- The Sainsbury Laboratory, University of Cambridge, Cambridge, United Kingdom
Transposable elements (TEs) are often regarded as harmful genomic factors and indeed they are strongly suppressed by the epigenetic silencing mechanisms. On the other hand, the mobilization of TEs brings about variability of genome and transcriptome which are essential in the survival and evolution of the host species. The vast majority of such controlling TEs influence the neighboring genes in cis by either promoting or repressing the transcriptional activities. Although TEs are highly repetitive in the genomes and transcribed in specific stress conditions or developmental stages, the trans-acting regulatory roles of TE-derived RNAs have been rarely studied. It was only recently that TEs were investigated for their regulatory roles as a form of RNA. Particularly in plants, TEs are ample source of small RNAs such as small interfering (si) RNAs and micro (mi) RNAs. Those TE-derived small RNAs have potentials to affect non-TE transcripts by sequence complementarity, thereby generating novel gene regulatory networks including stress resistance and hybridization barrier. Apart from the small RNAs, a number of long non-coding RNAs (lncRNAs) are originated from TEs in plants. For example, a retrotransposon-derived lncRNA expressed in rice root acts as a decoy RNA or miRNA target mimic which negatively controls miRNA171. The post-transcriptional suppression of miRNA171 in roots ensures the stabilization of the target transcripts encoding SCARECROW-LIKE transcription factors, the key regulators of root development. In this review article, the recent discoveries of the regulatory roles of TE-derived RNAs in plants will be highlighted.
Introduction
Transposable elements (TEs) are the major constituent of many eukaryotic genomes. Especially in the cereal crops (e.g., barley, wheat, and maize), more than 80% of their genomes are made up of transposons (Tenaillon et al., 2010). TEs are classified to two major classes depending on their modes of transposition; class I and class II (Feschotte et al., 2002; Wicker et al., 2007). Class I TEs, also known as retrotransposons, move through RNA intermediates that are later converted to cDNAs, creating extra copies in the genome. The long terminal repeat (LTR) retrotransposons and the long interspersed nuclear elements (LINEs) are the two main types of retrotransposon. Both LTR retrotransposons and LINEs are autonomous elements since they encode for the proteins necessary for transposition, while those that depend on the autonomous elements such as large retrotransposon derivatives (LARDs), terminal repeat retrotransposons in miniature (TRIMs) and short interspersed nuclear elements (SINEs) are non-autonomous retrotransposons. Unlike class I, class II transposons, or DNA TEs, are excised from one location and insert to another genomic position by the transposase protein which is encoded within DNA TEs. Class II TEs include another subclass, Helitrons, which replicate through rolling circle amplification. In many plant genomes, retrotransposons are more abundant compared to class II elements. Particularly, the LTR retrotransposons are the predominant families of TEs in many plants (Vitte et al., 2017). The replication cycle of the LTR retrotransposons initiates with transcription of genomic copy by the host’s RNA polymerase (Pol) II. The mRNAs of LTR retrotransposons are subjected to both translation and reverse-transcription (Grandbastien, 1998). Autonomous LTR retrotransposons produce multiple proteins including GAG, aspartic protease, reverse-transcriptase, RNase H and integrase which are required for the completion of retrotransposition cycle. As a result of reverse-transcription, the linear and double-stranded DNA is produced which is known as extrachromosomal DNA (ecDNA). The ecDNAs are then transported back to the nucleus and integrate to genomic chromosomal DNA by the integrase protein.
Since TE mobilization can be mutagenic, the host genomes have evolved elaborate mechanisms to suppress their activities (Matzke and Mosher, 2014). TEs are primarily repressed by the epigenetic silencing pathways including histone modification and DNA methylation. In plants, the RNA-directed DNA methylation (RdDM) pathway plays a central role in TE silencing. Genomic regions marked by DNA methylation are recognized by the plant-specific RNA polymerase, RNA PolIV, which transcribes relatively short stretches of RNAs (Blevins et al., 2015; Li et al., 2015; Zhai et al., 2015). The transcribed RNAs are then duplexed by the RNA-dependent RNA polymerase (RDR) 2 and subsequently sliced to 24 nucleotide (nt) small interfering (si) RNAs by the DICER-like (DCL) 3. These 24 nt-siRNAs are bound by the ARGONAUTE (AGO) 4 proteins and interact with the nascent RNA transcribed by the RNA PolV. AGO4 then recruits multiple proteins including SU(VAR)3-9 HOMOLOG (SUVH) 4/5/6 and DOMAINS REARRANGED METHYLASE (DRM) 1/2 that mediate repressive histone modification (H3K9me2) and DNA methylation, respectively, thus contributing to reinforcement of the silenced state of TE chromatins (Zilberman et al., 2004; Tran et al., 2005; Zhong et al., 2014). TEs escaped from silencing or newly introduced to the genome are recognized by the RDR6-RdDM pathway that post-transcriptionally degrades TE mRNAs. RNA PolII-transcribed TE mRNAs are processed to 21 or 22 nt-siRNAs by the RDR6 and DCL2/4 (Creasey et al., 2014). These 21 or 22 nt-siRNAs associate with AGO1 and target TE mRNAs for degradation. Intriguingly, TE-associated siRNAs can also interact with non-TE target transcripts exerting certain regulatory roles in various biological processes. In mammals, PIWI-interacting RNAs regulate a large number of mRNAs and long non-coding RNAs (lncRNAs) in testis, suggesting widespread regulatory roles of TE-derived small RNAs in both plants and animals (Watanabe et al., 2015).
In addition to siRNAs, many plant miRNAs have been suggested to be evolved from TEs (Piriyapongsa and Jordan, 2008; Li et al., 2011). Although miRNAs are distinct from siRNAs in origin and biogenesis by definition (Borges and Martienssen, 2015), the categorization of small RNAs identified by deep sequencing has not been done with sufficient precision. In fact, considerable number of siRNAs are mis-annotated to miRNAs (McCue et al., 2012). Nonetheless, multiple lines of evidence indicate that TEs have co-opted to miRNAs since the repeated sequences associated with TEs can readily form RNA hairpin structures that can be subsequently processed by miRNA biogenesis pathways (Piriyapongsa and Jordan, 2008; Li et al., 2011). Moreover, the vast majority of lncRNAs are originated from TEs in mammalian as well as plant genomes (Kelley and Rinn, 2012; Liu et al., 2012; Kapusta et al., 2013), suggesting dynamic evolutionary exaptation of TEs in the form of RNA. In the following two sections, several examples of TE domestication to functionally relevant regulatory RNAs in plants will be explained.
Transposon-Derived Small RNAs
TE-siRNA in Stress Response
In the mutant of Decreased DNA methylation 1 (DDM1), a gene encoding ATP-dependent chromatin remodeler in Arabidopsis, global DNA methylation level is dramatically reduced, thereby numerous TEs are reactivated (Vongs et al., 1993; Creasey et al., 2014). A large fraction of those reactivated TEs are accompanied with the production of 21 or 22 nt-siRNAs, known as epigenetically activated siRNAs (easiRNAs) (Creasey et al., 2014). The easiRNAs target TE transcripts for cleavage ensuring the silencing of TEs at the post-transcriptional step. Interestingly, a subset of the easiRNAs in ddm1 mutants can interact with genic mRNAs reducing their expression levels. For example, siRNA854 is one of the easiRNAs generated in ddm1 mutant and produced from Athila6A TE. It targets 3′ UTR of UBP1 transcript which encodes a stress granule protein (Figure 1A; McCue et al., 2012). Using the multiple reporter gene constructs containing the 3′ UTR of UBP1, it was demonstrated that siRNA854 represses non-TE targets as well. The expression levels of Athila6A and siRNA854 are increasingly upregulated in multiple generations of ddm1 mutation. For example, the plants with ddm1 homozygous mutation for six generations (ddm1 F6) have higher levels of siRNA854 compared to ddm1 F2. The ubp1 mutants show strong susceptibility to osmotic stress and similar phenotype was also observed in ddm1 F6 but not in ddm1 F2 plants. Therefore, the targeting of Athila6A-derived siRNA854 to UBP1 transcript and alteration of resistance to abiotic stresses provides insight into how TEs adapted to changing environment in plants. In addition to UBP1, Athila6A-derived easiRNAs can target other genic mRNAs including AMS and HHP2 (McCue et al., 2013). Several of those targets were experimentally validated for the easiRNA-mediated repression by the short tandem target mimic (STTM) transgenic approach, however, the biological relevance of this regulation is yet to be answered.
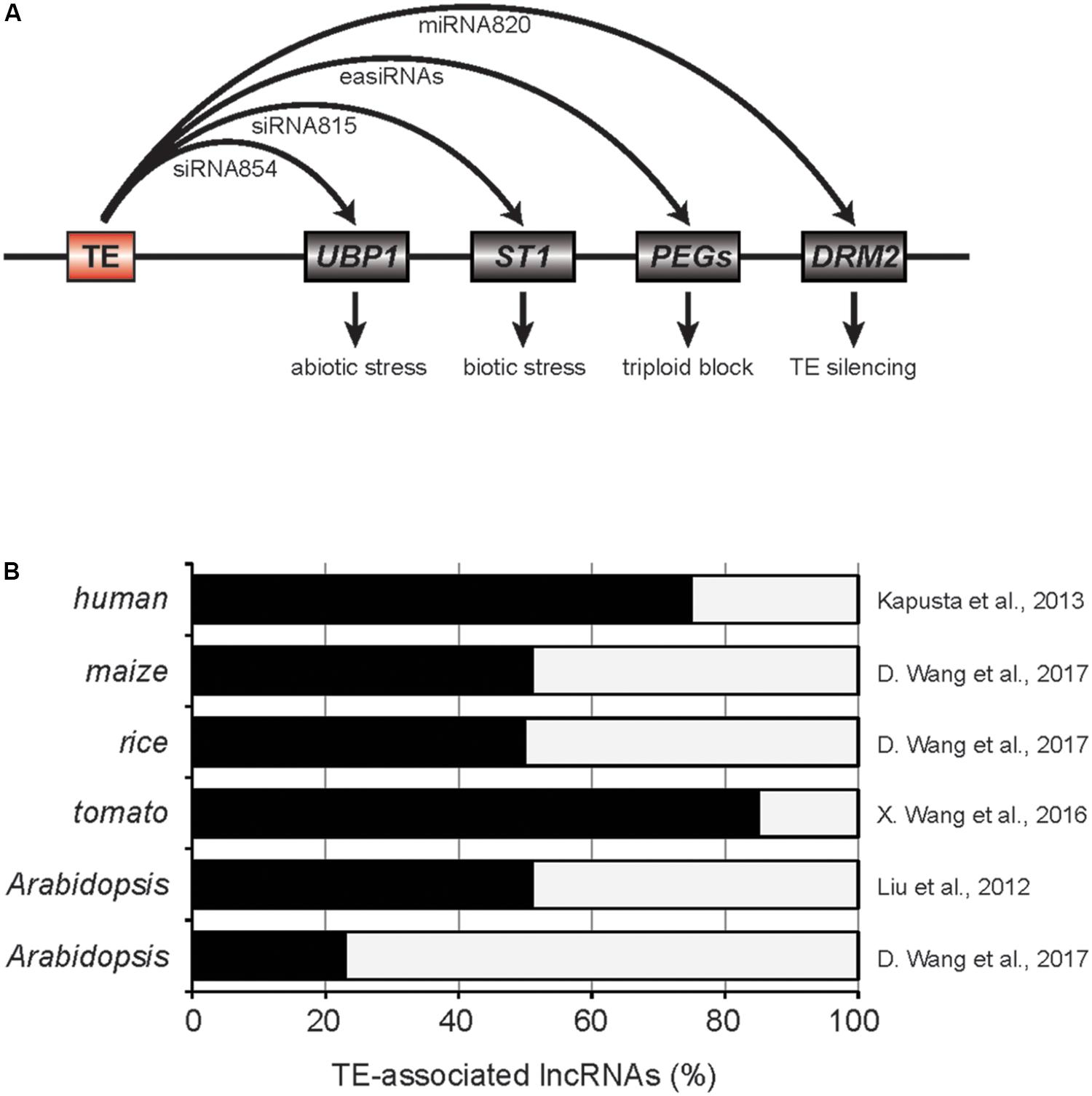
FIGURE 1. TE-associated small RNAs and long non-coding RNAs. (A) Roles of TE-derived small RNAs in plants. Red and gray boxes are TE and genes, respectively. PEGs, paternally expressed imprinted genes. (B) The fractions of lncRNAs associated to TEs. Related references are provided on the right.
A more recent paper by Zhang et al. (2016) suggested that TE-siRNA815 in rice can induce de novo DNA methylation in the target gene loci through RdDM pathway (Figure 1A). Two allelic transcription factor genes, WRKY45-1 and WRKY45-2, were previously shown to have opposing effects in the resistance to Xanthomonas oryzae pv. oryzae (Xoo). WRKY45-1 allele produces TE-siRNA815 from WANDERER_OS-type DNA TE located in the intron. TE-siRNA815 then recognizes the complementary sequence and deposits DNA methylation through RdDM pathway in the intron of ST1 locus, which is critical in the resistance against Xoo. On the other hand, WRKY45-2 allele lacks such siRNA producing region that ensures stable ST1 expression contributing to the pathogen resistance.
TE-siRNA in Hybridization Barrier
Since easiRNAs are mainly produced in the epigenetic mutants, it has been questioned if easiRNAs have a function in natural conditions. Two recent studies answered this question by demonstrating the roles of easiRNAs in the hybridization barrier in Arabidopsis (Borges et al., 2018; Martinez et al., 2018). The vegetative nuclei of pollen grains have reduced activity of DDM1 and numerous TEs are reactivated (Slotkin et al., 2009). Pollen-specific miRNA845b recognizes the conserved sequence [primer-binding site (PBS)] in the LTR retrotransposons activated in pollen and triggers the initial cleavage of LTR-TE mRNAs followed by the production of easiRNAs (Borges et al., 2018). Higher dosage of the paternal genome brings higher amount of easiRNAs in fertilization which gives rise to the unbalanced gametic siRNAs and ultimately seed failure (triploid block, Martinez et al., 2018). When the paternal easiRNA levels were suppressed by poliv mutation, the triploid blockage phenotype was partly restored, indicating a critical role of the paternal easiRNAs in the hybridization barrier (Martinez et al., 2018). The exact mechanism for the easiRNA-mediated triploid block is still unclear, however, it was suggested that the excess paternal easiRNAs might interfere with DNA methylation establishment around the paternally expressed imprinted genes by hijacking PolV-transcribed nascent transcripts (Figure 1A).
TE-Small RNA as Anti-silencing Factor
Transposable elements have often domesticated to miRNA genes in plants (Li et al., 2011). One example is miRNA820 of rice. miRNA820 is 22 or 24 nt in size and is originated from the internal region of CACTA DNA TE (Nosaka et al., 2012). Interestingly, miRNA820 targets the transcripts of DRM2 that encodes a de novo DNA methyltransferase (Figure 1A). The targeting of miRNA820 to DRM2 is evolutionarily conserved in the Oryza genus and the repression of DRM2 gene results in strong reduction in DNA methylation and transcriptional upregulation of many TEs. Therefore, miRNA820 can be seen as an anti-silencing factor encoded within a TE that works at the post-transcriptional level. Similarly, UBP1 is the Arabidopsis homolog of TIA-1 in mammals which is known to suppress the viral translation of Tick-Borne Encephalitis Virus (Albornoz et al., 2014). McCue et al. (2013) have also shown that UBP1 protein forms the cytoplasmic stress granules in abiotic stress condition or when heterochromatic TE silencing is released, for instance in ddm1 mutant. As is the case in mammals that TIA-1 inhibits the viral translation, the level of GAG protein encoded in Athila6A was elevated in ddm1 rdr6 ubp1 triple mutants (McCue et al., 2013). This data supports the notion that UBP1 acts on the activated TE mRNAs to suppress their translation and therefore TE-siRNAs are the repressors of the host TE silencing mechanism.
Transposon-Derived lncRNAs
There is emerging evidence of TE domestication to lncRNAs in both mammalian and plant genomes (Figure 1B; Kelley and Rinn, 2012; Kapusta et al., 2013; Johnson and Guigo, 2014; Liu et al., 2015; Wang et al., 2015, 2016; Quattro et al., 2017). LncRNA can be defined as a transcript of at least 200 bp in size but has low protein-coding potential (Liu et al., 2015). It is well-studied that lncRNAs perform various cellular function including the recruitment of the epigenetic regulators to target chromatin or the sequestration of miRNAs (Franco-Zorrilla et al., 2007; Heo and Sung, 2011; Csorba et al., 2014). In the past decade, transcriptomic analyses have dramatically expanded the catalog of the lncRNAs in various tissues and stress conditions of many plant species. Despite the large number of plant lncRNAs identified so far, however, the biological roles are still largely unexplored. In this section, the current status of plant TE-lncRNA studies will be discussed.
TE-lncRNA in Stress Response
Since many TEs in plants possess stress-responsive cis-acting elements within them (Paszkowski, 2015), TE-lncRNAs often appear in specific stress conditions (Liu et al., 2012; Quattro et al., 2017; Wang et al., 2017). In a recent report, Wang et al. (2017) interrogated the lncRNAs in Arabidopsis, rice and maize under various abiotic stresses. There was a large discrepancy in TE families that TE-lncRNAs are originated from; RC/Helitron in Arabidopsis, MITEs in rice and LTR retrotransposons in maize were predominantly overrepresented in TE-lncRNAs (Wang et al., 2017). Particularly, TE-lncRNA11195 in Arabidopsis contains an LTR-type retrotransposon and is activated after abiotic stresses or ABA treatment. The deletion of the LTR sequence compromised the ABA responsiveness, suggesting that LTR sequence conferred the stress responsiveness to TE-lncRNA11195 (Wang et al., 2017). TE-lncRNA11195 was tested for its role in stress response using T-DNA insertional mutants. Interestingly, two independent mutant lines showed marked increase in resistance to abscisic acid (ABA) in root elongation and shoot fresh weight (Wang et al., 2017). TE-lncRNAs in tomato were also described to be responsive for both abiotic and biotic stresses (Wang et al., 2015), however, the biological function has not been investigated as yet.
TE-lncRNA and Development
In mammals, the majority of lncRNAs are derived from TEs and exhibit strong tissue-specific expression pattern (Kelley and Rinn, 2012; Kapusta et al., 2013). Similarly, TE activation in plants is associated with specific development stages potentiating the emergence of tissue-specific TE-lncRNA (Hsieh et al., 2009; Slotkin et al., 2009; Baubec et al., 2014; Cho and Paszkowski, 2017). Recently, Cho and Paszkowski (2017) have investigated the expression pattern of TEs in various rice tissues and identified a retrotransposon-derived transcript called MIKKI which is specifically transcribed in rice roots. MIKKI contains multiple introns and has low coding potential, which is a strong sign of domestication to lncRNA. Intriguingly, the fourth intron of MIKKI is derived from an independent family of retrotransposon and the splicing of this intron generates a binding site for miR171 in the exon–exon junction. Despite the miR171-binding sequence, MIKKI mRNAs are not cleaved by miR171. The miR171-binding site of MIKKI does not perfectly base-pair with its cognate miRNA but has two mismatches at the positions where the cleavage is supposed to occur. It is very well-known that mismatches in the cleavage positions attenuate the cleavage activity of miRNA and is regarded as the signature of miRNA target mimic (Franco-Zorrilla et al., 2007; Yan et al., 2012; Reichel et al., 2015). Indeed, the knock-out mutants of MIKKI that had lost the target mimicking sequence showed higher levels of miR171, while overexpression of MIKKI resulted in the downregulation of miR171. miR171 targets the mRNAs encoding SCARECROW-Like (SCL) transcription factors which are critical regulators of root development (Wang et al., 2010). Therefore, MIKKI evolved from retrotransposons in rice and was positively selected to suppress miR171 in root. This in turn stabilizes the mRNAs of SCLs which are essential in the root development. Similarly, Quattro et al., 2017 also identified multiple TE-derived lncRNAs from Brachypodium genome that are able to interact with miRNAs, however, their target mimicry activities are yet to be confirmed.
Concluding Remarks
Transposon-derived RNAs have been underestimated for a long time and their biological function have just started to be unveiled. The fact that TEs are repeated in the genome has significantly hampered the investigation of transposons so far. For example, the short length of the next-generation sequencing reads causes drastic ambiguity and imprecision in mapping the TE reads. Recent advance of the long read sequencing of PacBio (Disdero and Filée, 2017) and Oxford Nanopore (Debladis et al., 2017) is expected to overcome this shortcoming. In addition, due to the multiplicity of TEs in the genome and possible redundancy between them, the genetic analyses of TEs have been challenging. CRISPR-mediated mutagenesis has become more efficient and even a large deletion of an entire TE can be made by triggering the double-strand breaks in the flanking regions of the targeted TE (Gao et al., 2016; Ordon et al., 2017). Another option worth to consider is the population genetics approach. There is increasing number of available genome sequences and the number will grow exponentially as the sequencing cost drops. A large scale genome resequencing data analysis performed in the Arabidopsis natural accessions revealed that the TE landscape is very dynamic and the transcriptomic, epigenomic as well as phenotypic variations are attributed to TEs (Quadrana et al., 2016; Stuart et al., 2016). Taken all together, it seems that now is the best time to explore the hidden roles of TEs in plants by applying the new technologies developed recently.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Funding
This work was supported by the European Research Council (EVOBREED) [322621] and the Gatsby Charitable Foundation [AT3273/GLE].
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I thank Dr. Jayne Griffiths for critical reading.
References
Albornoz, A., Carletti, T., Corazza, G., and Marcello, A. (2014). The stress granule component TIA-1 binds tick-borne encephalitis virus RNA and is recruited to perinuclear sites of viral replication to inhibit viral translation. J. Virol. 88, 6611–6622. doi: 10.1128/JVI.03736-13
Baubec, T., Finke, A., Mittelsten Scheid, O., and Pecinka, A. (2014). Meristem-specific expression of epigenetic regulators safeguards transposon silencing in Arabidopsis. EMBO Rep. 15, 446–452. doi: 10.1002/embr.201337915
Blevins, T., Podicheti, R., Mishra, V., Marasco, M., Wang, J., Rusch, D., et al. (2015). Identification of Pol IV and RDR2-dependent precursors of 24 nt siRNAs guiding de novo DNA methylation in Arabidopsis. Elife 4:e09591. doi: 10.7554/eLife.09591
Borges, F., and Martienssen, R. A. (2015). The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 16, 727–741. doi: 10.1038/nrm4085
Borges, F., Parent, J. S., Van Ex, F., Wolff, P., Martínez, G., Köhler, C., et al. (2018). Transposon-derived small RNAs triggered by miR845 mediate genome dosage response in Arabidopsis. Nat. Genet. 50, 186–192. doi: 10.1038/s41588-017-0032-5
Cho, J., and Paszkowski, J. (2017). Regulation of rice root development by a retrotransposon acting as a microRNA sponge. Elife 6:e30038. doi: 10.7554/eLife.30038
Creasey, K. M., Zhai, J., Borges, F., Van Ex, F., Regulski, M., Meyers, B. C., et al. (2014). miRNAs trigger widespread epigenetically activated siRNAs from transposons in Arabidopsis. Nature 508, 411–415. doi: 10.1038/nature13069
Csorba, T., Questa, J. I., Sun, Q., and Dean, C. (2014). Antisense COOLAIR mediates the coordinated switching of chromatin states at FLC during vernalization. Proc. Natl. Acad. Sci. U.S.A. 111, 16160–16165. doi: 10.1073/pnas.1419030111
Debladis, E., Llauro, C., Carpentier, M. C., Mirouze, M., and Panaud, O. (2017). Detection of active transposable elements in Arabidopsis thaliana using Oxford Nanopore Sequencing technology. BMC Genomics 18:537. doi: 10.1186/s12864-017-3753-z
Disdero, E., and Filée, J. (2017). LoRTE: detecting transposon-induced genomic variants using low coverage PacBio long read sequences. Mob. DNA 8:5. doi: 10.1186/s13100-017-0088-x
Feschotte, C., Jiang, N., and Wessler, S. R. (2002). Plant transposable elements: where genetics meets genomics. Nat. Rev. Genet. 3, 329–341. doi: 10.1038/nrg793
Franco-Zorrilla, J. M., Valli, A., Todesco, M., Mateos, I., Puga, M. I., Rubio-Somoza, I., et al. (2007). Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 39, 1033–1037. doi: 10.1038/ng2079
Gao, X., Chen, J., Dai, X., Zhang, D., and Zhao, Y. (2016). An effective strategy for reliably isolating heritable and Cas9-free Arabidopsis mutants generated by CRISPR/Cas9-mediated genome editing. Plant Physiol. 171, 1794–1800. doi: 10.1104/pp.16.00663
Grandbastien, M. A. (1998). Activation of plant retrotransposons under stress conditions. Trends Plant Sci. 3, 181–187. doi: 10.1016/S1360-1385(98)01232-1
Heo, J. B., and Sung, S. (2011). Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 331, 76–80. doi: 10.1126/science.1197349
Hsieh, T. F., Ibarra, C. A., Silva, P., Zemach, A., Eshed-Williams, L., Fischer, R. L., et al. (2009). Genome-wide demethylation of Arabidopsis endosperm. Science 324, 1451–1454. doi: 10.1126/science.1172417
Johnson, R., and Guigo, R. (2014). The RIDL hypothesis: transposable elements as functional domains of long noncoding RNAs. RNA 20, 959–976. doi: 10.1261/rna.044560.114
Kapusta, A., Kronenberg, Z., Lynch, V. J., Zhuo, X., Ramsay, L., Bourque, G., et al. (2013). Transposable elements are major contributors to the origin, diversification, and regulation of vertebrate long noncoding RNAs. PLoS Genet. 9:e1003470. doi: 10.1371/journal.pgen.1003470
Kelley, D. R., and Rinn, J. L. (2012). Transposable elements reveal a stem cell-specific class of long noncoding RNAs. Genome Biol. 13:R107. doi: 10.1186/gb-2012-13-11-r107
Li, S., Vandivier, L. E., Tu, B., Gao, L., Won, S. Y., Li, S., et al. (2015). Detection of Pol IV/RDR2-dependent transcripts at the genomic scale in Arabidopsis reveals features and regulation of siRNA biogenesis. Genome Res. 25, 235–245. doi: 10.1101/gr.182238.114
Li, Y., Li, C., Xia, J., and Jin, Y. (2011). Domestication of transposable elements into microRNA genes in plants. PLoS One 6:e19212. doi: 10.1371/journal.pone.0019212
Liu, J., Jung, C., Xu, J., Wang, H., Deng, S., Bernad, L., et al. (2012). Genome-wide analysis uncovers regulation of long intergenic noncoding RNAs in Arabidopsis. Plant Cell 24, 4333–4345. doi: 10.1105/tpc.112.102855
Liu, J., Wang, H., and Chua, N. H. (2015). Long noncoding RNA transcriptome of plants. Plant Biotechnol. J. 13, 319–328. doi: 10.1111/pbi.12336
Martinez, G., Wolff, P., Wang, Z., Moreno-romero, J., Santos-gonzález, J., Conze, L. L., et al. (2018). Paternal easiRNAs regulate parental genome dosage in Arabidopsis. Nat. Genet. 50, 193–198. doi: 10.1038/s41588-017-0033-4
Matzke, M. A., and Mosher, R. A. (2014). RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat. Rev. Genet. 15, 394–408. doi: 10.1038/nrg3683
McCue, A. D., Nuthikattu, S., Reeder, S. H., and Slotkin, R. K. (2012). Gene expression and stress response mediated by the epigenetic regulation of a transposable element small RNA. PLoS Genet. 8:e1002474. doi: 10.1371/journal.pgen.1002474
McCue, A. D., Nuthikattu, S., Reeder, S. H., and Slotkin, R. K. (2013). Genome-wide identification of genes regulated in trans by transposable element small interfering RNAs. RNA Biol. 10, 1379–1395. doi: 10.4161/rna.25555
Nosaka, M., Itoh, J. I., Nagato, Y., Ono, A., Ishiwata, A., and Sato, Y. (2012). Role of transposon-derived small RNAs in the interplay between genomes and parasitic DNA in rice. PLoS Genet. 8:e1002953. doi: 10.1371/journal.pgen.1002953
Ordon, J., Gantner, J., Kemna, J., Schwalgun, L., Reschke, M., Streubel, J., et al. (2017). Generation of chromosomal deletions in dicotyledonous plants employing a user-friendly genome editing toolkit. Plant J. 89, 155–168. doi: 10.1111/tpj.13319
Paszkowski, J. (2015). Controlled activation of retrotransposition for plant breeding. Curr. Opin. Biotechnol. 32, 200–206. doi: 10.1016/j.copbio.2015.01.003
Piriyapongsa, J., and Jordan, I. K. (2008). Dual coding of siRNAs and miRNAs by plant transposable elements. RNA 14, 814–821. doi: 10.1261/rna.916708.ferred
Quadrana, L., Silveira, A. B., Mayhew, G. F., LeBlanc, C., Martienssen, R. A., Jeddeloh, J. A., et al. (2016). The Arabidopsis thaliana mobilome and its impact at the species level. Elife 5:e15716. doi: 10.7554/eLife.15716
Quattro, C. D., Enrico Pè, M., and Bertolini, E. (2017). Long noncoding RNAs in the model species Brachypodium distachyon. Sci. Rep. 7:11252. doi: 10.1038/s41598-017-11206-z
Reichel, M., Li, Y., Li, J., and Millar, A. A. (2015). Inhibiting plant microRNA activity: molecular SPONGEs, target MIMICs and STTMs all display variable efficacies against target microRNAs. Plant Biotechnol. J. 13, 915–926. doi: 10.1111/pbi.12327
Slotkin, R. K., Vaughn, M., Borges, F., Tanurdžić, M., Becker, J. D., Feijó, J. A., et al. (2009). Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136, 461–472. doi: 10.1016/j.cell.2008.12.038
Stuart, T., Eichten, S. R., Cahn, J., Karpievitch, Y. V., Borevitz, J. O., and Lister, R. (2016). Population scale mapping of transposable element diversity reveals links to gene regulation and epigenomic variation. Elife 5:e20777. doi: 10.7554/eLife.20777
Tenaillon, M. I., Hollister, J. D., and Gaut, B. S. (2010). A triptych of the evolution of plant transposable elements. Trends Plant Sci. 15, 471–478. doi: 10.1016/j.tplants.2010.05.003
Tran, R. K., Zilberman, D., De Bustos, C., Ditt, R. F., Henikoff, J. G., Lindroth, A. M., et al. (2005). Chromatin and siRNA pathways cooperate to maintain DNA methylation of small transposable elements in Arabidopsis. Genome Biol. 6:R90. doi: 10.1186/gb-2005-6-11-r90
Vitte, C., Fustier, M. A., Alix, K., and Tenaillon, M. I. (2017). The bright side of transposons in crop evolution. Brief. Funct. Genomics 13, 276–295. doi: 10.1093/bfgp/elu002
Vongs, A., Kakutani, T., Martienssen, R. A., and Richardstt, E. J. (1993). Arabidopsis thaliana DNA methylation mutants. Science 260, 1926–1928. doi: 10.1126/science.8316832
Wang, D., Qu, Z., Yang, L., Zhang, Q., Liu, Z. H., Do, T., et al. (2017). Transposable elements (TEs) contribute to stress-related long intergenic noncoding RNAs in plants. Plant J. 90, 133–146. doi: 10.1111/tpj.13481
Wang, L., Mai, Y. X., Zhang, Y. C., Luo, Q., and Yang, H. Q. (2010). MicroRNA171c-targeted SCL6-II, SCL6-III, and SCL6-IV genes regulate shoot branching in Arabidopsis. Mol. Plant 3, 794–806. doi: 10.1093/mp/ssq042
Wang, X., Ai, G., Zhang, C., Cui, L., Wang, J., Li, H., et al. (2015). Expression and diversification analysis reveals transposable elements play important roles in the origin of Lycopersicon-specific lncRNAs in tomato. New Phytol. 209, 1442–1455. doi: 10.1111/nph.13718
Wang, Z., Schwacke, R., and Kunze, R. (2016). DNA damage-induced transcription of transposable elements and long non-coding RNAs in Arabidopsis is rare and ATM-dependent. Mol. Plant 9, 1142–1155. doi: 10.1016/j.molp.2016.04.015
Watanabe, T., Cheng, E. C., Zhong, M., and Lin, H. (2015). Retrotransposons and pseudogenes regulate mRNAs and lncRNAs via the piRNA pathway in the germline. Genome Res. 25, 368–380. doi: 10.1101/gr.180802.114
Wicker, T., Sabot, F., Hua-Van, A., Bennetzen, J. L., Capy, P., Chalhoub, B., et al. (2007). A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 8, 973–982. doi: 10.1038/nrg2165
Yan, J., Gu, Y., Jia, X., Kang, W., Pan, S., Tang, X., et al. (2012). Effective small RNA destruction by the expression of a short tandem target mimic in Arabidopsis. Plant Cell 24, 415–427. doi: 10.1105/tpc.111.094144
Zhai, J., Bischof, S., Wang, H., Feng, S., Lee, T. F., Teng, C., et al. (2015). A one precursor one siRNA model for pol IV-dependent siRNA biogenesis. Cell 163, 445–455. doi: 10.1016/j.cell.2015.09.032
Zhang, H., Tao, Z., Hong, H., Chen, Z., Wu, C., Li, X., et al. (2016). Transposon-derived small RNA is responsible for modified function of WRKY45 locus. Nat. Plants 2:16016. doi: 10.1038/nplants.2016.16
Zhong, X., Du, J., Hale, C. J., Gallego-Bartolome, J., Feng, S., Vashisht, A. A., et al. (2014). Molecular mechanism of action of plant DRM de novo DNA methyltransferases. Cell 157, 1050–1060. doi: 10.1016/j.cell.2014.03.056
Keywords: transposable elements, domestication, small RNA, long non-coding RNA, microRNA target mimic
Citation: Cho J (2018) Transposon-Derived Non-coding RNAs and Their Function in Plants. Front. Plant Sci. 9:600. doi: 10.3389/fpls.2018.00600
Received: 28 February 2018; Accepted: 16 April 2018;
Published: 03 May 2018.
Edited by:
Dora Szakonyi, Instituto Gulbenkian de Ciência (IGC), PortugalReviewed by:
German Martinez, Swedish University of Agricultural Sciences, SwedenDeqiang Zhang, Beijing Forestry University, China
Copyright © 2018 Cho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jungnam Cho, jungnam.cho@slcu.cam.ac.uk
 Jungnam Cho
Jungnam Cho