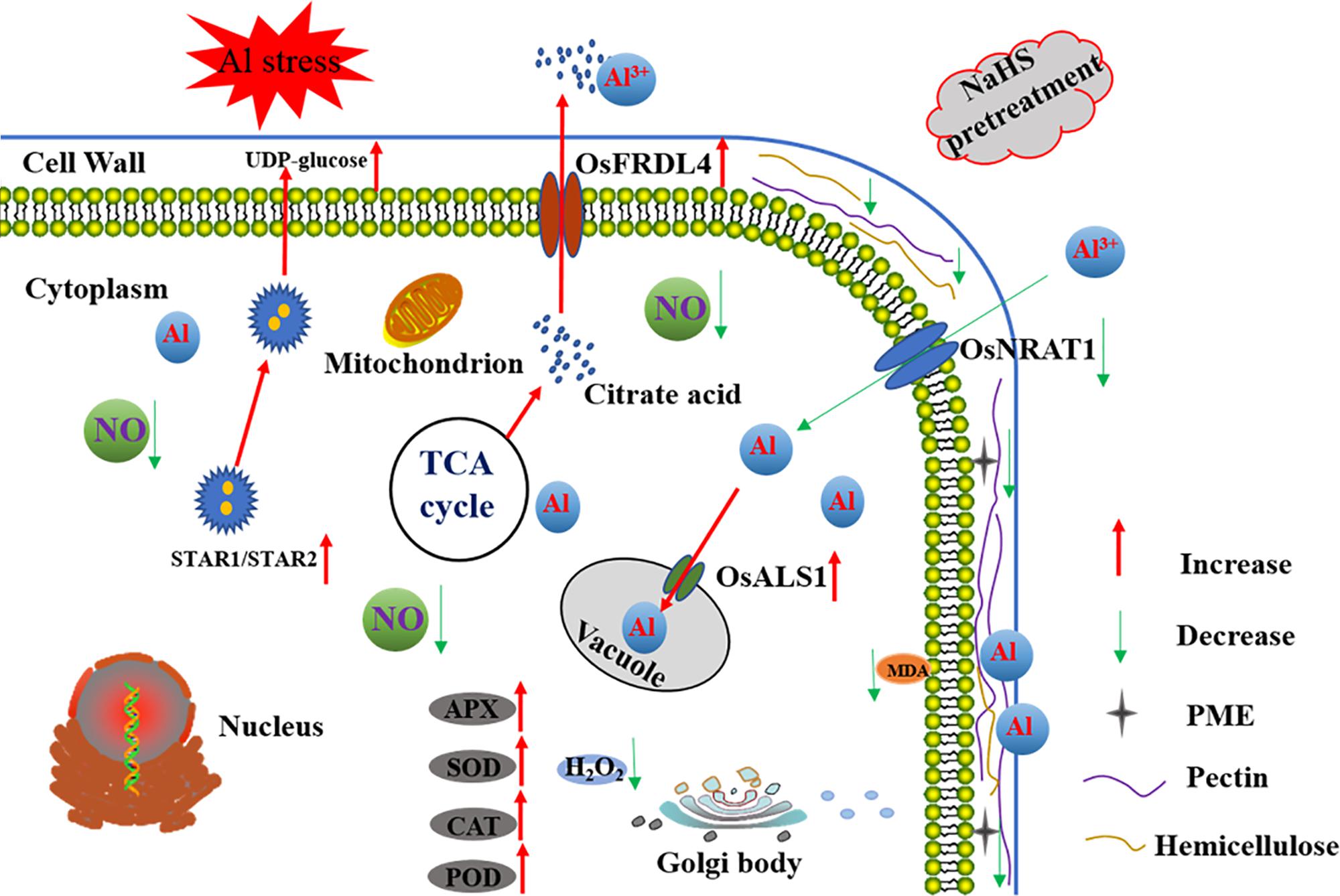
FIGURE 10. A schematic model for H2S-mediated improvement of root elongation in the face of Al toxicity in rice. Pretreatment with NaHS significantly decreased the cell wall Al content via decreasing the cell wall pectin and hemicellulose content and the activity of PME, and increasing the secretion of citrate acid and the expression of OsSTAR1 and OsSTAR2. The pretreatment with NaHS also significantly decreased the translocation of Al from the environment to the cell cytoplasm and promoted its detoxification via increasing the activity of antioxidative enzymes as well as Al transfer from the cytoplasm to the vacuole. The red arrow in the figure denotes increased or enhanced processes, the green arrow in the figure denotes decreased or weakened processes.
Author Contributions
CQZ, JZ, LS, LZ, and BA performed the research. CQZ analyzed the data and wrote the draft. WH, CZ, ZB, and HS revised the article. CQZ, XC, and QJ designed the research. QJ wrote the article.
Funding
This work was funded by the Basic Research Foundation of National Commonweal Research Institute (No. 2017RG004-2), the Zhejiang Provincial Natural Science Foundation of China (Nos. LY18C130005, Q15C130006), and the National Key Research and Development Program of China (No. 2017YFD0300100).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Anthon, G. E., and Barrett, D. M. (2004). Comparison of three colorimetric reagents in the determination of methanol with alcohol oxidase. Application to the assay of pectin methylesterase. J. Agric. Food Chem. 52, 3749–3753. doi: 10.1021/jf035284w
Beauchamp, C., and Fridovich, I. (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287. doi: 10.1016/0003-2697(71)90370-8
Besson-Bard, A., Gravot, A., Richaud, P., Auroy, P., Duc, C., Gaymard, F., et al. (2009). Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol. 149, 1302–1315. doi: 10.1104/pp.108.133348
Blamey, F. P. C., and Dowling, A. J. (1995). Antagonism between aluminium and calcium for sorption by calcium pectate. Plant Soil 171, 137–140. doi: 10.1007/BF00009576
Blamey, F. P. C., Nishizawa, N. K., and Yoshimura, E. (2004). Timing, magnitude, and location of initial soluble aluminum injuries to mungbean roots. Soil Sci. Plant Nutr. 50, 67–76. doi: 10.1080/00380768.2004.10408453
Blumenkrantz, N., and Asboe-Hansen, G. (1973). New method for quantitative determination of uronic acids. Anal. Biochem. 54, 484–489. doi: 10.1016/0003-2697(73)90377-1
Chang, Y. C., Yamamoto, Y., and Matsumoto, H. (1999). Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ. 22, 1009–1017. doi: 10.1046/j.1365-3040.1999.00467.x
Chen, J., Wang, W. H., Wu, F. H., You, C. Y., Liu, T. W., Dong, X. J., et al. (2013). Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362, 301–318. doi: 10.1007/s11104-012-1275-7
Clarkson, D. T. (1967). Interactions between aluminium and phosphorus on root surfaces and cell wall material. Plant Soil 27, 347–356. doi: 10.1007/BF01376328
Dawood, M., Cao, F., Jahangir, M. M., Zhang, G., and Wu, F. (2012). Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. J. Hazard. Mater. 20, 121–128. doi: 10.1016/j.jhazmat.2011.12.076
Degenhardt, J., Larsen, P. B., Howell, S. H., and Kochian, L. V. (1998). Aluminum resistance in the Arabidopsis mutantalr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol. 117, 19–27. doi: 10.1104/pp.117.1.19
Delhaize, E., Ma, J. F., and Ryan, P. R. (2012). Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 17, 341–348. doi: 10.1016/j.tplants.2012.02.008
Devi, S. R., Yamamoto, Y., and Matsumoto, H. (2003). An intracellular mechanism of aluminum tolerance associated with high antioxidant status in cultured tobacco cells. J. Inorg. Biochem. 97, 59–68. doi: 10.1016/S0162-0134(03)00182-X
Dhindsa, R. S., Plumb-Dhindsa, P., and Thorpe, T. A. (1981). Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32, 93–101. doi: 10.1093/jxb/32.1.93
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Ezaki, B., Gardner, R. C., Ezaki, Y., and Matsumoto, H. (2000). Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol. 122, 657–666. doi: 10.1104/pp.122.3.657
García-Mata, C., and Lamattina, L. (2013). Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci. 201, 66–73. doi: 10.1016/j.plantsci.2012.11.007
He, H. Y., He, L. F., Gu, M. H., and Li, X. F. (2012). Nitric oxide improves aluminum tolerance by regulating hormonal equilibrium in the root apices of rye and wheat. Plant Sci. 183, 123–130. doi: 10.1016/j.plantsci.2011.07.012
Horst, W., Asher, C., Cakmak, I., Szulkiewicz, P., and Wissemeier, A. (1992). Short-term responses of soybean roots to aluminium. J. Plant Physiol. 140, 174–178. doi: 10.1016/S0176-1617(11)80930-2
Huang, C. F., Yamaji, N., Chen, Z., and Ma, J. F. (2011). A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 69, 857–867. doi: 10.1111/j.1365-313X.2011.04837.x
Huang, C. F., Yamaji, N., Chen, Z., and Ma, J. F. (2012). A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 69, 857–867. doi: 10.1111/j.1365-313X.2011.04837.x
Huang, C. F., Yamaji, N., Mitani, N., Yano, M., Nagamura, Y., and Ma, J. F. (2009). A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell Online 21, 655–667. doi: 10.1105/tpc.108.064543
Ishikawa, S., Wagatsuma, T., Sasaki, R., and Ofei-Manu, P. (2000). Comparison of the amount of citric and malic acids in Al media of seven plant species and two cultivars each in five plant species. Soil Sci. Plant Nutr. 46, 751–758. doi: 10.1080/00380768.2000.10409141
Jin, Z., Xue, S., Luo, Y., Tian, B., Fang, H., Li, H., et al. (2013). Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol. Biochem. 62, 41–46. doi: 10.1016/j.plaphy.2012.10.017
Kengo, Y., Naoki, Y., and Jian Feng, M. (2011). An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 68, 1061–1069. doi: 10.1111/j.1365-313X.2011.04757.x
Kobayashi, Y., Yamamoto, Y., and Matsumoto, H. (2003). Aluminum-triggered production of superoxide anions and its possible involvement in root elongation inhibition in pea (Pisum sativum). Plant Cell Physiol. 44, S165–S165.
Kuo, M., and Kao, C. (2003). Aluminum effects on lipid peroxidation and antioxidative enzyme activities in rice leaves. Biol. Plant. 46, 149–152. doi: 10.1023/A:1022356322373
Li, L., Rose, P., and Moore, P. K. (2011). Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 51, 169–187. doi: 10.1146/annurev-pharmtox-010510-100505
Li, Z. G., and Gong, M. (2011). Mechanical stimulation-induced cross-adaptation in plants: an overview. J. Plant Biol. 54, 358–364. doi: 10.1007/s12374-011-9178-3
Li, Z. G., Gong, M., and Liu, P. (2012). Hydrogen sulfide is a mediator in H2O2-induced seed germination in Jatropha curcas. Acta Physiol. Plant. 34, 2207–2213. doi: 10.1007/s11738-012-1021-z
Li, Z. G., Yang, S. Z., Long, W. B., Yang, G. X., and Shen, Z. Z. (2013). Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 36, 1564–1572. doi: 10.1111/pce.12092
Lisjak, M., Teklic, T., Wilson, I. D., Whiteman, M., and Hancock, J. T. (2013). Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell Environ. 36, 1607–1616. doi: 10.1111/pce.12073
Liu, J., Hou, L., Liu, G., Liu, X., and Wang, X. (2011). Hydrogen sulfide induced by nitric oxide mediates ethylene-induced stomatal closure of Arabidopsis thaliana. Chin. Sci. Bull. 56, 3547–3553. doi: 10.1007/s11434-011-4819-y
Ma, J. F. (2005). Plant root responses to three abundant soil minerals: silicon, aluminum and iron. Crit. Rev. Plant Sci. 24, 267–281. doi: 10.1080/07352680500196017
Ma, J. F., and Hiradate, S. (2000). Form of aluminium for uptake and translocation in buckwheat (Fagopyrum esculentum Moench). Planta 211, 355–360. doi: 10.1007/s004250000292
Ma, J. F., Hiradate, S., and Matsumoto, H. (1998). High aluminum resistance in buckwheat II. Oxalic acid detoxifies aluminum internally. Plant Physiol. 117, 753–759. doi: 10.1104/pp.117.3.753
Ma, J. F., Ryan, P. R., and Delhaize, E. (2001). Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 6, 273–278. doi: 10.1016/S1360-1385(01)01961-6
Ma, J. F., Shen, R., Nagao, S., and Tanimoto, E. (2004). Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol. 45, 583–589. doi: 10.1093/pcp/pch060
Ma, J. F., Shen, R., Zhao, Z., Wissuwa, M., Takeuchi, Y., Ebitani, T., et al. (2002). Response of rice to Al stress and identification of quantitative trait loci for Al tolerance. Plant Cell Physiol. 43, 652–659. doi: 10.1093/pcp/pcf081
Ma, J. F., Zheng, S. J., Matsumoto, H., and Hiradate, S. (1997). Detoxifying aluminium with buckwheat. Nature 390, 569–570. doi: 10.1038/37518
Ma, Z., and Miyasaka, S. C. (1998). Oxalate exudation by taro in response to Al. Plant Physiol. 118, 861–865. doi: 10.1104/pp.118.3.861
Micheli, F. (2001). Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 6, 414–419. doi: 10.1016/S1360-1385(01)02045-3
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. doi: 10.1016/S1360-1385(02)02312-9
Nakamura, M., Kuramata, M., Kasugai, I., Abe, M., and Youssefian, S. (2009). Increased thiol biosynthesis of transgenic poplar expressing a wheat O-acetylserine (thiol) lyase enhances resistance to hydrogen sulfide and sulfur dioxide toxicity. Plant Cell Rep. 28, 313–323. doi: 10.1007/s00299-008-0635-5
Nakano, Y., and Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880.
Papernik, L. A., Bethea, A. S., Singleton, T. E., Magalhaes, J. V., Garvin, D. F., and Kochian, L. V. (2001). Physiological basis of reduced Al tolerance in ditelosomic lines of Chinese spring wheat. Planta 212, 829–834. doi: 10.1007/s004250000444
Pineros, M. A., Magalhaes, J. V., Alves, V. M. C., and Kochian, L. V. (2002). The physiology and biophysics of an aluminum tolerance mechanism based on root citrate exudation in maize. Plant Physiol. 129, 1194–1206. doi: 10.1104/pp.002295
Rengel, Z. (2004). Aluminium cycling in the soil-plant-animal-human continuum. Biometals 17, 669–689. doi: 10.1007/s10534-004-1201-4
Rengel, Z., and Reid, R. J. (1997). Uptake of Al across the plasma membrane of plant cells. Plant Soil 192, 31–35. doi: 10.1023/A:1004265913770
Schreiber, H. D., Jones, A. H., Lariviere, C. M., Mayhew, K. M., and Cain, J. B. (2011). Role of aluminum in red-to-blue color changes inHydrangea macrophylla sepals. Biometals 24, 1005–1015. doi: 10.1007/s10534-011-9458-x
Shan, C. J., Zhang, S. L., Li, D. F., Zhao, Y. Z., Tian, X. L., Zhao, X. L., et al. (2011). Effects of exogenous hydrogen sulfide on the ascorbate and glutathione metabolism in wheat seedlings leaves under water stress. Acta Physiol. Plant. 33, 2533–2540. doi: 10.1007/s11738-011-0746-4
Sharma, P., and Dubey, R. S. (2007). Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep. 26, 2027–2038. doi: 10.1007/s00299-007-0416-6
Shen, R., Ma, J., Kyo, M., and Iwashita, T. (2002). Compartmentation of aluminium in leaves of an Al-accumulator, Fagopyrum esculentum Moench. Planta 215, 394–398. doi: 10.1007/s00425-002-0763-z
Silva, I. R., Smyth, T. J., Raper, C. D., Carter, T. E., and Rufty, T. W. (2001). Differential aluminum tolerance in soybean: an evaluation of the role of organic acids. Physiol. Plant. 112, 200–210. doi: 10.1034/j.1399-3054.2001.1120208.x
Sivaguru, M., Fujiwara, T., Samaj, J., Baluska, F., Yang, Z. M., Osawa, H., et al. (2000). Aluminum-induced 1–>3-beta-D-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol. 124, 991–1005. doi: 10.1104/pp.124.3.991
Sun, C., Lu, L., Yu, Y., Liu, L., Hu, Y., Ye, Y., et al. (2016). Decreasing methylation of pectin caused by nitric oxide leads to higher aluminium binding in cell walls and greater aluminium sensitivity of wheat roots. J. Exp. Bot. 67, 979–989. doi: 10.1093/jxb/erv514
Tahara, K., Yamanoshita, T., Norisada, M., Hasegawa, I., Kashima, H., Sasaki, S., et al. (2008). Aluminum distribution and reactive oxygen species accumulation in root tips of two Melaleuca trees differing in aluminum resistance. Plant Soil 307, 167–178. doi: 10.1007/s11104-008-9593-5
Wang, H., Li, Y., Hou, J., Huang, J., and Liang, W. (2016). Nitrate reductase-mediated nitric oxide production alleviates Al-induced inhibition of root elongation by regulating the ascorbate-glutathione cycle in soybean roots. Plant Soil 410, 453–465. doi: 10.1007/s11104-016-3045-4
Wang, H. H., Hou, J. J., Li, Y., Zhang, Y. Y., Huang, J. J., and Liang, W. H. (2017). Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration. Plant Soil 416, 39–52. doi: 10.1007/s11104-017-3197-x
Wang, H. H., Huang, J. J., and Bi, Y. R. (2010). Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Sci. 179, 281–288. doi: 10.1016/j.plantsci.2010.05.014
Wang, W., Zhao, X. Q., Chen, R. F., Dong, X. Y., Lan, P., Ma, J. F., et al. (2015). Altered cell wall properties are responsible for ammonium-reduced aluminium accumulation in rice roots. Plant Cell Environ. 38, 1382–1390. doi: 10.1111/pce.12490
Wang, Y., Li, L., Cui, W., Xu, S., Shen, W., and Wang, R. (2012). Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 351, 107–119. doi: 10.1007/s11104-011-0936-2
Wang, Y. S., and Yang, Z. M. (2005). Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol. 46, 1915–1923. doi: 10.1093/pcp/pci202
Xia, J., Yamaji, N., Kasai, T., and Ma, J. F. (2010). Plasma membrane-localized transporter for aluminum in rice. Proc. Natl. Acad. Sci. U.S.A. 107, 18381–18385. doi: 10.1073/pnas.1004949107
Xia, J., Yamaji, N., and Ma, J. F. (2013). A plasma membrane-localized small peptide is involved in rice aluminum tolerance. Plant J. 76, 345–355. doi: 10.1111/tpj.12296
Xiong, J., Yang, Y., Fu, G., and Tao, L. (2015). Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 206, 118–126. doi: 10.1111/nph.13285
Yamamoto, Y., Kobayashi, Y., Devi, S. R., Rikiishi, S., and Matsumoto, H. (2002). Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 128, 63–72. doi: 10.1104/pp.010417
Yang, J. L., Li, Y. Y., Zhang, Y. J., Zhang, S. S., Wu, Y. R., Wu, P., et al. (2008). Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 146, 602–611. doi: 10.1104/pp.107.111989
Yang, J. L., Zhu, X. F., Peng, Y. X., Zheng, C., Li, G. X., Liu, Y., et al. (2011). Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol. 155, 1885–1892. doi: 10.1104/pp.111.172221
Yang, X. Y., Zeng, Z. H., Yan, J. Y., Fan, W., Bian, H. W., Zhu, M. Y., et al. (2013). Association of specific pectin methylesterases with Al-induced root elongation inhibition in rice. Physiol. Plant. 148, 502–511. doi: 10.1111/ppl.12005
Yokosho, K., Yamaji, N., Kashino-Fujii, M., and Ma, J. F. (2016). Retrotransposonmediated aluminum tolerance through enhanced expression of the citrate transporter OsFRDL4. Plant Physiol. 172, 2327–2336. doi: 10.1104/pp.16.01214
Yokosho, K., Yamaji, N., and Ma, J. F. (2011). An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 68, 1061–1069. doi: 10.1111/j.1365-313X.2011.04757.x
Yong, Q. C., Choo, C. H., Tan, B. H., Low, C.-M., and Bian, J.-S. (2010). Effect of hydrogen sulfide on intracellular calcium homeostasis in neuronal cells. Neurochem. Int. 56, 508–515. doi: 10.1016/j.neuint.2009.12.011
Zhang, H., Hu, L. Y., Hu, K. D., He, Y. D., Wang, S. H., and Luo, J. P. (2008a). Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J. Integr. Plant Biol. 50, 1518–1529. doi: 10.1111/j.1744-7909.2008.00769.x
Zhang, H., Li, Y. H., Hu, L. Y., Wang, S. H., Zhang, F. Q., and Hu, K. D. (2008b). Effects of exogenous nitric oxide donor on antioxidant metabolism in wheat leaves under aluminum stress. Russ. J. Plant Physiol. 55, 469–474. doi: 10.1134/S1021443708040067
Zhang, H., Hu, L. Y., Li, P., Hu, K. D., Jiang, C. X., and Luo, J. P. (2010a). Hydrogen sulfide alleviated chromium toxicity in wheat. Biol. Plant. 54, 743–747. doi: 10.1007/s10535-010-0133-9
Zhang, H., Tan, Z. Q., Hu, L. Y., Wang, S. H., Luo, J. P., and Jones, R. L. (2010b). Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J. Integr. Plant Biol. 52, 556–567. doi: 10.1111/j.1744-7909.2010.00946.x
Zhang, H., Wang, M., Hu, L., Wang, S., Hu, K., Bao, L., et al. (2010c). Hydrogen sulfide promotes wheat seed germination under osmotic stress. Russ. J. Plant Physiol. 57, 532–539. doi: 10.1134/S1021443710040114
Zhang, H., Tang, J., Liu, X. P., Wang, Y., Yu, W., Peng, W. Y., et al. (2009). Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J. Integr. Plant Biol. 51, 1086–1094. doi: 10.1111/j.1744-7909.2009.00885.x
Zheng, S. J., Ma, J. F., and Matsumoto, H. (1998a). Continuous secretion of organic acids is related to aluminium resistance during relatively long-term exposure to aluminium stress. Physiol. Plant. 103, 209–214. doi: 10.1034/j.1399-3054.1998.1030208.x
Zheng, S. J., Ma, J. F., and Matsumoto, H. (1998b). High aluminum resistance in buckwheat I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol. 117, 745–751.
Zhong, H., and Lauchli, A. (1993). Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. J. Exp. Bot. 44, 773–778. doi: 10.1093/jxb/44.4.773
Zhou, Y., Xu, X. Y., Chen, L. Q., Yang, J. L., and Zheng, S. J. (2012). Nitric oxide exacerbates Al-induced inhibition of root elongation in rice bean by affecting cell wall and plasma membrane properties. Phytochemistry 76, 46–51. doi: 10.1016/j.phytochem.2011.12.004
Zhu, C. Q., Zhu, X. F., Hu, A. Y., Wang, C., Wang, B., Dong, X. Y., et al. (2016). Differential effects of nitrogen forms on cell wall phosphorus remobilization in rice (Oryza sativa) are mediated by nitric oxide, pectin content and the expression of the phosphate transporter OsPT2. Plant Physiol. 171, 1407–1417. doi: 10.1104/pp.16.00176
Zhu, X. F., Lei, G. J., Jiang, T., Liu, Y., Li, G. X., and Zheng, S. J. (2012a). Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 236, 989–997.
Zhu, X. F., Shi, Y. Z., Lei, G. J., Fry, S. C., Zhang, B. C., Zhou, Y. H., et al. (2012b). XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 24, 4731–4747. doi: 10.1105/tpc.112.106039
Zhu, X. F., Wang, Z. W., Wan, J. X., Sun, Y., Wu, Y. R., Li, G. X., et al. (2014). Pectin enhances rice (Oryza sativa) root phosphorus remobilization. J. Exp. Bot. 66, 1017–1024. doi: 10.1093/jxb/eru461
Keywords: aluminum toxicity, hydrogen sulfide, cell wall, root elongation, gene expression, rice, antioxidant enzymes
Citation: Zhu CQ, Zhang JH, Sun LM, Zhu LF, Abliz B, Hu WJ, Zhong C, Bai ZG, Sajid H, Cao XC and Jin QY (2018) Hydrogen Sulfide Alleviates Aluminum Toxicity via Decreasing Apoplast and Symplast Al Contents in Rice. Front. Plant Sci. 9:294. doi: 10.3389/fpls.2018.00294
Received: 09 December 2017; Accepted: 20 February 2018;
Published: 06 March 2018.
Edited by:
Sergey Shabala, University of Tasmania, AustraliaReviewed by:
Jayakumar Bose, University of Adelaide, AustraliaJian Li Yang, Zhejiang University, China
Copyright © 2018 Zhu, Zhang, Sun, Zhu, Abliz, Hu, Zhong, Bai, Sajid, Cao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao C. Cao, caoxiaochuang@126.com Qian Y. Jin, jinqianyu@caas.cn;, jinqianyu1956@163.com
 Chun Q. Zhu
Chun Q. Zhu Jun H. Zhang1
Jun H. Zhang1 Chu Zhong
Chu Zhong Hussain Sajid
Hussain Sajid Xiao C. Cao
Xiao C. Cao