- 1Department of Psychiatry, Melbourne Neuropsychiatry Centre, The University of Melbourne, Melbourne, VIC, Australia
- 2Division of Medical Imaging and Technology, Department of Clinical Science, Intervention and Technology (CLINTEC), Karolinska Institutet, Stockholm, Sweden
- 3Unit of Metabolism, Department of Medicine Huddinge, Karolinska Institutet, Stockholm, Sweden
Psychomotor disturbances (PMD) are a classic feature of depressive disorder that provides rich clinical information. The aim our narrative review was to characterize the functional anatomy of PMD by summarizing findings from neuroimaging studies. We found evidence across several neuroimaging modalities that suggest involvement of fronto-striatal neurocircuitry, and monoaminergic pathways and metabolism. We suggest that PMD in major depressive disorder emerge from an alteration of limbic signals, which influence emotion, volition, higher-order cognitive functions, and movement.
Introduction
Psychomotor signs are a classic feature of major depressive disorder that already attracted attention over a century ago (1). Emil Kraepelin gave a vivid and still valid description of psychomotor disturbances (PMD) in his chapter on general symptomatology in Lehrbuch des Psychiatrie, 1907: “The psychomotor retardation, which is the most important disturbance in the depressed states of manic-depressive insanity, is probably due to a […] increase in resistance […] In spite of every apparent exertion, the patients cannot utter a word or at best answer only in monosyllables, and are unable to eat, stand up, or dress. As a rule they clearly recognize the enormous pressure lying upon them, which they are unable to overcome” (2).
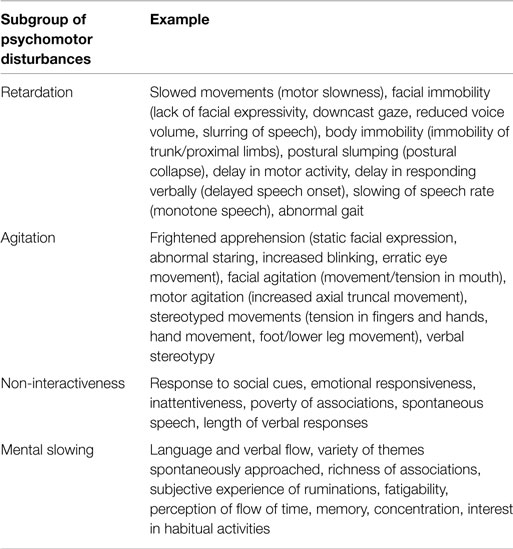
Psychomotor disturbances in depressive disorder can be broadly classified in to four subgroups of symptoms and signs based on three available clinical rating scales designed to characterize them [CORE, motor agitation and retardation scale (MARS), Widlöcher scale] (3–5): retardation, agitation, non-interactiveness, and mental slowing (Table 1). The symptoms and signs of PMD therefore entail a wide range of brain functions including motor performance, executive function, volition, and drive. These provide rich clinical information (i.e., diagnostic subgroup, prognosis, treatment) (6, 7).
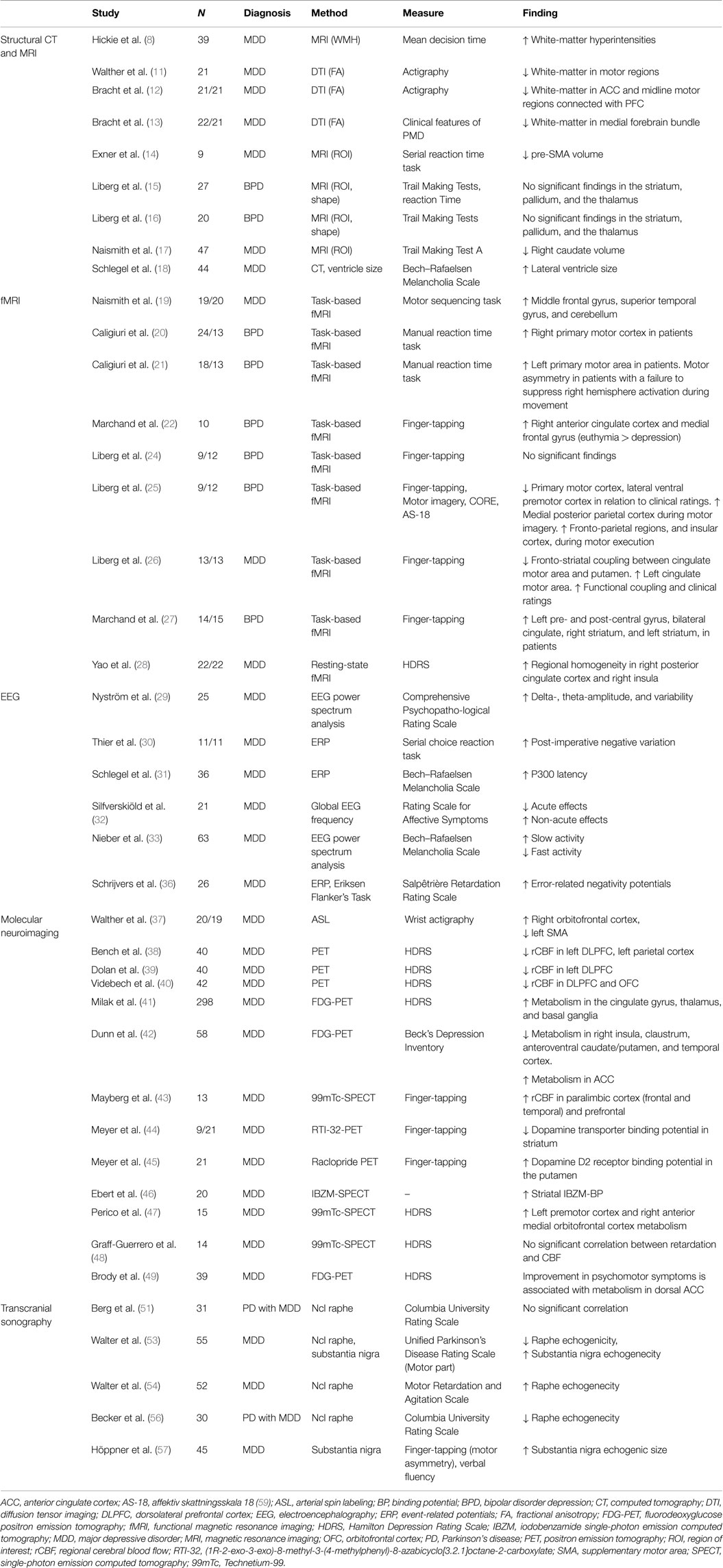
No previous review has focused specifically on neuroimaging findings related to PMD in major depressive disorder. The aim of this narrative review is to characterize the functional anatomy of PMD in major depressive disorder by summarizing findings from human neuroimaging studies that probe structure, function, neurochemistry, and connectivity.
Structural Neuroimaging
Structural aberrations in white matter are the most prominent structural neuroimaging findings associated with PMD in depressive disorder.
White-matter alterations (hyperintensities, WHI; and white-matter fiber integrity), are one of the most reproduced findings in mood disorders. White-matter hyperintensities (WHIs) are radiological hyperintense regions of white matter with elusive etiology in MRI images. They are primarily associated with late-life depression, but are also more common in major depressive disorder in younger age groups. The extent of WHIs correlates with illness severity, poor treatment response, and decreased psychomotor speed on several neuropsychological tests (8). White-matter tissue broadly comprises glial cells with myelin surrounding axons. Currently, the general understanding is that the WHIs alterations observed in depression arise from small vessel disease that lead to disruption of white-matter pathways (9). However, other disease mechanisms involving white-matter tissue may also lead to disruptions of specific neurocircuits and lead to psychiatric symptoms such as PMD (10).
White-matter fiber integrity can be assessed with diffusion-weighted imaging. One study by Walther et al. (11) who specifically addressed psychomotor functioning in depressive disorder used diffusion-weighted magnetic resonance imaging and actigraphy – an objective measure of the general activity level in an individual. It showed that lower activity levels correlate with measures of differential myelinization in the frontal lobe and posterior cingulate region, and that there is a negative correlation between the same measures in the white matter beneath the primary motor cortex and in the parahippocampal region. The authors conclude that changes in psychomotor function in depressive disorder may be linked to changes in white matter in motor regions. Bracht et al. used diffusion-weighted imaging to investigate white-matter microstructure in relation to PMD. They found a positive association between decreased physical motor activity and alterations in paralimbic and motor midline regions not only involved in volitional movement but also involvement of ascending mesocortical dopamine pathways in clinical states with prominent PMD (12, 13).
To this date, few studies have investigated the relation between gray matter volume and PMD in major depressive disorder. Current findings involve volume reductions in several pre-executive parts of the motor system. One volumetric study showed that thinning of the right presupplementary motor cortex (pre-SMA) is associated with impaired performance on a motor learning test (14). The pre-SMA is a part of the mesial premotor cortex that advances signals from the prefrontal regions, engaged in higher-order cognitive functions. In studies measuring subcortical volumes and regional shape alterations, no significant associations could be found between performance on a psychomotor task (trail making test variations) and the volumes of striatum, pallidum, and thalamus in depressed subjects (15, 16). Another study found that reduced caudate nucleus volumes predicts decreased psychomotor speed in depressed subjects >50 years old (17).
Only one study, using CT, has assessed cerebrospinal fluid space size. This study found that the size of the third ventricle was associated with clinical ratings of psychomotor retardation (18).
Functional Neuroimaging
Blood–oxygen-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) is currently the most prevalent method for studying neural activation patterns during experimental tasks in patients with depressive disorder. A few research teams have specifically addressed PMD using fMRI and experimental motor tasks, clinical ratings of psychomotor disturbance, or motor physiology metrics (i.e., actigraphy, reaction time). Two types of studies have been employed – task and non-task based studies. Naismith et al. (19) used a motor sequence task (button press response) to study motor learning, and found increased activation of lateral prefrontal cortex, superior temporal regions, and the cerebellum. Caligiuri et al. (20, 21) studied motor execution using a manual reaction time task, and found increased activation during movement in the primary motor cortex, alongside motor asymmetry. Five other studies investigated motor speed using different finger-tapping variations (22–27), and suggest an increased activation in both motor and paralimbic regions, and with altered fronto-striatal coupling among patients. One non-task, resting-state study, by Yao et al. (28) corroborates the hyperactivation of paralimbic regions in patients.
Electroencephalography
Electroencephalography (EEG) is used to study power amplitude of particular frequency spectrums, hemisphere asymmetry, and chronometric features of cortical neural activation. PMD have been associated with greater variability and increased amplitudes in the delta (<4 Hz) and theta (4–7 Hz) spectrum, but not with hemisphere asymmetry (29). The post-imperative negative variation is a metric related to frontal lobe function, and has been associated with psychomotor slowing in a choice reaction task (30). Another frontal metric (P300) has also been correlated positively correlated with PMD (31). Interestingly, this study also showed that only clinical ratings more focused on PMD than the Hamilton depression ratings scale (HDRS) predicted P300 latency. In a group of patients receiving electroconvulsive treatment, clinical ratings of PMD were positively correlated with frequency decreases during initial improvement, whereas the reverse relationship was found during the later partial remission phase (32). One study by Nieber et al. (33) showed a positive correlation between decreased frequencies in particular regions of the theta and alpha (7–13 Hz) spectrum and overall retardation, with motor retardation, in particular. In that study, increased frequency in particular regions of in the alpha and beta spectrum was negatively correlated with PMD. Error-related negativity and positive-negativity are metrics associated with anterior and posterior cingulate cortex function, respectively (34, 35). These metrics have been associated with a slowing of psychomotor performance in subjects during action monitoring, but only positive-negativity differentiated patients and controls (36).
Molecular Neuroimaging
Single-photon emission tomography (SPECT), positron emission tomography (PET), and arterial spin labeling (ASL) are the three molecular neuroimaging methods that have been used to study PMD. These three methods measure regional cerebral blood flow, glucose metabolism, oxygen consumption, or synaptic transmission factors. Walther et al. (37) used ASL and actigraphy to measure the correlation between regional cerebral blood flow and general motor activity outside of the scanner environment in depressed subjects. The study showed a positive correlation between physical activity and blood perfusion in the right orbitofrontal cortex, and a negative correlation with left supplementary motor area perfusion. The available evidence from PET and SPECT studies also suggests that PMD in depression are associated with decreased DLPFC metabolism (38–40), increased ACC metabolism (41–43), and a lower dopaminergic tone and altered metabolism in striatal regions (41, 42, 44–47). However, a SPECT study by Graff-Guerrero et al. (48) failed to reproduce these associations between clinical rating of PMD and cerebral blood flow. One longitudinal study also suggests that improvement of psychomotor slowing is associated with increased activation in the dorsal ACC (49).
Transcranial Ultrasound
Hypo- or hyperechogenicity measured by transcranial sonography in vivo reflect changes in tissue impedance, likely due to alterations of microarchitecture such as shifts in cell density, changes in interstitial matrix composition, or alterations of fiber tract integrity (50, 51). Those transcranial ultrasound studies that have investigated PMD in major depression have focused on the serotonergic raphe nuclei and the dopaminergic substantia nigrae. A significantly reduced echogenicity of the mesencephalic midline raphe nuclei has been reported in depressed subjects (52). Hypoechogenicity of the raphe nuclei can be found in 50–70% of unipolar depressed subjects compared to 10% in healthy subjects (53). Hypoechogenicity of the raphe nuclei of the brain stem is associated with better treatment response to serotonin reuptake inhibitors (54) and with symptom severity in suicidal ideation (55). One study could not find any association between echogenicity of the raphe nuclei and PMD (51), another found a positive correlation with the degree of psychomotor retardation (56), and a third a negative correlation with psychomotor retardation (54). Hoeppner et al. showed that substantia nigra echogenic size correlates with motor asymmetry and reduced verbal fluency in unipolar depression. In that study, the association was stronger in patients ≥50 years, and in patients with reduced brain stem raphe nuclei hypogenicity (57).
Conclusion
In this review, we summarize the literature on the functional neuroanatomy of PMD in major depressive disorder (Table 2). Despite the clinical importance of PMD, we found relatively few studies. Indeed, the motor system has been relatively neglected in brain imaging studies of psychiatric disorders in general (58). We conclude that structural alterations that correlate with PMD have been found in gray- and white-matter regions within several nodes of cortico-subcortical circuits. Findings in functional neuroimaging studies show involvement of the same neurocircuitry nodes (along with their white-matter connections) as in structural neuroimaging studies, and further that limbic influences on the motor system may be important in the emergence of PMD. EEG studies suggest that frequency variations across many spectra, and an involvement of the frontal cortex, anterior, and posterior cingulate cortex, are associated with PMD. The molecular neuroimaging correlates of PMD resemble the functional anatomy of major depression described with functional and structural methods, but in addition also implicate disrupted monoamine transmission in PMD. The few available studies that use transcranial ultrasound primarily show an association between PMD and echogenic features of the substantia nigra, which then corroborates molecular neuroimaging findings of disrupted dopamine transmission.
Structural and functional neuroimaging studies suggest that PMD involve alterations in large-scale cortico-striato-thalamo-cortical neurocircuits, and in particular fronto-striatal subdivisions. Findings from transcranial ultrasound, and molecular neuroimaging studies, suggest a putative underlying factor for these alterations in the form of disrupted influence of ascending dopamine tracts that emanate from deeper midbrain nuclei. This notion also fits with the broader picture of a depressive disorder with psychomotor disturbances, which also include alterations in cognitive function, drive, and emotional expression – phenomena that also map onto ascending monoamine tracts with targets in the frontal lobe. Taken together, the broad picture suggests that PMD in major depressive disorder emerges from altered limbic signals at the interface of emotion, volition, higher-order cognitive function, and movement.
Our review shows that PMD is an emerging field of research that has kept growing since over 20 years. However, the currently available studies also preclude firmer evidence when evaluated in the context of general research methodology. Most studies are cross-sectional, have <25 participants, and have not been reproduced. Furthermore, a wide variety of clinical psychomotor measures have been used. Thus, information about the anatomical specificity of PMD from future studies could be improved by the use of objective measurements of motor performance (i.e., finger-tapping, actigraphy) when investigating the different dimensions of PMD delineated by current clinical measurements (i.e., CORE, MARS), and using rating scales that probe PMD specifically. Further studies would also benefit from longitudinal experimental designs that disentangle the effects of brain changes on the functional components of PMD, and assess differences across neuropsychiatric disorders.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
BL received funding from Svenska Läkaresällskapet (The Swedish Society of Medicine, SLS-403101), and the Strategic Research Committee, Karolinska Institutet/Stockholm County Council, Sweden. CR received funding from Schizofreniförbundet, Sweden. We also thank Dr. Caroline Wachtler for language revisions of the manuscript.
References
2. Kraepelin E, Diefendorf AR. Clinical Psychiatry. (Vol. xvii). New York, NY: The Macmillan Company (1907). 562 p.
3. Parker G, Hadzi-Pavlovic D, Brodaty H, Boyce P, Mitchell P, Wilhelm K, et al. Psychomotor disturbance in depression: defining the constructs. J Affect Disord (1993) 27(4):255–65. doi: 10.1016/0165-0327(93)90049-P
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Sobin C, Mayer L, Endicott J. The motor agitation and retardation scale: a scale for the assessment of motor abnormalities in depressed patients. J Neuropsychiatry Clin Neurosci (1998) 10(1):85–92. doi:10.1176/jnp.10.1.85
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Widlocher DJ. Psychomotor retardation: clinical, theoretical, and psychometric aspects. Psychiatr Clin North Am (1983) 6(1):27–40.
6. Malhi GS, Berk M. Does dopamine dysfunction drive depression? Acta Psychiatr Scand Suppl (2007) 433:116–24. doi:10.1111/j.1600-0447.2007.00969.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Malhi GS, Parker GB, Greenwood J. Structural and functional models of depression: from sub-types to substrates. Acta Psychiatr Scand (2005) 111(2):94–105. doi:10.1111/j.1600-0447.2004.00475.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Hickie I, Scott E, Mitchell P, Wilhelm K, Austin MP, Bennett B. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry (1995) 37(3):151–60. doi:10.1016/0006-3223(94)00174-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Wang L, Leonards CO, Sterzer P, Ebinger M. White matter lesions and depression: a systematic review and meta-analysis. J Psychiatr Res (2014) 56:56–64. doi:10.1016/j.jpsychires.2014.05.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res (2015) 161(1):102–12. doi:10.1016/j.schres.2014.04.041
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Walther S, Hugli S, Hofle O, Federspiel A, Horn H, Bracht T, et al. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol Dis (2012) 47(1):13–9. doi:10.1016/j.nbd.2012.03.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Bracht T, Federspiel A, Schnell S, Horn H, Hofle O, Wiest R, et al. Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PLoS One (2012) 7(12):e52238. doi:10.1371/journal.pone.0052238
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Bracht T, Horn H, Strik W, Federspiel A, Schnell S, Hofle O, et al. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J Affect Disord (2014) 155:186–93. doi:10.1016/j.jad.2013.10.048
14. Exner C, Lange C, Irle E. Impaired implicit learning and reduced pre-supplementary motor cortex size in early-onset major depression with melancholic features. J Affect Disord (2009) 119(1–3):156–62. doi:10.1016/j.jad.2009.03.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Liberg B, Ekman CJ, Sellgren C, Johansson A, Landen M. Vertex-based morphometry in euthymic bipolar disorder implicates striatal regions involved in psychomotor function. Psychiatry Res (2014) 221(3):173–8. doi:10.1016/j.pscychresns.2014.01.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Liberg B, Ekman CJ, Sellgren C, Johansson AG, Landen M. Subcortical morphometry and psychomotor function in euthymic bipolar disorder with a history of psychosis. Brain Imaging Behav (2014). doi:10.1007/s11682-014-9313-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Naismith S, Hickie I, Ward PB, Turner K, Scott E, Little C, et al. Caudate nucleus volumes and genetic determinants of homocysteine metabolism in the prediction of psychomotor speed in older persons with depression. Am J Psychiatry (2002) 159(12):2096–8. doi:10.1176/appi.ajp.159.12.2096
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Schlegel S, Maier W, Philipp M, Aldenhoff JB, Heuser I, Kretzschmar K, et al. Computed tomography in depression: association between ventricular size and psychopathology. Psychiatry Res (1989) 29(2):221–30. doi:10.1016/0165-1781(89)90037-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Naismith SL, Lagopoulos J, Ward PB, Davey CG, Little C, Hickie IB. Fronto-striatal correlates of impaired implicit sequence learning in major depression: an fMRI study. J Affect Disord (2010) 125(1–3):256–61. doi:10.1016/j.jad.2010.02.114
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Caligiuri MP, Brown GG, Meloy MJ, Eberson SC, Kindermann SS, Frank LR, et al. An fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorder. Psychiatry Res (2003) 123(3):171–82. doi:10.1016/S0925-4927(03)00075-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Caligiuri MP, Brown GG, Meloy MJ, Eyler LT, Kindermann SS, Eberson S, et al. A functional magnetic resonance imaging study of cortical asymmetry in bipolar disorder. Bipolar Disord (2004) 6(3):183–96. doi:10.1111/j.1399-5618.2004.00116.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Marchand WR, Lee JN, Thatcher J, Thatcher GW, Jensen C, Starr J. A preliminary longitudinal fMRI study of frontal-subcortical circuits in bipolar disorder using a paced motor activation paradigm. J Affect Disord (2007) 103(1–3):237–41. doi:10.1016/j.jad.2007.01.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Marchand WR, Lee JN, Thatcher JW, Thatcher GW, Jensen C, Starr J. A longitudinal functional magnetic resonance imaging study of frontal-subcortical circuits in bipolar disorder using a paced motor activation paradigm. Bipolar Disord (2007) 9:74–5.
24. Liberg B, Adler M, Jonsson T, Landen M, Rahm C, Wahlund LO, et al. The neural correlates of self-paced finger tapping in bipolar depression with motor retardation. Acta Neuropsychiatr (2013) 25(1):43–51. doi:10.1111/j.1601-5215.2012.00659.x
25. Liberg B, Adler M, Jonsson T, Landen M, Rahm C, Wahlund LO, et al. Motor imagery in bipolar depression with slowed movement. J Nerv Ment Dis (2013) 201(10):885–93. doi:10.1097/NMD.0b013e3182a5c2a7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Liberg B, Klauser P, Harding IH, Adler M, Rahm C, Lundberg J, et al. Functional and structural alterations in the cingulate motor area relate to decreased fronto-striatal coupling in major depressive disorder with psychomotor disturbances. Front Psychiatry (2014) 5:176. doi:10.3389/fpsyt.2014.00176
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Marchand WR, Lee JN, Thatcher GW, Jensen C, Stewart D, Dilda V, et al. A functional MRI study of a paced motor activation task to evaluate frontal-subcortical circuit function in bipolar depression. Psychiatry Res (2007) 155(3):221–30. doi:10.1016/j.pscychresns.2007.03.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Yao Z, Wang L, Lu Q, Liu H, Teng G. Regional homogeneity in depression and its relationship with separate depressive symptom clusters: a resting- state fMRI study. J Affect Disord (2009) 115(3):430–8. doi:10.1016/j.jad.2008.10.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Nystrom C, Matousek M, Hallstrom T. Relationships between EEG and clinical characteristics in major depressive disorder. Acta Psychiatr Scand (1986) 73(4):390–4. doi:10.1111/j.1600-0447.1986.tb02700.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Thier P, Axmann D, Giedke H. Slow brain potentials and psychomotor retardation in depression. Electroencephalogr Clin Neurophysiol (1986) 63(6):570–81. doi:10.1016/0013-4694(86)90144-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Schlegel S, Nieber D, Herrmann C, Bakauski E. Latencies of the P300 component of the auditory event-related potential in depression are related to the Bech-Rafaelsen melancholia scale but not to the Hamilton rating scale for depression. Acta Psychiatr Scand (1991) 83(6):438–40. doi:10.1111/j.1600-0447.1991.tb05571.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Silfverskiold P, Rosen I, Risberg J, Gustafson L. Changes in psychiatric symptoms related to EEG and cerebral blood flow following electroconvulsive therapy in depression. Eur Arch Psychiatry Neurol Sci (1987) 236(4):195–201. doi:10.1007/BF00383849
33. Nieber D, Schlegel S. Relationships between psychomotor retardation and EEG power spectrum in major depression. Neuropsychobiology (1992) 25(1):20–3. doi:10.1159/000118804
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Miltner WH, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MG. Implementation of error-processing in the human anterior cingulate cortex: a source analysis of the magnetic equivalent of the error-related negativity. Biol Psychol (2003) 64(1–2):157–66. doi:10.1016/S0301-0511(03)00107-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Vocat R, Pourtois G, Vuilleumier P. Unavoidable errors: a spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia (2008) 46(10):2545–55. doi:10.1016/j.neuropsychologia.2008.04.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Schrijvers D, de Bruijn ER, Maas Y, De Grave C, Sabbe BG, Hulstijn W. Action monitoring in major depressive disorder with psychomotor retardation. Cortex (2008) 44(5):569–79. doi:10.1016/j.cortex.2007.08.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Walther S, Hofle O, Federspiel A, Horn H, Hugli S, Wiest R, et al. Neural correlates of disbalanced motor control in major depression. J Affect Disord (2012) 136(1–2):124–33. doi:10.1016/j.jad.2011.08.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med (1993) 23(3):579–90. doi:10.1017/S0033291700025368
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Dolan RJ, Bench CJ, Liddle PF, Friston KJ, Frith CD, Grasby PM, et al. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? J Neurol Neurosurg Psychiatry (1993) 56(12):1290–4. doi:10.1136/jnnp.56.12.1290
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Videbech P, Ravnkilde B, Pedersen TH, Hartvig H, Egander A, Clemmensen K, et al. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr Scand (2002) 106(1):35–44. doi:10.1034/j.1600-0447.2002.02245.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Milak MS, Parsey RV, Keilp J, Oquendo MA, Malone KM, Mann JJ. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry (2005) 62(4):397–408. doi:10.1001/archpsyc.62.4.397
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Dunn RT, Kimbrell TA, Ketter TA, Frye MA, Willis MW, Luckenbaugh DA, et al. Principal components of the Beck depression inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol Psychiatry (2002) 51(5):387–99. doi:10.1016/S0006-3223(01)01244-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Mayberg HS, Lewis PJ, Regenold W, Wagner HN Jr. Paralimbic hypoperfusion in unipolar depression. J Nucl Med (1994) 35(6):929–34.
44. Meyer JH, Kruger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, et al. Lower dopamine transporter binding potential in striatum during depression. Neuroreport (2001) 12(18):4121–5. doi:10.1097/00001756-200112210-00052
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff NP, et al. Elevated putamen D(2) receptor binding potential in major depression with motor retardation: an [11C]raclopride positron emission tomography study. Am J Psychiatry (2006) 163(9):1594–602. doi:10.1176/ajp.2006.163.9.1594
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. Ebert D, Feistel H, Loew T, Pirner A. Dopamine and depression – striatal dopamine D2 receptor SPECT before and after antidepressant therapy. Psychopharmacology (1996) 126(1):91–4. doi:10.1007/BF02246416
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
47. Perico CA, Skaf CR, Yamada A, Duran F, Buchpiguel CA, Castro CC, et al. Relationship between regional cerebral blood flow and separate symptom clusters of major depression: a single photon emission computed tomography study using statistical parametric mapping. Neurosci Lett (2005) 384(3):265–70. doi:10.1016/j.neulet.2005.04.088
48. Graff-Guerrero A, Gonzalez-Olvera J, Mendoza-Espinosa Y, Vaugier V, Garcia-Reyna JC. Correlation between cerebral blood flow and items of the Hamilton rating scale for depression in antidepressant-naive patients. J Affect Disord (2004) 80(1):55–63. doi:10.1016/S0165-0327(03)00049-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, Baxter LR. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol Psychiatry (2001) 50(3):171–8. doi:10.1016/S0006-3223(01)01117-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
50. Becker G, Berg D, Lesch KP, Becker T. Basal limbic system alteration in major depression: a hypothesis supported by transcranial sonography and MRI findings. Int J Neuropsychopharmacol (2001) 4(1):21–31. doi:10.1017/S1461145701002164
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
51. Berg D, Supprian T, Hofmann E, Zeiler B, Jager A, Lange KW, et al. Depression in Parkinson’s disease: brainstem midline alteration on transcranial sonography and magnetic resonance imaging. J Neurol (1999) 246(12):1186–93. doi:10.1007/s004150050541
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Becker G, Struck M, Bogdahn U, Becker T. Echogenicity of the brainstem raphe in patients with major depression. Psychiatry Res (1994) 55(2):75–84. doi:10.1016/0925-4927(94)90002-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
53. Walter U, Hoeppner J, Prudente-Morrissey L, Horowski S, Herpertz SC, Benecke R. Parkinson’s disease-like midbrain sonography abnormalities are frequent in depressive disorders. Brain (2007) 130(Pt 7):1799–807. doi:10.1093/brain/awm017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
54. Walter U, Prudente-Morrissey L, Herpertz SC, Benecke R, Hoeppner J. Relationship of brainstem raphe echogenicity and clinical findings in depressive states. Psychiatry Res (2007) 155(1):67–73. doi:10.1016/j.pscychresns.2006.12.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
55. Budisic M, Karlovic D, Trkanjec Z, Lovrencic-Huzjan A, Vukovic V, Bosnjak J, et al. Brainstem raphe lesion in patients with major depressive disorder and in patients with suicidal ideation recorded on transcranial sonography. Eur Arch Psychiatry Clin Neurosci (2010) 260(3):203–8. doi:10.1007/s00406-009-0043-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
56. Becker T, Becker G, Seufert J, Hofmann E, Lange KW, Naumann M, et al. Parkinson’s disease and depression: evidence for an alteration of the basal limbic system detected by transcranial sonography. J Neurol Neurosurg Psychiatry (1997) 63(5):590–6. doi:10.1136/jnnp.63.5.590
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Hoeppner J, Prudente-Morrissey L, Herpertz SC, Benecke R, Walter U. Substantia nigra hyperechogenicity in depressive subjects relates to motor asymmetry and impaired word fluency. Eur Arch Psychiatry Clin Neurosci (2009) 259(2):92–7. doi:10.1007/s00406-008-0840-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. Kupferschmidt DA, Zakzanis KK. Toward a functional neuroanatomical signature of bipolar disorder: quantitative evidence from the neuroimaging literature. Psychiatry Res (2011) 193(2):71–9. doi:10.1016/j.pscychresns.2011.02.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Adler M, Liberg B, Andersson S, Isacsson G, Hetta J. Development and validation of the affective self rating scale for manic, depressive, and mixed affective states. Nord J Psychiatry (2008) 62(2):130–5. doi:10.1080/08039480801960354
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: psychomotor performance, major depressive disorder, neuroimaging, frontal lobe, basal ganglia, monoamines
Citation: Liberg B and Rahm C (2015) The functional anatomy of psychomotor disturbances in major depressive disorder. Front. Psychiatry 6:34. doi: 10.3389/fpsyt.2015.00034
Received: 19 December 2014; Accepted: 19 February 2015;
Published: 10 March 2015.
Edited by:
Sebastian Walther, University Hospital of Psychiatry, SwitzerlandReviewed by:
Bernhard J. Mitterauer, Volitronics-Institute for Basic Research Psychopathology and Brain Philosophy, AustriaJessica A. Turner, Georgia State University, USA
Sebastian Walther, University Hospital of Psychiatry, Switzerland
Copyright: © 2015 Liberg and Rahm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benny Liberg, Department of Psychiatry, Melbourne Neuropsychiatry Centre, Alan Gilbert Building, Level 3, 161 Barry Street, Carlton South, Melbourne, VIC 3053, Australia benny.liberg@gmail.com
 Benny Liberg
Benny Liberg Christoffer Rahm
Christoffer Rahm