- 1Department of Psychology and Neuroscience, University of Colorado Boulder, Boulder, CO, USA
- 2Department of Psychology, University of Oregon, Eugene, OR, USA
Emerging lines of research suggest that both testosterone and maladaptive reward processing can modulate behavioral dysregulation. Yet, to date, no integrative account has been provided that systematically explains neuroendocrine function, dysregulation of reward, and behavioral dysregulation in a unified perspective. This is particularly important given specific neuroendocrine systems are potential mechanisms underlying and giving rise to reward-relevant behaviors. In this review, we propose a forward-thinking approach to study the mechanisms of reward and behavioral dysregulation from a positive affective neuroendocrinology (PANE) perspective. This approach holds that testosterone increases reward processing and motivation, which increase the likelihood of behavioral dysregulation. Additionally, the PANE framework holds that reward processing mediates the effects of testosterone on behavioral dysregulation. We also explore sources of potential sex differences and the roles of age, cortisol, and individual differences within the PANE framework. Finally, we discuss future prospects for research questions and methodology in the emerging field of affective neuroendocrinology.
Introduction
In recent decades, separate lines of research have investigated the psychological, neural, and neuroendocrine mechanisms of behavioral dysregulation, defined here as appetitive, risky behaviors, such as sexual risk-taking (e.g., unprotected sex), dangerous driving, risky financial decision making, and substance use. Two bodies of research of relevance have independently examined the hormonal mechanisms of behavioral dysregulation. One perspective investigates the hormonal predictors and mechanisms (particularly testosterone), while another has focused on reward dysregulation, defined by researchers as the pursuit of pleasurable feelings and stimuli and heightened responsiveness to positive, reward-related stimuli [e.g., Ref. (1–3)]. As we argue, these systems share overlapping psychological and physiological mechanisms, yet have not been simultaneously deployed to understand behavioral dysregulation. Thus, there is a need to integrate these disparate lines of work into a common theoretical framework. This framework should not only be consistent with extant findings but also make novel predictions to be tested in future research.
In this paper, we propose a forward-thinking approach to study reward motivation and behavioral dysregulation, referred to as the positive affective neuroendocrinology (PANE) approach. The PANE approach incorporates existing research in the hormonal mechanisms of behavioral dysregulation with research on reward dysregulation and related positive affectivity. This approach suggests that reward dysregulation underlies the established links between testosterone and behavioral dysregulation. The PANE approach also holds that testosterone increases reward processing – or neural activity in the reward-relevant regions of the brain – and reward motivation, which in turn increase the likelihood of behavioral dysregulation. More specifically, this framework posits that increases in reward mediate the effects of testosterone on behavioral dysregulation. In this paper, we provide a focused review of the roles of testosterone in modulating behavioral dysregulation and then discuss how reward dysregulation represents a crucial mechanism in this relationship. We also explore the potential sources of sex differences and the effects of age, cortisol, and individual differences within a PANE perspective. Finally, we close with a discussion of future research prospects in the emerging field of affective neuroendocrinology.
Evidence for PANE
What is the evidence for the PANE framework of reward and behavioral dysregulation? In the following sections, we discuss the evidence from three areas for why the association between testosterone and behavioral dysregulation may be mediated by elevated reward dysregulation: (1) evidence showing that testosterone is a predictor and mechanism of behavioral dysregulation, (2) evidence for how reward dysregulation is a critical mechanism of behavioral dysregulation, and (3) evidence that testosterone increases reward dysregulation.
Testosterone and Behavioral Dysregulation
Testosterone, a steroid hormone and end-product of the hypothalamic–pituitary–gonadal (HPG) axis, is of prime relevance to behavioral dysregulation. In men, testosterone is primarily produced in the testes, while women’s testosterone is produced in smaller quantities by the ovaries and adrenal cortex (4). Testosterone also has a diurnal cycle, where testosterone is highest upon waking and decreases across the day, flattening in the afternoon (5). Researchers often distinguish between organizational effects of testosterone – the “permanent modification of brain structure and function during prenatal and early postnatal life due to exposure to testosterone” [Ref. (6), p. 15268] – and activational effects of testosterone – temporary, non-developmental moment-to-moment effects of testosterone that modulate affect, cognition, and behavior upon administration or release of testosterone.
Research on the dysregulatory behavioral effects and correlates of testosterone confirms both stable and dynamic, contextual psychological effects of the HPG axis. Studying the stable, trait-like elements of testosterone involves inferring stable levels of testosterone from either multiple samples at the same time of day [e.g., Ref. (7)], or taking a sample at one time of the day for all participants, after a period of neutral activity (8). Support for this approach comes from reports that testosterone concentrations are relatively stable when measured at the same time of day (9). Thus, baseline testosterone can be considered a trait-like index of testosterone. Large-scale studies have linked baseline testosterone to several dysregulatory behaviors in army veterans, such as substance use, previous juvenile delinquency, and law breaking [e.g., Ref. (10)]. Baseline testosterone is also positively associated with risky financial decision making and preferences [see Ref. (11), for a review; e.g., Ref. (12, 13)].
Collectively, there is a weak positive association between stable, trait-like testosterone concentrations and risk-taking, with some inconsistent findings. For instance, Stanton et al. (14) report a non-linear relationship between testosterone and risk-taking, suggesting that risk-taking is elevated in low and high testosterone individuals, but not those with middle-range testosterone concentrations. Additionally, Sapienza et al. (6) report a positive association between testosterone and risk-taking in women, but not men. Furthermore, Schipper (15) found a negative association between testosterone and risk-aversion for gains, but not losses, in men.
The lack of strong effects of baseline testosterone on risk-taking in humans may be due to the potential for testosterone concentrations to alter in response to social events. Although baseline testosterone may predict how individuals generally respond and act across a wide variety of contexts and self-reported psychological traits, a more fine-tuned assessment of testosterone may be needed for assessing situation-specific behaviors. For example, previous work has examined the behavioral effects of testosterone responses to competitions (16–19), opposite sex interactions (20), men’s interactions with women (21, 22), social exclusion (23), holding dominant vs. submissive postures (24), and aggressive provocation (25). These dynamic effects of testosterone are theorized to be more robustly associated with context-specific social behaviors than baseline testosterone (26), and this notion is supported by several emerging studies [e.g., Ref. (16, 27, 28)] showing robust effects of testosterone changes predicting aggressive behavior in social contexts. This work is also bolstered by a recent study showing that acute changes in testosterone in response to monetary wins and losses also are associated with increased financial risk-taking in men (29). Collectively, these studies suggest that both baseline and dynamic changes in testosterone are positively related to a range of dysregulatory behaviors, particularly risk-taking.
Reward-Seeking and Behavioral Dysregulation
Theories of behavioral dysregulation (e.g., risk-taking) have distinguished between appetitive, approach-oriented, reward motivations based on achieving satisfaction and avoidance motivations based on reducing or avoiding negative consequences, such as pain, punishment, or losses [e.g., Ref. (30–32)]. Affective and motivational accounts of risk-taking specify reward dysregulation as a critical component [e.g., Ref. (33)]. Additionally, elevations in reward-seeking facilitate the heightened risk-taking behaviors associated with adolescence [see Ref. (34), for review] and underlie a host of dysregulatory behaviors, such as addictive gambling (35), substance abuse (36), traffic violations (37), and childhood obesity (38). In this work, both the elevated experience of positive emotions and the experience of excessive reward motivation are critical components to behavioral dysregulation.
The deleterious effects of reward motivation and excessive positive emotion also emerge in clinical disorders. Disorders associated with risk-taking behavior, such as bipolar disorder (BD), are characterized by elevated and abnormally persistent positive emotions (39), excessive reward pursuit and deficits in reward-related learning [e.g., Ref. (40)], and deficits in positive emotion regulation [e.g., Ref. (41–43)]. BD is often characterized by elevated risk-taking behaviors and impulsivity (44, 45), such as substance use (46), impulsive gambling behavior (47), aggressive behavior (48), and harmful substance use (46). Broadly, deficits in the behavioral approach system (BAS) are thought to characterize BD and elevated behavioral dysregulation (49).
The effects of positive emotion regulation and elevated, persistent positive affect on behavioral dysregulation are becoming increasingly known. When experiencing urgent positive emotions, people are more likely to engage in a variety of dysregulatory behaviors, such as substance use and risky-sexual behavior (50, 51). Elevated reward processing and positive affect have a robust association with risk-taking (52). Additionally, dysregulatory behaviors such as substance use, binge eating, and risky-sexual behavior, are more likely to occur in the context of positive emotions (50, 51). Elevated reward processing and positive affect have a robust association with risk-taking (52). Additionally, elevated reward-sensitivity uniquely characterizes a subpopulation of drug addicts that are motivated toward drug addiction through the presence of potential for rewards (53, 54).
Research on clinical disorders has also provided insights into the fundamental affective mechanisms of behavioral dysregulation. This research suggests that reward dysregulation is a critical component of behavioral dysregulation. Affective accounts of risk-taking specify reward dysregulation as a critical component [e.g., Ref. (33)]. Clinical disorders associated with risk-taking behavior, such as BD, are characterized by elevated and abnormally persistent positive emotions (39), excessive reward pursuits and deficits in reward-related learning [e.g., Ref. (40)], and deficits in positive emotion regulation [e.g., Ref. (3, 41, 42)]. BD is often characterized by elevated risk-taking behaviors and impulsivity (44, 45), such as substance use (46), impulsive gambling behavior (47), aggressive behavior (48), and harmful substance use (46). In addition to excessive positive emotion, this heightened irritability may also potentiate behavioral dysregulation, such as impulsive aggression (55).
Reward-Related Neural Function and Behavioral Dysregulation
In addition to elevated positive affect and reward motivation, neural structures related to positive affect and reward also predict behavioral dysregulation. The reward system has been broadly thought to be the neural basis of the BAS, which operates via the mesolimbic dopaminergic network (56, 57). Connectivity between these dopaminergic regions is theorized to form the basis of the neural circuits of reward and appetitive behavior [see Ref. (58) for a review]. Broadly, this reward system of the brain utilizes several key dopamine-linked structures, such as the ventral tegmental area (VTA) and nucleus accumbens (NAcc), the latter of which is nested in the ventral striatum (59–61). Within these regions, the VTA has numerous dopaminergic pathways with output to the hippocampus, amygdala, medial pre-frontal cortex (PFC) ventral pallidum, and of prime relevance, the NAcc [Ref. (62), for a review]. For example, dopaminergic neuron activation in the VTA that stimulates the NAcc aids in reinforcing responses to food and drugs used in substance abuse [see Ref. (63), for a review], as well as reward cues (64). The NAcc also plays a critical role in affect and appetitive motivation, reaction to novel stimuli, reward-related learning, responses to delayed reward, controlling feeding, and hedonic taste preferences [e.g., Ref. (65–70)]. More broadly, dopamine release in the ventral striatum is associated with self-reported euphoria in humans [e.g., Ref. (71)] and is thought to be a critical modulator of reward anticipation in mammals (72).
Dysregulation in the dopaminergic system has attracted considerable attention in researching behavioral dysregulation and related psychiatric disorders [see Ref. (59, 73), for a review]. For instance, the dopaminergic reward system and dopamine receptor polymorphisms have been linked to the crucial rewarding effects of substance abuse and addiction (74) and pathological gambling [e.g., Ref. (75–77)]. The dopamine system and receptors also modulate increased risky-decision making in humans [e.g., Ref. (78, 79)] and impulsive behavior in rodents [e.g., Ref. (80)]. Furthermore, neural theories of self-regulation (81, 82) hold that self-control is a function of the balance of activation and connectivity between the mesolimbic dopaminergic regions and regions of the PFC – a putative mechanism of self-control, inhibiting craving, and emotional control (74, 83–85). As we will discuss, testosterone modulates activity in these regions (see Figure 1).
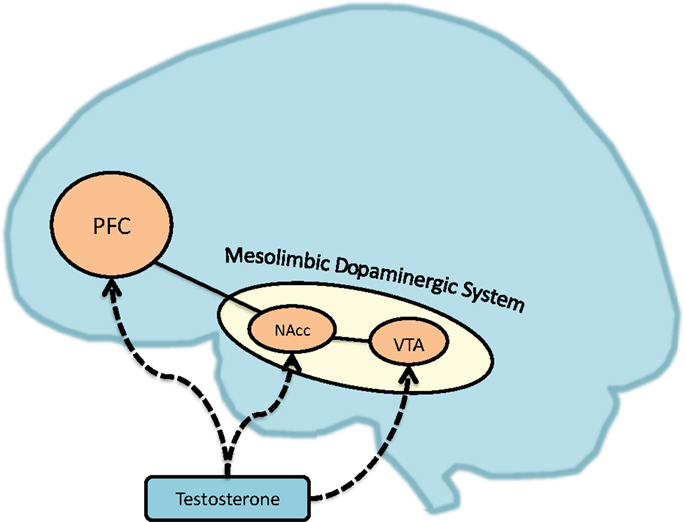
Figure 1. Testosterone’s effects on brain regions associated with reward dysregulation. PFC, prefrontal cortex; NAcc, nucleus accumbens; VTA, ventral tegmental area.
Testosterone and Reward
Our evidence for testosterone’s role in reward-seeking comes from three areas of research: testosterone and reward-seeking behavior, testosterone and reward-related affect, and testosterone and the neural circuitry of reward.
Testosterone and Reward-Seeking Behavior and Traits
Without involving affective and neural processes, testosterone is associated with increased reward-focused traits, sensation seeking, and impulsive behaviors in humans and animals [e.g., Ref. (86–92)]. Additionally, previous work suggests that exogenous testosterone administration can shift sensitivity from punishment to reward dependency (93). Testosterone changes are also associated with increased monetary gains in stock traders (94). Broadly, this work suggests that testosterone increases motivation to seek rewards.
Testosterone and Reward-Related Affect
Testosterone may be related to reward-seeking behaviors, but is it associated with reward-related affect? Work using exogenously administered testosterone suggests that testosterone may shift focus away from withdrawal-related emotions to approach-related, reward-focused aggression (95, 96), and increase subjective and physiological measures of sexual-arousal (97). Testosterone may also be associated with approach-related positive affect. Indeed, testosterone increases are correlated with increased enjoyment of competition in decisive victories (98). Additionally, there is a well-established negative correlation between testosterone and depressive symptoms [see Ref. (99), for a review]. Previous work also suggests that exogenously administered testosterone can also decrease depression (100, 101) and increase manic symptoms (102). Furthermore, in women with BD, testosterone concentrations positively predict the number of manic episodes and suicide attempts (103).
Testosterone and Reward-Related Neural Function
Broadly, both testosterone’s organizational and activational effects on the brain are associated with neural regions linked to increased dominance, reward, and approach behaviors [see Ref. (26, 104–106) for reviews; Ref. (107)]. Relevant to the current framework, an expansive literature suggests that testosterone is linked to reward-related neural function, both within animal and human literature. We summarize these associations in Figure 1. Animal research suggests testosterone modulates the dopaminergic system (108–110) and dopamine-linked sexual behaviors in rodents [e.g., Ref. (111, 112)], and has rewarding effects via the mesolimbic dopaminergic system [see Ref. (113), for a review]. For instance, rats show conditioned place preference for regions where they received testosterone injections, and this effect is mediated by dopamine function in the ventral striatum and NAcc (114, 115). Supporting this idea, research in hamsters also suggests testosterone can facilitate dopaminergic activity in the NAcc (116). Furthermore, research with California mice suggests testosterone increases in response to victories facilitate future aggression through the expression of androgen receptors in the ventral striatum (117), potentially through dopaminergic activity.
The association between testosterone and reward-related neural activity parallels that of rodent research. In humans, adolescents’ hormonal changes in puberty have also been theorized to increase appetitive motivation by influencing reward-linked brain structures and dopaminergic pathways (118–122). In humans, testosterone administration increases functional connectivity in neural circuits linked with reduced depression (123). Additionally, exogenous testosterone administrations in humans increase ventral striatal responses to financial reward cues in adolescents and adults receiving monetary rewards (124, 125).
In summary, testosterone may increase reward motivation by acting directly on dopaminergic neural structures in the BAS. However, less work has focused on the effects of testosterone and the BAS beyond dopamine-dependent regions. Although some work suggests, for instance, that testosterone is associated with elevated dorsolateral pre-frontal cortex (DLPFC) activation during an anger control induction (126), other work has not revealed associations between testosterone and the DLPFC during aggressive interactions (127). Future research is needed to extend the specificity of the effects of testosterone beyond reward function to the BAS.
Reciprocal Associations Between Reward and Testosterone
Overall, the literature reviewed above suggests that testosterone can increase reward processing and dysregulation. However, there also may be a reciprocal effect of reward on testosterone increases. Reciprocal associations are consistent with existing neuroendocrine theories that posit hormones and behavior reciprocally affect each other through feedback loops [e.g., Ref. (128)]. Broadly, contexts that modulate testosterone responses, such as competitive outcomes and sexually attractive individuals, have rewarding properties. For instance, testosterone responses to competitive victories that facilitate aggressive and risk-taking behavior may occur because winning a competition is an enjoyable experience. Research supporting this possibility suggests a positive association between testosterone responses in winners of competitions and enjoyment of the competition (98). Additionally, the dynamic increases in testosterone following winning a competition and decreases following losing have been thought to facilitate changes in reward-dependent learning (129). However, to fully test this hypothesis, research experimentally manipulates reward in multiple contexts while measuring the effects on testosterone fluctuations is needed.
Critical Moderators
In the following section, we highlight potential critical moderators for within the PANE framework, including cortisol, sex, age, and individual differences linked to reward sensitivity and motivation.
Interactive Effects with Cortisol
Within the PANE framework, testosterone may interact with other hormones to predict behavioral dysregulation. Emerging work also suggests that cortisol – a glucocorticoid steroid hormone released as the end-product of the HPG axis – interacts with testosterone to modulate dysregulatory behavior [see Ref. (130), for a review]. From a neurobiological perspective, cortisol downregulates androgen receptors, inhibits HPG activity, and inhibits the effects of testosterone on specific tissues [e.g., Ref. (131–134)]. Additionally, the HPG and HPA axes are thought to have mutually inhibitory effects on each other (135). Therefore, it is possible that cortisol may also moderate the psychological and behavioral effects of testosterone. This notion is supported by psychological literature, finding that when cortisol levels are low, but not high, testosterone levels are positively associated with dominance (136), risk-taking (137), perceived status (138), violent crime (139, 140), and externalizing psychopathology in adolescents (141), although others did not find similar associations (10, 142). Recent research also suggests that acute testosterone changes are positively related to earnings in bargaining contexts when cortisol levels decrease, but not increase (143). In summary, not only do cortisol and testosterone have independent effects on costly behavioral dysregulation but these hormones may also co-regulate risk-taking behavior and impulsive traits. Future research is needed to further investigate the extent to which testosterone and cortisol jointly influence self-control related behaviors (144).
Sex Differences in the Psychoneuroendocrinology of Behavioral Dysregulation
A large literature suggests men are more impulsive, punishment insensitive, and sensation seeking than women [see Ref. (145), for a meta-analysis; Ref. (146)], and are on average, more risk-taking (147), although the effect sizes are small (148). Men also typically die earlier than women (149, 150), are more likely to die from violent deaths (151), are more aggressive [e.g., Ref. (152–155)], and are more likely to abuse alcohol (156). Additionally, relative to women, men have more psychopathological traits and disorders linked increased impulsivity (39, 157–160).
Sex differences in testosterone are thought to account for sex differences in risk-taking (6). Work by Sapienza and colleagues indicates that both the organizational functions of testosterone in prenatal development – indexed by the ratio of the second to fourth finger digits (161, 162) – and circulating levels of testosterone account for sex differences in risky-decision making. On the level of prenatal exposure to testosterone, previous work has found physiological indicators of prenatal testosterone exposure can alter children’s social and empathic abilities (163). Although numerous cultural and social factors explain gender differences in behavioral dysregulation, both organizational and activational effects of testosterone likely explain a portion of this variability. It is important, however, not to rule out social and cultural factors facilitating differences in behavioral dysregulation between men and women. Gender roles often guide behavior through social pressures and conformity [see Ref. (164, 165), for reviews]. To explain sex differences in dysregulatory impulsivity and risk-taking, it is necessary to account for not only both the nature and nurture, but the interaction between the two (164–166).
The effects of dynamic changes in testosterone on behavior may be specific to men. For example, Carré et al. (16) find that testosterone reactivity mediates the effect of competitive outcomes on aggressive behavior specifically in men but not women. Although several studies investigating the effects of testosterone reactivity on dysregulatory behaviors have primarily focused on samples of men [e.g., Ref. (23, 28)], future research is needed to establish whether the dynamic effects of testosterone on dysregulatory behavior are sex-specific. This work does not imply, however, that testosterone cannot have behavioral and psychological effects in women. Several testosterone administration studies, for example, have produced behavioral and psychological effects of testosterone in samples of exclusively women [e.g., Ref. (167–170)]. Additionally, previous work has identified interactive effects of testosterone and cortisol in samples of both men and women (136, 137).
Several factors may additionally explain smaller psychological and behavioral effects of testosterone in women. First, animal research suggests that females may have less androgen sensitivity compared to males. Although exogenous androgens can influence sexual mounting behaviors in female hamsters, female hamsters are less responsive to the effects of androgens on neuroendocrine function and sexual behavior than males (171, 172). Females have also been found to have decreased androgen receptor immunoreactivity and density compared to males in several regions of the brain (173). Second, compared men, women produce far less testosterone and have less variability in testosterone. This restricted range reduces the statistical power to detect testosterone’s psychological and behavioral effects (174) and this may hinder the detection of these effects in women. Additionally, the type of methodology used to measure testosterone can have sensitivity at different ranges (175) and may not always be well-suited for measuring the decreased concentrations of testosterone in women.
In summary, testosterone explains both intersex and intrasex variability in dysregulatory behavior. Researchers have several obstacles in measuring testosterone, which hopefully will be curtailed with the advent of greater precision in measurement and the accumulation of more data. Further exploring the role of testosterone in reward dysregulation within men and women would advance the study of the psychological effects of testosterone.
Age
Another potential moderator of the PANE framework is age. Post-adolescence aging coincides with decreases in testosterone [e.g., Ref. (176)], decline in neural reward-related function [e.g., Ref. (177)], increased preferences for delayed rewards (178), and decreased risk-taking behavior [e.g., Ref. (147)]. Furthermore, developmental researchers have proposed that pubertal increases in sex hormones including testosterone are linked to elevated risk-taking in adolescents (179, 180). Additionally, research on risk-taking suggest that both male and female adolescents engage in more risk-taking compared to adults [e.g., Ref. (181, 182)], leaving greater potential for testosterone to explain risk-taking in adolescents compared to adults. Thus, it is possible that age may moderate associations in the PANE framework and also modulate differences in the mechanisms of testosterone, reward function, and behavioral dysregulation.
Individual Differences
Although work on individual differences moderators of testosterone, reward, and behavioral dysregulation is preliminary, the associations in the PANE framework may be modulated by individual differences. For instance, Norman et al. (183) found that trait anxiety moderates the association between testosterone dynamics and impulsive aggression, while Schultheiss and colleagues (184, 185) report that implicit power motive can modulate testosterone responses to competitive contexts. Additionally, the mechanisms in PANE may also be affected by other individual differences related to reward motivation, such as the behavioral inhibition and activation scales [BIS/BAS; (186)] or regulatory focus (187).
The PANE Framework – A Summary
The PANE framework provides an organizing framework of existing research showing that the association between testosterone and behavioral dysregulation is mediated by increased reward motivation and reward dysregulation (see Figure 2). Specifically, the PANE approach primarily holds that both stable, trait-like levels and moment-to-moment dynamic changes in testosterone can increase reward dysregulation. Enhanced reward processing – a psychological and neural mechanism of behavioral dysregulation – then increases the likelihood of behavioral dysregulation. Because reward function is affected by testosterone and also serves as a key mechanism of behavioral dysregulation, we argue that reward function is a prime candidate for a mediator of the association between testosterone and behavioral dysregulation. Consistent with contemporary accounts of mediation (188), by specifying reward function as a mediator of the association between testosterone and behavioral dysregulation, we mean that reward function is a causal mechanism in this association. As we review above, testosterone and reward function and motivation modulate a common set of reward-dependent behaviors, and well-established causal directions among testosterone, reward, and behavioral dysregulation suggest that this network of relations is mediated.
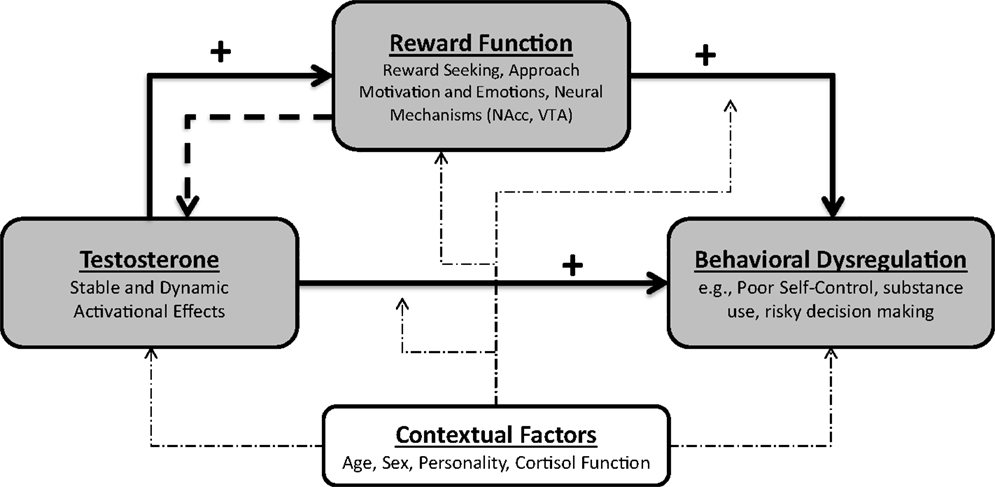
Figure 2. The PANE framework of reward and behavioral dysregulation. The PANE framework specifies that elevated stable levels and dynamic increases of testosterone facilitate increased reward function. This increased reward function then facilitates behavioral dysregulation and behaviors indicative of excessive reward pursuit. This perspective also allows for the possibility that reward function can increase testosterone.
The PANE approach is currently at a preliminary state in understanding neuroendocrine function, reward-dysfunction, and behavioral dysregulation. The current paper provides a rationale for why reward function may mediate the association between testosterone and behavioral dysregulation. However, as a whole, this mediation is untested by empirical articles. Future work is needed for researchers to test the overall mediation proposed by the PANE framework. This framework can be measured and tested in numerous forms and contexts, including using both human and animal samples, experimental manipulations of reward, pharmacological testosterone manipulations, testosterone modulating experimental paradigms (e.g., competitive outcomes), and multiple measures of behavioral dysregulation (e.g., poor financial decision making, substance abuse, risky-sexual behaviors). This flexibility allows for the PANE model to be tested and applied by researchers from many backgrounds.
It is necessary to note where the PANE approach differs from other neuroendocrine accounts of behavior. Previous accounts of testosterone and social behavior often indicate testosterone as a biomarker and mechanism of dominance and reproductive behaviors [e.g., Ref. (189, 190)], while recent research has also linked testosterone to threat-based neural function [e.g., Ref. (191)]. It is clear that testosterone modulates these psychological functions in addition to purely reward-related function. However, the literature we review suggests that dominance and sexual behavior are not the only variables regulated by testosterone. As we reviewed, testosterone is related to reward-related neural function, affect, and behaviors, as well as multiple phenotypes of behavioral dysregulation more distal to sexual behavior and dominance, such as substance use, risky-decision making, and sensation seeking. Thus, these behaviors are unlikely to be guided completely by the dominance and sexual behavior-related functions of testosterone. It is possible, instead, that rewards of status-seeking and sexual behavior may actually be a function of the reward-related function of testosterone, but future work is needed to test this possibility. At this point, the PANE framework is designed to be an additive perspective of the effects of testosterone on behavior in addition to other existing accounts.
Future Directions for Research on Reward Dysregulation, Testosterone, and Behavioral Dysregulation
Although the PANE approach proposes that reward function is a critical mediator in the association of testosterone and dysregulatory behaviors, this research can be developed in several ways. In the following sections, we also propose additional ways the PANE perspective can be expanded: (1) the role of social functioning, (2) translational work in psychiatric populations, (3) integration with neuroendocrine models of aggressive behavior, (4) the positive effects of behavioral dysregulation, and (5) integration with other systems of behavioral dysregulation.
Decreased Social Functioning
Testosterone may also increase risk-taking by decreasing social connections with others. It has been long known that socially isolated or disconnected individuals are more likely to engage in reckless behaviors, such as aggression, violence, and drug use (192, 193). This idea is consistent with recent reports suggesting a robust association between having social connections and decreased risk-taking. For instance, having better quality peer and family relationships is associated with decreased risk-taking in adolescents (194, 195) and higher levels of social support are linked with decreased risk-taking in stigmatized sexual minorities [e.g., Ref. (196, 197)]. Additionally, self-regulation has been found to be impaired by social exclusion (198). Socially excluded people are also more likely to engage in financial risk-taking (199).
How might testosterone decrease social relationship quality? Broadly, low testosterone is associated with nurturant, pro-social, relationship-promoting behavior (200–202). Basal testosterone is positively associated with having an avoidant, disconnected interpersonal approach, and greater loneliness (203). Testosterone is also positively related to decreased relationship satisfaction and commitment in couples, in both individuals and their romantic partners (204). Additionally, exogenous testosterone can decrease empathy and trust (168, 205), which may impair social relations. Furthermore, the increased risk-taking associated with testosterone function may also in turn decrease relationship quality, further impairing this process.
In summary, by decreasing the quality of social relationships, testosterone may increase the likelihood individuals engage in dysregulatory behaviors, such as maladaptive substance use to cope with poor social relationships [e.g., Ref. (206)]. We additionally suggest this association may be mediated by other processes we previously reviewed. For instance, research suggests having more meaningful family relationships can decrease risk-taking through neural activation indicative of decreased reward sensitivity and increased cognitive control [dorsolateral pre-frontal cortex, Ref. (194)]. Likewise, decreased empathy – an emergent property of reward and positive emotions (207, 208) – may also be related to the association between testosterone and reward processing.
Translational Implications for Psychiatric Illnesses
Numerous psychological disorders are characterized by trait impulsivity and behavioral dysregulation, such as BD, borderline personality disorder, and attention deficit hyperactivity disorder (39). From the PANE perspective, targeting hormones and affective states leading to behavioral dysregulation presents a novel, translational approach to understanding and treating these disorders. In particular, BD is a prime candidate to investigate the PANE approach. BD is characterized by increased reward sensitivity and difficulties down-regulating reward (2, 41), which may be of particular interest and application for the PANE approach. A chronic, severe, and often fatal psychiatric illness, BD ranks in the top 10 leading causes of worldwide disability by the World Health Organization. The core diagnostic criterion for BD involves periods of abnormally and persistently elevated positive mood (39) and impairments in reward processing have been proposed as a putative endophenotype for BD (209).
Three lines of evidence suggest that BD is a target population for studying testosterone and reward function. First, BD is associated with increased reward sensitivity. For example, people with BD exhibit increased reward reactivity (2, 210, 211), excessive pursuits aimed at obtaining rewards (1, 212), and impairments in reward-related learning (213). Second, empirical models of BD stress the importance of reward dysregulation in the causes and course of the disorder (1, 2, 210–212). Troubles with reward processing persist in BD, even during periods of symptom remission. For example, remitted BD patients report trouble decreasing or down-regulating reward (42), and engage in maladaptive strategies that amplify reward-relevant responses (43, 214), compared with healthy controls. Third, increased reward sensitivity is associated with clinical impairment in BD. Sensitivity to reward predicts increases in manic symptoms over time in BD (215).
Preliminary evidence also suggests that testosterone is important factor for understanding the course and symptom severity in BD. For instance, heightened testosterone levels are associated with significant increases in mania symptoms and severity in BD (103, 216), and oral administration of testosterone has been causally linked to the onset of manic symptoms (102). Future research is needed to understand the hormonal and reward-related mechanisms of BD and other disorders.
Integration with Theories of Aggressive Behavior
Much of research and theory links aggressive behavior to negative affective systems and threat processing [e.g., Ref. (217–221); see Ref. (26), for a review]. However, the reward systems are also implicated in aggressive behavior [e.g., Ref. (117, 222)]. A model of aggressive behavior accounting for reward and threat-processing may help explain mixed evidence for testosterone and aggressive behavior in neuroendocrine research. Although threat-function and negative affective systems undoubtedly play a critical role in facilitating aggressive behavior, a PANE approach to aggression may help enhance neuroendocrine models of aggressive behavior beyond just accounting for negative affect.
Exploring the “Light Side” of Behavioral Dysregulation
So far, the primary discussion of the PANE approach to impulsive behavioral dysregulation has focused on impulsive behaviors. However, just as calculated, non-impulsive behaviors can have antisocial consequences, not all impulsive acts have negative effects and many can be generous or pro-social to others [e.g., Ref. (223, 224)]. Emerging research suggests testosterone is positively associated with pro-social acts of fairness, cooperation, and reciprocity (205, 225, 226). Because positive emotionality has been found to be linked to pro-social behavior and because neural systems linked to reward are also related to pro-social behavior (227), the PANE approach may also explain the how testosterone can increase pro-social behaviors through positive emotions and reward motivation. Future research is needed to uncover further associations between testosterone, positive emotions, and pro-social behavior.
Integration with Other Systems of Behavioral Dysregulation
Although the PANE perspective specifies reward processing as a central mediator to the association between testosterone and behavioral dysregulation, reward is likely not the only mechanism. For example, one potential mechanism of increased risk-taking and impulsive behavior implicated are the pre-frontal regions of the brain linked to impulse control and self-regulation, such as the orbitofrontal cortex (OFC), which is related to risky-decision making [Ref. (228); see Ref. (179), for a review]. Although the literature suggesting testosterone can modulate the OFC is not as expansive as the testosterone-reward literature, the association testosterone has with aggression and risk-taking has been in part explained by decreased OFC activation (127) and volume in males (179). Furthermore, research suggests testosterone decreases connectivity between the OFC and subcortical areas like the amygdala (229, 230).
The effects of testosterone on the reward and self-control systems fit well with established dual-systems models of self-control. Hofmann et al. (231) specify that two systems modulate self-control: an impulsive associate system that automatically triggers impulsive responses to the environment and a reflective system providing executive control of overriding impulses and implementing strategic plans for goal pursuit. Based on what is known of the neural effects of testosterone, testosterone changes may modulate the activation of both impulsive and reflective systems. As more research emerges, one broad goal of the PANE perspective and surrounding research will be to integrate more with other mechanisms and perspectives of behavioral dysregulation, such as the dual-systems approach.
Conclusion
The PANE perspective is designed to organize the work on testosterone, reward dysregulation, and behavioral dysregulation into one coherent framework to stimulate research on behavioral dysregulation. The endocrine mechanisms discussed in this paper may also influence behavioral dysregulation through other mechanisms than reward [such as self-control systems and the OFC, Ref. (127)]. However, the evidence is clear that reward dysregulation is a principal mechanism modulating dysregulatory behaviors and it is necessary to unify this work into a larger framework. Broadly, researchers need to identify mediating psychological and neural mechanisms for the association between testosterone and behavioral dysregulation, and to unify these processes in an elegant, unified model. Together, both neuroendocrine and reward motivation accounts of behavioral dysregulation may hold promise in explaining poor self-control and impulsive behaviors across a wide range of clinical, health, and social contexts.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Alloy LB, Abramson LA. The role of the behavioral approach system (BAS) in bipolar spectrum disorders. Curr Dir Psychol Sci (2010) 19(3):189–94. doi: 10.1177/0963721410370292
2. Gruber J. Can feeling too good be bad? Positive emotion persistence (PEP) in bipolar disorder. Curr Dir Psychol Sci (2011) 20(4):217–21. doi:10.1177/0963721411414632
3. Johnson SL, Gruber J, Eisner L. Emotion in bipolar disorder. In: Rottenberg J, Johnson SL, editors. Emotion and Psychopathology: Bridging Affective and Clinical Science. Washington, DC: American Psychological Association (APA) Books (2007). p. 123–50.
4. Nelson RJ. Chapter 2: the endocrine system. 3rd ed. An Introduction to Behavioral Endocrinology. Sunderland, MA: Sinauer Associates (2005). p. 37–88.
5. Dabbs JM. Salivary testosterone measurements: reliability across hours, days, and weeks. Physiol Behav (1990) 48:83–6. doi:10.1016/0031-9384(90)90265-6
6. Sapienza P, Zingales L, Maestripieri D. Gender differences in financial risk aversion and career choices are affected by testosterone. Proc Natl Acad Sci U S A (2009) 106(36):15268–73. doi:10.1073/pnas.0907352106
7. Glenn AL, Raine A, Schug RA, Gao Y, Granger DA. Increased testosterone-to-cortisol ratio in psychopathy. J Abnorm Psychol (2011) 120(2):389. doi:10.1037/a0021407
8. Josephs RA, Telch MJ, Hixon JG, Evans JJ, Lee H, Knopik VS, et al. Genetic and hormonal sensitivity to threat: testing a serotonin transporter genotype × testosterone interaction. Psychoneuroendocrinology (2012) 37(6):752–61. doi:10.1016/j.psyneuen.2011.09.006
9. Liening SH, Stanton SJ, Saini EK, Schultheiss OC. Salivary testosterone, cortisol, and progesterone: two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiol Behav (2010) 99(1):8–16. doi:10.1016/j.physbeh.2009.10.001
10. Mazur A, Booth A. Testosterone is related to deviance in male army veterans, but relationships are not moderated by cortisol. Biol Psychol (2014) 96:72–6. doi:10.1016/j.biopsycho.2013.11.015
11. Apicella CL, Carré JM, Dreber A. Testosterone and economic risk taking: a review. Adapt Human Behav Physiol (2015):1–28. doi:10.1007/s40750-014-0020-2
12. Evans KL, Hampson E. Does risk-taking mediate the relationship between testosterone and decision-making on the Iowa gambling task? Pers Individ Differ (2014) 61:57–62. doi:10.1016/j.paid.2014.01.011
13. Stanton SJ, Liening SH, Schultheiss OC. Testosterone is positively associated with risk taking in the Iowa gambling task. Horm Behav (2011) 59(2):252–6. doi:10.1016/j.yhbeh.2010.12.003
14. Stanton SJ, Mullette-Gillman OA, McLaurin RE, Kuhn CM, LaBar KS, Platt ML, et al. Low- and high-testosterone individuals exhibit decreased aversion to economic risk. Psychol Sci (2011) 22(4):447–53. doi:10.1177/0956797611401752
16. Carré JM, Campbell JA, Lozoya E, Goetz SMM, Welker KM. Changes in testosterone mediate the effect of winning on subsequent aggressive behaviour. Psychoneuroendocrinology (2013) 38(10):2034–41. doi:10.1016/j.psyneuen.2013.03.008
17. Maner JK, Miller SL, Schmidt NB, Eckel LA. Submitting to defeat social anxiety, dominance threat, and decrements in testosterone. Psychol Sci (2008) 19(8):764–8. doi:10.1111/j.1467-9280.2008.02154.x
18. Welker KM, Carré JM. Individual differences in testosterone predict persistence in men. Eur J Pers (2015) 29:83–9. doi:10.1002/per.1958
19. Zilioli S, Mehta PH, Watson NV. Losing the battle but winning the war: uncertain outcomes reverse the usual effect of winning on testosterone. Biol Psychol (2014) 103:54–62. doi:10.1016/j.biopsycho.2014.07.022
20. Ronay R, von Hippel W. The presence of an attractive woman elevates testosterone and physical risk taking in young men. Soc Psychol Pers Sci (2010) 1(1):57–64. doi:10.1177/1948550609352807
21. Roney J, Mahler S, Maestripieri D. Behavioural and hormonal responses of men to brief interactions with women. Evol Hum Behav (2003) 24:365–75. doi:10.1016/s1090-5138(03)00053-9
22. Roney J, Lukaszewski A, Simmons Z. Rapid endocrine responses of young men to social interactions with young women. Horm Behav (2007) 52:326–33. doi:10.1016/j.yhbeh.2007.05.008
23. Geniole SN, Carré JM, McCormick CM. State, not trait, neuroendocrine function predicts costly reactive aggression in men after social exclusion and inclusion. Biol Psychol (2011) 87(1):137–45. doi:10.1016/j.biopsycho.2011.02.020
24. Carney DR, Cuddy AJC, Yap AJ. Power posing: brief nonverbal displays affect neuroendocrine levels and risk tolerance. Psychol Sci (2010) 21(10):1363–8. doi:10.1177/0956797610383437
25. Carré JM, Iselin AR, Welker KM, Hariri AR, Dodge KA. Testosterone reactivity to provocation mediates the effect of early intervention on aggressive behavior. Psychol Sci (2014) 25(5):1140–6. doi:10.1177/0956797614525642
26. Carré JM, McCormick CM, Hariri AR. The social neuroendocrinology of human aggression. Psychoneuroendocrinology (2011) 36(7):935–44. doi:10.1016/j.psyneuen.2011.02.001
27. Carré JM, Putnam SK, McCormick CM. Testosterone responses to competition predict future aggressive behaviour at a cost to reward in men. Psychoneuroendocrinology (2009) 34:561–70. doi:10.1016/j.psyneuen.2008.10.018
28. Carré JM, Baird-Rowe CD, Hariri AR. Testosterone responses to competition predict decreased trust ratings of emotionally neutral faces. Psychoneuroendocrinology (2014) 49:79–83. doi:10.1016/j.psyneuen.2014.06.011
29. Apicella CL, Dreber A, Mollerstrom J. Salivary testosterone change following monetary wins and losses predicts future financial risk-taking. Psychoneuroendocrinology (2014) 39:58–64. doi:10.1016/j.psyneuen.2013.09.025
30. Atkinson JW. Motivational determinants of risk-taking behavior. Psychol Rev (1957) 64(6):359–72. doi:10.1037/h0043445
31. Gray JA. Précis of the neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. Behav Brain Sci (1982) 5:469–534. doi:10.1017/s0140525x00013066
32. Gray JA. Perspectives on anxiety and impulsivity: a commentary. J Res Pers (1987) 21(4):493–509. doi:10.1016/0092-6566(87)90036-5
33. Mason L, El-Deredy W, Richard B. Reward dysfunction in mania: neural correlates of risk and impulsivity in individuals vulnerable to bipolar disorder. Int Clin Psychopharmacol (2011) 26:e46. doi:10.1097/01.yic.0000405710.14232.bf
34. Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev (2008) 28(1):78–106. doi:10.1016/j.dr.2007.08.002
35. Loxton NJ, Nguyen D, Casey L, Dawe S. Reward drive, rash impulsivity and punishment sensitivity in problem gamblers. Pers Individ Differ (2008) 45(2):167–73. doi:10.1016/j.paid.2008.03.017
36. Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addict Behav (2004) 29(7):1389–405. doi:10.1016/j.addbeh.2004.06.004
37. Castellà J, Pérez J. Sensitivity to punishment and sensitivity to reward and traffic violations. Accid Anal Prev (2004) 36(6):947–52. doi:10.1016/j.aap.2003.10.003
38. Van den Berg L, Pieterse K, Malik JA, Luman M, van Dijk KW, Oosterlaan J, et al. Association between impulsivity, reward responsiveness and body mass index in children. Int J Obes (Lond) (2011) 35(10):1301–7. doi:10.1038/ijo.2011.116
39. American Psychiatric Association. Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association (2000).
40. Gruber J, Gilbert KE, Youngstrom EA, Kogos Youngstrom J, Feeny NC, Findling RL. Reward dysregulation and mood symptoms in an adolescent outpatient sample. J Abnorm Child Psychol (2013) 41(7):1053–65. doi:10.1007/s10802-013-9746-8
41. Gruber J. A review and synthesis of positive emotion and reward disturbance in bipolar disorder. Clin Psychol Psychother (2011) 18(5):356–65. doi:10.1002/cpp.776
42. Gruber J, Harvey AG, Purcell A. What goes up can come down? A preliminary investigation of emotion reactivity and emotion recovery in bipolar disorder. J Affect Disord (2011) 133(3):457–66. doi:10.1007/s10802-013-9746-8
43. Johnson SL, McKenzie G, McMurrich S. Ruminative responses to negative and positive affect among students diagnosed with bipolar disorder and major depressive disorder. Cognit Ther Res (2008) 32(5):702–13. doi:10.1007/s10608-007-9158-6
44. Giovanelli A, Hoerger M, Johnson SL, Gruber J. Impulsive responses to positive mood and reward are related to mania risk. Cogn Emot (2013) 27(6):1091–104. doi:10.1080/02699931.2013.772048
45. Reddy LF, Lee J, Davis MC, Altshuler L, Glahn DC, Miklowitz DJ, et al. Impulsivity and risk taking in bipolar disorder and schizophrenia. Neuropsychopharmacology (2013) 39:456–63. doi:10.1038/npp.2013.218
46. Kathleen HM, Bearden CE, Barguil M, Fonseca M, Serap Monkul E, Nery FG, et al. Conceptualizing impulsivity and risk taking in bipolar disorder: importance of history of alcohol abuse. Bipolar Disord (2009) 11(1):33–40. doi:10.1111/j.1399-5618.2009.00720.x
47. McIntyre RS, McElroy SL, Konarski JZ, Soczynska JK, Wilkins K, Kennedy SH. Problem gambling in bipolar disorder: results from the Canadian community health survey. J Affect Disord (2007) 102(1):27–34. doi:10.1016/j.jad.2006.12.005
48. Garno JL, Gunawardane N, Goldberg JF. Predictors of trait aggression in bipolar disorder. Bipolar Disord (2008) 10(2):285–92. doi:10.1111/j.1399-5618.2007.00489.x
49. Urošević S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: review of theory and evidence. Clin Psychol Rev (2008) 28(7):1188–205. doi:10.1016/j.cpr.2008.04.004
50. Cyders MA, Smith GT. Clarifying the role of personality dispositions in risk for increased gambling behavior. Pers Individ Differ (2008) 45(6):503–8. doi:10.1016/j.paid.2008.06.002
51. Cyders MA, Smith GT. Emotion-based dispositions to rash action: positive and negative urgency. Psychol Bull (2008) 134(6):807–28. doi:10.1037/a0013341
52. Isen AM, Patrick R. The effect of positive feelings on risk taking: when the chips are down. Organ Behav Human Perform (1983) 31(2):194–202. doi:10.1016/0030-5073(83)90120-4
53. Bechara A. Risky business: emotion, decision-making, and addiction. J Gambl Stud (2003) 19(1):23–51. doi:10.1023/A:1021223113233
54. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia (2002) 40(10):1675–89. doi:10.1016/S0028-3932(02)00015-5
55. Stanford MS, Greve KW, Dickens TJ. Irritability and impulsiveness: relationship to self-reported impulsive aggression. Pers Individ Differ (1995) 19:757–60. doi:10.1016/0191-8869(95)00144-u
56. Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci (1999) 22(3):491–517. doi:10.1017/S0140525X99002046
57. Pickering AD, Gray JA. The neuroscience of personality. In: Pervin LA, John SP, editors. Handbook of Personality: Theory and Research. New York: Guilford Publications (1999). p. 277–99.
58. Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci (2007) 30(5):220–7. doi:10.1016/j.tins.2007.03.003
59. Chau DT, Roth RM, Green AI. The neural circuitry of reward and its relevance to psychiatric disorders. Curr Psychiatry Rep (2004) 6:391–9. doi:10.1007/s11920-004-0026-8
60. Gregorios-Pippas L, Tobler PN, Schultz W. Short-term temporal discounting of reward value in human ventral striatum. J Neurophysiol (2009) 101(3):1507–23. doi:10.1152/jn.90730.2008
61. Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci (2004) 24(47):10652–9. doi:10.1523/jneurosci.3179-04.2004
62. Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev (2006) 30:215–38. doi:10.1016/j.neubiorev.2005.04.016
63. Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci (1996) 19:319–40. doi:10.1146/annurev.neuro.19.1.319
64. Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci (1992) 12(12):4595–610. doi:10.1016/j.neures.2009.09.526
65. Berridge KC, Robinson TE. Parsing reward. Trends Neurosci (2003) 26:507–13. doi:10.1016/s0166-2236(03)00233-9
66. Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev (2002) 26:321–52. doi:10.1016/s0149-7634(02)00007-6
67. Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res (2002) 137:75–114. doi:10.1016/s0166-4328(02)00286-3
68. Kelley AE. Ventral striatal control of appetitive motivation role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev (2004) 27:765–76. doi:10.1016/j.neubiorev.2003.11.015
69. Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opiod modulation of taste hedonics within the ventral striatum. Physiol Behav (2002) 76:365–77. doi:10.1016/s0031-9384(02)00751-5
70. Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience (1997) 76:707–14. doi:10.1016/s0306-4522(96)00382-x
71. Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry (2001) 49:81–96. doi:10.1016/S0006-3223(00)01038-6
72. Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev (1999) 31:6–41. doi:10.1016/S0165-0173(99)00023-5
73. Berk M, Dodd S, Kauer-Sant’Anna M, Malhi GS, Bourin M, Kapczinski F, et al. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand (2007) 116(s434):41–9. doi:10.1111/j.1600-0447.2007.01058.x
74. Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A (2011) 108(37):15037–42. doi:10.1073/pnas.1010654108
75. Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and incertainty by dopamine neurons. Science (2003) 299:1898–902. doi:10.1126/science.1077349
76. Joutsa J, Johansson J, Niemelä S, Ollikainen A, Hirvonen MM, Piepponen P, et al. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. Neuroimage (2012) 60(4):1992–9. doi:10.1016/j.neuroimage.2012.02.006
77. Kohno M, Ghahremani DG, Morales AM, Robertson CL, Ishibashi K, Morgan AT, et al. Risk-taking behavior: dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cereb Cortex (2013) 25:236–45. doi:10.1093/cercor/bht218
78. Mitchell MR, Weiss VG, Beas BS, Morgan D, Bizon JL, Setlow B. Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacology (2014) 39(4):955–62. doi:10.1038/npp.2013.295
79. Norbury A, Manohar S, Rogers RD, Husain M. Dopamine modulates risk-taking as a function of baseline sensation-seeking trait. J Neurosci (2013) 33(32):12982–6. doi:10.1523/jneurosci.5587-12.2013
80. Pattij T, Schetters D, Schoffelmeer AN. Dopaminergic modulation of impulsive decision making in the rat insular cortex. Behav Brain Res (2014) 270:118–24. doi:10.1016/j.bbr.2014.05.010
81. Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci (2011) 15(3):132–9. doi:10.1016/j.tics.2010.12.005
82. Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med (2006) 12(12):559–66. doi:10.1016/j.molmed.2006.10.005
83. Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science (2009) 324(5927):646–8. doi:10.1126/science.1168450
84. Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci (2004) 24(49):11017–22. doi:10.1523/JNEUROSCI.3321-04.2004
85. Van Honk J, Harmon-Jones E, Morgan BE, Schutter DJ. Socially explosive minds: the triple imbalance hypothesis of reactive aggression. J Pers (2010) 78(1):67–94. doi:10.1111/j.1467-6494.2009.00609.x
86. Aluja A, García LF. Sensation seeking, sexual curiosity and testosterone in inmates. Neuropsychobiology (2005) 51(1):28–33. doi:10.1159/000082852
87. Alvergne A, Jokela M, Faurie C, Lummaa V. Personality and testosterone in men from a high-fertility population. Pers Individ Differ (2010) 49:840–4. doi:10.1016/j.paid.2010.07.006
88. Bayless DW, Darling JS, Daniel JM. Mechanisms by which neonatal testosterone exposure mediates sex differences in impulsivity in prepubertal rats. Horm Behav (2013) 64(5):764–9. doi:10.1016/j.yhbeh.2013.10.003
89. Campbell BC, Dreber A, Apicella CL, Eisenberg DT, Gray PB, Little AC, et al. Testosterone exposure, dopaminergic reward, and sensation-seeking in young men. Physiol Behav (2010) 99(4):451–6. doi:10.1016/j.physbeh.2009.12.011
90. Coccaro EF, Beresford B, Minar P, Kaskow J, Geracioti T. CSF testosterone: relationship to aggression, impulsivity, and venturesomeness in adult males with personality disorder. J Psychiatr Res (2007) 41(6):488–92. doi:10.1016/j.jpsychires.2006.04.009
91. Daitzman R, Zuckerman M. Disinhibitory sensation seeking, personality and gonadal hormones. Pers Individ Differ (1980) 1(2):103–10. doi:10.1016/0191-8869(80)90027-6
92. Määttänen I, Jokela M, Hintsa T, Firtser S, Kähönen M, Jula A, et al. Testosterone and temperament traits in men: longitudinal analysis. Psychoneuroendocrinology (2013) 38(10):2243–8. doi:10.1016/j.psyneuen.2013.04.009
93. Van Honk J, Schutter DJ, Hermans EJ, Putman P, Tuiten A, Koppeschaar H. Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology (2004) 29:937–43. doi:10.1016/j.psyneuen.2003.08.007
94. Coates JM, Herbert J. Endogenous steroids and financial risk taking on a London trading floor. Proc Natl Acad Sci U S A (2008) 105(16):6167–72. doi:10.1073/pnas.0704025105
95. Van Honk J, Peper JS, Schutter DJ. Testosterone reduces unconscious fear but not consciously experienced anxiety: implications for the disorders of fear and anxiety. Biol Psychiatry (2005) 58(3):218–25. doi:10.1016/j.biopsych.2005.04.003
96. Van Honk J, Schutter DJ. Vigilant and avoidant responses to angry facial expressions. In: Harmon-Jones E, Vinkielman P, editors. Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior. New York, NY: Guilford Press (2007). p. 197–223.
97. Tuiten A, Van Honk J, Verbaten R, Laan E, Everaerd W, Stam H. Can sublingual testosterone increase subjective and physiological measures of laboratory-induced sexual arousal? Arch Gen Psychiatry (2002) 59(5):465–6. doi:10.1001/archpsyc.59.5.465
98. Mehta PH, Snyder NA, Knight EL, Lassetter B. Close versus decisive victory moderates the effect of testosterone change on competitive decisions and task enjoyment. Adapt Human Behav Physiol (2014). doi:10.1007/s40750-014-0014-0
99. Ebinger M, Sievers C, Ivan D, Schneider HJ, Stalla GK. Is there a neuroendocrinological rationale for testosterone as a therapeutic option in depression? J Psychopharmacol (2009) 23:841–53. doi:10.1177/0269881108092337
100. Amanatkar HR, Chibnall JT, Seo B, Manepalli JN, Grossberg GT. Impact of exogenous testosterone on mood: a systematic review and meta-analysis of randomized placebo-controlled trials. Ann Clin Psychiatry (2014) 26:19–32. doi:10.1016/S0084-4071(08)70175-1
101. Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract (2009) 15(4):289–305. doi:10.1097/01.pra.0000358315.88931.fc
102. Pope HG, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men a randomized controlled trial. Arch Gen Psychiatry (2000) 57(2):133–40. doi:10.1001/archpsyc.57.2.133
103. Sher L, Grunebaum MF, Sullivan GM, Burke GM, Cooper TB, Mann JJ, et al. Testosterone levels in suicide attempters with bipolar disorder. J Psychiatr Res (2012) 46:1267–71. doi:10.1016/j.jpsychires.2012.06.016
104. Mehta PH, Goetz SM, Carré JM. Genetic, hormonal, and neural underpinnings of human aggressive behavior. Handbook of Neurosociology. Springer (2013). p. 47–65.
105. Terburg D, Van Honk J. Approach-avoidance versus dominance-submissiveness: a multilevel neural framework on how testosterone promotes social status. Emot Rev (2013) 5(3):296–302. doi:10.1177/1754073913477510
106. Van Honk J, Bos PA, Terburg D. Testosterone and dominance in humans: behavioral and brain mechanisms. New Frontiers in Social Neuroscience. Springer International Publishing (2014). p. 201–14.
107. Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Lai MC, Taylor K, et al. Fetal programming effects of testosterone on the reward system and behavioral approach tendencies in humans. Biol Psychiatry (2012) 72(10):839–47. doi:10.1016/j.biopsych.2012.05.027
108. Aubele T, Kritzer MF. Androgen influence on prefrontal dopamine systems in adult male rats: localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated extracellular dopamine level. Cereb Cortex (2011) 22:1799–812. doi:10.1093/cercor/bhr258
109. Bell MR, Sisk CL. Dopamine mediates testosterone-induced social reward in male Syrian hamsters. Endocrinology (2013) 154:1225–34. doi:10.1210/en.2012-2042
110. Hernandez L, Gonzalez L, Murzi E, Páez X, Gottberg E, Baptista T. Testosterone modulates mesolimbic dopaminergic activity in male rats. Neurosci Lett (1994) 171(1):172–4. doi:10.1016/0304-3940(94)90632-7
111. Hull EM, Du J, Lorrain DS, Matuszewich L. Testosterone, preoptic dopamine, and copulation in male rats. Brain Res Bull (1997) 44(4):327–33. doi:10.1016/j.yhbeh.2003.06.006
112. Szczypka MS, Zhou QY, Palmiter RD. Dopamine-stimulated sexual behavior is testosterone dependent in mice. Behav Neurosci (1998) 112(5):1229–35. doi:10.1037//0735-7044.112.5.1229
113. Wood RI. Anabolic-androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrinol (2008) 29:490–506. doi:10.1016/j.yfrne.2007.12.002
114. Packard MG, Cornell AH, Alexander GM. Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behav Neurosci (1997) 111(1):219. doi:10.1037//0735-7044.111.1.219
115. Packard MG, Schroeder JP, Alexander GM. Expression of testosterone conditioned place preference is blocked by peripheral or intra-accumbens injection of α-flupenthixol. Horm Behav (1998) 34(1):39–47. doi:10.1006/hbeh.1998.1461
116. DiMeo AN, Wood RI. ICV testosterone induces Fos in male Syrian hamster brain. Psychoneuroendocrinology (2006) 31(2):237–49. doi:10.1016/j.psyneuen.2005.08.001
117. Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci U S A (2010) 107(27):12393–8. doi:10.3410/f.4098956.4799055
118. Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp (2010) 31:926–33. doi:10.1002/hbm.21052
119. Forbes E, Dahl R. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn (2010) 72:66–72. doi:10.1016/j.bandc.2009.10.007
120. Graber J, Nichols T, Brooks-Gunn J. Putting pubertal timing in developmental context: implications for prevention. Dev Psychobiol (2010) 52:254–62. doi:10.1002/dev.20438
121. Nelson E, Lieibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med (2005) 35:163–74. doi:10.1017/S0033291704003915
122. Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn (2010) 72:146–59. doi:10.1016/j.bandc.2009.10.013
123. Schutter DJ, Peper JS, Koppeschaar HP, Kahn RS, Van Honk J. Administration of testosterone increases functional connectivity in a cortico-cortical depression circuit. J Neuropsychiatry Clin Neurosci (2005) 17(3):372–7. doi:10.1176/appi.neuropsych.17.3.372
124. Hermans EJ, Bos PA, Ossewaarde L, Ramsey NF, Fernández G, Van Honk J. Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. Neuroimage (2010) 52(1):277–83. doi:10.1016/j.neuroimage.2010.04.019
125. Op de Macks ZA, Moor BG, Overgaauw S, Güroğlu B, Dahl RE, Crone EA. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev Cogn Neurosci (2011) 1(4):506–16. doi:10.1016/j.dcn.2011.06.003
126. Denson TF, Ronay R, von Hippel W, Schira MM. Endogenous testosterone and cortisol modulate neural responses during induced anger control. Soc Neurosci (2013) 8(2):165–77. doi:10.1080/17470919.2012.655425
127. Mehta PH, Beer J. Neural mechanisms of the testosterone-aggression relation: the role of orbitofrontal cortex. J Cogn Neurosci (2010) 22(10):2357–68. doi:10.1162/jocn.2009.21389
128. Mazur A, Booth A. Testosterone and dominance in men. Behav Brain Sci (1998) 21(3):353–97. doi:10.1017/S0140525X98001228
129. Schultheiss OC, Wirth MM, Torges CM, Pang JS, Villacorta MA, Welsh KM. Effects of implicit power motivation on men’s and women’s implicit learning and testosterone changes after social victory or defeat. J Pers Soc Psychol (2005) 88(1):174. doi:10.1037/0022-3514.88.1.174
130. Mehta PH, Prasad S. The dual-hormone hypothesis: a brief review and future research agenda. Curr Opin Behav Sci (2015) 3:163–68. doi:10.1016/j.cobeha.2015.04.008
131. Chen SY, Wang J, Yu GQ, Liu W, Pearce D. Androgen and glucocorticoid receptor heterodimer formation: a possible mechanism for mutual inhibition of transcriptional activity. J Biol Chem (1997) 272(22):14087–92. doi:10.1074/jbc.272.22.14087
132. Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev (1992) 16(2):115–30. doi:10.1016/s0149-7634(05)80175-7
133. Smith RG, Syms AJ, Nag A, Lerner S, Norris JS. Mechanism of the glucocorticoid regulation of growth of the androgen-sensitive prostate-derived R3327H-G8-A1 tumor cell line. J Biol Chem (1985) 260(23):12454–63. doi:10.1002/pros.2990090305
134. Tilbrook AJ, Turner AI, Clarke IJ. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod (2000) 5(2):105–13. doi:10.1530/revreprod/5.2.105
135. Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol (2002) 14:506–13. doi:10.1046/j.1365-2826.2002.00798.x
136. Mehta PH, Josephs RA. Testosterone and cortisol jointly regulate dominance: evidence for a dual-hormone hypothesis. Horm Behav (2010) 58(5):898–906. doi:10.1016/j.yhbeh.2010.08.020
137. Mehta PH, Welker KM, Zilioli S, Carré JM. Testosterone and cortisol jointly modulate risk-taking. Psychoneuroendocrinology (2015) 56:88–99. doi:10.1016/j.psyneuen.2015.02.023
138. Edwards DA, Casto KV. Women’s intercollegiate athletic competition: cortisol, testostorone, and the dual-hormone hypothesis as it relates to status among teammates. Horm Behav (2013) 64:153–60. doi:10.1016/j.yhbeh.2013.03.003
139. Dabbs JM, Jurkovic GJ, Frady RL. Salivary testosterone and cortisol among late adolescent male offenders. J Abnorm Child Psychol (1991) 19(4):469–78. doi:10.1007/bf00919089
140. Popma A, Vermeiren R, Geluk CA, Rinne T, van den Brink W, Knol DL, et al. Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biol Psychiatry (2007) 61(3):405–11. doi:10.1016/j.biopsych.2006.06.006
141. Tackett JL, Herzhoff K, Harden KP, Page-Gould E, Josephs RA. Personality×hormone interactions in adolescent externalizing psychopathology. Personal Disord (2014) 5(3):235. doi:10.1037/per0000075
142. Welker KM, Lozoya E, Campbell JA, Carré JM, Neumann C. Testosterone, cortisol, and psychopathic traits in men and women. Physiol Behav (2014) 129:230–6. doi:10.1016/j.physbeh.2014.02.057
143. Mehta PH, Mor S, Yap AJ, Prasad S. Dual-hormone changes are related to bargaining performance. Psychol Sci (2015) 26(6):866–76. doi:10.1177/0956797615572905
144. Carré JM, Mehta PH. Importance of considering testosterone-cortisol interactions in predicting human aggression and dominance. Aggress Behav (2011) 37:1–3. doi:10.1002/ab.20407
145. Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol Bull (2011) 137(1):97. doi:10.1037/a0021591
146. Zuckerman M, Kuhlman DM. Personality and risk-taking: common biosocial factors. J Pers (2000) 68:999–1029. doi:10.1111/1467-6494.00124
147. Byrnes JP, Miller DC, Schafer WD. Gender differences in risk taking: a meta-analysis. Psychol Bull (1999) 125(3):367. doi:10.1037/0033-2909.125.3.367
148. Nelson JA. Are women really more risk-averse than men? SSRN Electron J (2012). doi:10.2139/ssrn.2158950
149. Kruger DJ, Nesse RM. An evolutionary life-history framework for understanding sex differences in human mortality rates. Hum Nat (2006) 17(1):74–97. doi:10.1007/s12110-006-1021-z
150. Pampel FC. Gender equality and the sex differential in mortality from accidents in high income nations. Popul Res Policy Rev (2001) 20:397–421. doi:10.1023/A:1013307620643
151. Maxim PS, Keane C. Gender, age, and the risk of violent death in Canada, 1950-1986. Can Rev Sociol (1992) 29(3):329–45. doi:10.1111/j.1755-618x.1992.tb02442.x
152. Archer J. Sex differences in aggression in real-world settings: a meta-analytic review. Rev Gen Psychol (2004) 8(4):291. doi:10.1037/1089-2680.8.4.291
153. Bettencourt B, Miller N. Gender differences in aggression as a function of provocation: a meta-analysis. Psychol Bull (1996) 119(3):422. doi:10.1037/0033-2909.119.3.422
154. Eagly AH, Steffen VJ. Gender and aggressive behaviour: a meta-analytic review of the social psychological literature. Psychol Bull (1986) 100(3):309. doi:10.1037/0033-2909.100.3.309
155. Knight GP, Guthrie IK, Page MC, Fabes RA. Emotional arousal and gender differences in aggression: a meta-analysis. Aggress Behav (2002) 28(5):366–93. doi:10.1002/ab.80011
156. Hill EM, Chow K. Life-history theory and risky drinking. Addiction (2002) 97(4):401–13. doi:10.1046/j.1360-0443.2002.00020.x
157. Frank E, editor. Gender and Its Effects on Psychopathology. Arlington, VA: American Psychiatric Press (2000).
158. Gershon J, Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord (2002) 5:143–54. doi:10.1177/108705470200500302
159. Moffitt TE, editor. Sex Differences in Antisocial Behaviour: Conduct Disorder, Delinquency, and Violence in the Dunedin Longitudinal Study. Cambridge: Cambridge University Press (2001).
160. Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J Child Psychol Psychiatry (2003) 44(8):1092–115. doi:10.1111/1469-7610.00194
161. Manning JT. Digit Ratio: A Pointer to Fertility, Behavior, and Health. New Brunswick, NJ: Rutgers University Press (2002).
162. Manning JT, Bundred PE, Newton DJ, Flanigan BF. The second to fourth digit ratio and variation in the androgen receptor gene. Evol Hum Behav (2003) 24:399–405. doi:10.1016/s1090-5138(03)00052-7
163. Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K, Hackett G. Fetal testosterone and empathy. Horm Behav (2006) 49:282–92. doi:10.1016/j.yhbeh.2005.08.010
164. Wood W, Eagly AH. Biosocial construction of sex differences and similarities in behavior. Adv Exp Soc Psychol (2012) 46:55. doi:10.1016/b978-0-12-394281-4.00002-7
165. Wood W, Eagly AH. Biology or culture alone cannot account for human sex differences and similarities. Psychol Inq (2013) 24(3):241–7. doi:10.1080/1047840x.2013.815034
166. Eagly AH, Wood W. The nature-nurture debates 25 years of challenges in understanding the psychology of gender. Perspect Psychol Sci (2013) 8(3):340–57. doi:10.1177/1745691613484767
167. Bos PA, Terburg D, Van Honk J. Testosterone decreases trust in socially naive humans. Proc Natl Acad Sci U S A (2010) 107(22):9991–5. doi:10.1073/pnas.0911700107
168. Bos PA, Panksepp J, Bluthé RM, Honk JV. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Front Neuroendocrinol (2012) 33(1):17–35. doi:10.1016/j.yfrne.2011.01.002
169. Terburg D, Aarts H, Van Honk J. Testosterone affects gaze aversion from angry faces outside of conscious awareness. Psychol Sci (2012) 23(5):459–63. doi:10.1177/0956797611433336
170. Van Honk J, Tuiten A, Hermans E, Putnam P, Koppeschaar H, Thijssen J, et al. A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Behav Neurosci (2001) 115(1):238. doi:10.1037/0735-7044.115.1.238
171. DeBold JF, Clemens LG. Aromatization and the induction of male sexual behavior in male, female, and androgenized female hamsters. Horm Behav (1978) 11:401–13. doi:10.1016/0018-506x(78)90040-5
172. Yellon SM, Hutchison JS, Goldman BD. Sexual differentiation of the steroid feedback mechanism regulating follicle-stimulating hormone secretion in the Syrian hamster. Biol Reprod (1989) 40:7–14. doi:10.1095/biolreprod41.1.7
173. Wood RI, Newman SW. Androgen receptor immunoreactivity in the male and female Syrian hamster brain. J Neurobiol (1999) 39(3):359–70. doi:10.1002/(SICI)1097-4695(19990605)39:3<359::AID-NEU3>3.0.CO;2-W
174. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum (1988).
175. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Utility, limitations, and pitfalls in measuring testosterone: an endocrine society position statement. J Clin Endocrinol Metab (2007) 92(2):405–13. doi:10.1210/jc.2006-1864
176. Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev (2005) 26(6):833–76. doi:10.1210/er.2004-0013
177. Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Acad Sci U S A (2008) 105(39):15106–11. doi:10.1073/pnas.0802127105
178. Mischel W, Metzner R. Preference for delayed reward as a function of age, intelligence, and length of delay interval. J Abnorm Soc Psychol (1962) 64(6):425. doi:10.1037/h0045046
179. Peper JS, Koolschijn PCM, Crone EA. Development of risk taking: contributions from adolescent testosterone and the orbito-frontal cortex. J Cogn Neurosci (2013) 25(12):2141–50. doi:10.1162/jocn_a_00445
180. Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn (2010) 72(1):124–33. doi:10.1016/j.bandc.2009.07.003
181. Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia card task. J Exp Psychol Learn Mem Cogn (2009) 35(3):709. doi:10.1037/a0014983
182. Figner B, Weber EU. Who takes risks when and why? Determinants of risk taking. Curr Dir Psychol Sci (2011) 20(4):211–6. doi:10.1177/0963721411415790
183. Norman RE, Moreau BJP, Welker KM, Carré JM. Trait anxiety moderates the relationship between testosterone responses to competition and aggressive behavior. Adapt Human Behav Physiol (2014). doi:10.1007/s40750-014-0016-y
184. Schultheiss OC, Campbell KL, McClelland DC. Implicit power motivation moderates men’s testosterone responses to imagined and real dominance success. Horm Behav (1999) 36:234–41. doi:10.1006/hbeh.1999.1542
185. Schultheiss OC, Rhode W. Implicit power motivation predicts men’s testosterone changes and implicit learning in a contest situation. Horm Behav (2002) 41:195–202. doi:10.1006/hbeh.2001.1745
186. Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol (1994) 67(2):319. doi:10.1037/0022-3514.67.2.319
187. Crowe E, Higgins ET. Regulatory focus and strategic inclinations: promotion and prevention in decision-making. Organ Behav Hum Decis Process (1997) 69(2):117–32. doi:10.1006/obhd.1996.2675
188. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. New York: Guilford Press (2013).
189. Mazur A. A biosocial model of status in face-to-face primate groups. Soc Forces (1985) 64(2):377–402. doi:10.1093/sf/64.2.377
190. Wingfield JC, Hegner RE, Dufty AM Jr, Ball GF. The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat (1990) 136:829–46. doi:10.1086/285134
191. Goetz SM, Tang L, Thomason ME, Diamond MP, Hariri AR, Carré JM. Testosterone rapidly increases neural reactivity to threat in healthy men: a novel two-step pharmacological challenge paradigm. Biol Psychiatry (2014) 76(4):324–31. doi:10.1016/j.biopsych.2014.01.016
194. Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Meaningful family relationships: neurocognitive buffers of adolescent risk taking. J Cogn Neurosci (2013) 25(3):374–87. doi:10.1162/jocn_a_00331
195. Telzer EH, Fuligni AJ, Lieberman MD, Miernicki M, Galván A. The quality of adolescents’ peer relationships modulates neural sensitivity to risk taking. Soc Cogn Affect Neurosci (2014) 10:389–98. doi:10.1093/scan/nsu064
196. Kapadia F, Siconolfi DE, Barton S, Olivieri B, Lombardo L, Halkitis PN. Social support network characteristics and sexual risk taking among a racially/ethnically diverse sample of young, urban men who have sex with men. AIDS Behav (2013) 17(5):1819–28. doi:10.1007/s10461-013-0468-2
197. Kimberly JA, Serovich JM. The role of family and friend social support in reducing risk behaviors among HIV-positive gay men. AIDS Educ Prev (1999) 11(6):465–75.
198. Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. J Pers Soc Psychol (2005) 88(4):589. doi:10.1037/e501232006-005
199. Duclos R, Wan EW, Jiang Y. Show me the honey! Effects of social exclusion on financial risk-taking. J Consum Res (2013) 40(1):122–35. doi:10.1086/668900
200. Harris JA, Rushton JP, Hampson E, Jackson DN. Salivary testosterone and self-report aggressive and pro-social personality characteristics in men and women. Aggress Behav (1996) 22:321–31. doi:10.1002/(SICI)1098-2337(1996)22:5<321::AID-AB1>3.0.CO;2-M
201. van Anders SM, Goldey KL, Kuo PX. The steroid/peptide theory of social bonds: integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology (2011) 36:1265–75. doi:10.1016/j.psyneuen.2011.06.001
202. van Anders SM, Tolman RM, Volling BL. Baby cries and nurturance affect testosterone in men. Horm Behav (2012) 61:31–6. doi:10.1016/j.yhbeh.2011.09.012
203. Turan B, Guo J, Boggiano MM, Bedgood D. Dominant, cold, avoidant, and lonely: basal testosterone as a biological marker for an interpersonal style. J Res Pers (2014) 50:84–9. doi:10.1016/j.jrp.2014.03.008
204. Edelstein RS, van Anders SM, Chopik WJ, Goldey KL, Wardecker BM. Dyadic associations between testosterone and relationship quality in couples. Horm Behav (2014) 65(4):401–7. doi:10.1016/j.yhbeh.2014.03.003
205. Boksem MA, Mehta PH, Van den Bergh B, van Son V, Trautmann ST, Roelofs K, et al. Testosterone inhibits trust but promotes reciprocity. Psychol Sci (2013) 24(11):2306–14. doi:10.1177/0956797613495063
206. Grunbaum JA, Tortolero S, Weller N, Gingiss P. Cultural, social, and intrapersonal factors associated with substance use among alternative high school students. Addict Behav (2000) 25(1):145–51. doi:10.1016/s0306-4603(99)00006-4
207. Devlin HC, Zaki J, Ong DC, Gruber J. Not as good as you think? Trait positive emotion is associated with increased self-reported empathy, but decreased empathic performance. PLoS One (2014) 9(10):e110470. doi:10.1371/journal.pone.0110470
208. Forgas JP, East R. On being happy and gullible: mood effects on skepticism and the detection of deception. J Exp Soc Psychol (2008) 44:1362–7. doi:10.1016/j.jesp.2008.04.010
209. Hasler G, Drevets WC, Gould TD, Gotteseman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry (2006) 60(2):93–105. doi:10.1590/S1516-44462006000200003
210. Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: relations of the BIS/BAS scales with symptoms. J Psychopathol Behav Assess (2001) 23:133–43. doi:10.1023/A:1010929402770
211. Nusslock R, Almeida JRC, Forbes EE, Versace A, Frank E, LaBarbara EJ, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord (2012) 14:249–60. doi:10.1111/j.1399-5618.2012.01012.x
212. Johnson SL. Mania and dysregulation in goal pursuit: a review. Clin Psychol Rev (2005) 25(2):241–62. doi:10.1016/j.cpr.2004.11.002
213. Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning on a probabilistic reward task. Biol Psychiatry (2008) 64(2):162–8. doi:10.1016/j.biopsych.2007.12.001
214. Gruber J, Eidelman P, Johnson SL, Smith B, Harvey AG. Hooked on a feeling: rumination about positive and negative emotion in inter-episode bipolar disorder. J Abnorm Psychol (2011) 120(4):956–61. doi:10.1037/a0023667
215. Gruber J, Culver JL, Johnson SL, Nam J, Keller KL, Ketter TK. Do positive emotions predict symptomatic change in bipolar disorder? Bipolar Disord (2009) 11:330–6. doi:10.1111/j.1399-5618.2009.00679.x
216. Weiss EL, Bowers MB, Mazure CM. Testosterone-patch-induced psychotic mania. Am J Psychiatry (1999) 156(6):969–969. doi:10.1176/ajp.156.6.969
217. Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry (2007) 62(2):168–78. doi:10.1016/j.biopsych.2006.08.024
218. Josephs RA, Sellers JG, Newman ML, Mehta PH. The mismatch effect: when testosterone and status are at odds. J Pers Soc Psychol (2006) 90(6):999. doi:10.1037/0022-3514.90.6.999
219. Newman ML, Sellers JG, Josephs RA. Testosterone, cognition, and social status. Horm Behav (2005) 47(2):205–11. doi:10.1016/j.yhbeh.2004.09.008
220. Mehta PH, Jones AC, Josephs RA. The social endocrinology of dominance: basal testosterone predicts cortisol changes and behavior following victory and defeat. J Pers Soc Psychol (2008) 94(6):1078. doi:10.1037/0022-3514.94.6.1078
221. Zilioli S, Watson NV. Winning isn’t everything: mood and testosterone regulate the cortisol response in competition. PLoS One (2013) 8(1):e52582. doi:10.1371/journal.pone.0052582
222. Schlüter T, Winz O, Henkel K, Prinz S, Rademacher L, Schmaljohann J, et al. The impact of dopamine on aggression: an [18F]-FDOPA PET Study in healthy males. J Neurosci (2013) 33(43):16889–96. doi:10.1523/jneurosci.1398-13.2013
223. O’Sullivan SS, Evans AH, Quinn NP, Lawrence AD, Lees AJ. Reckless generosity in Parkinson’s disease. Mov Disord (2010) 25(2):221–3. doi:10.1002/mds.22687
224. Taute H, McQuitty S. Feeling good! Doing good! An exploratory look at the impulsive purchase of the social good. J Mark Theory Pract (2004) 12:16–27. doi:10.1086/662199
225. Eisenegger C, Naef M, Snozzi R, Heinrichs M, Fehr E. Prejudice and truth about the effect of testosterone on human bargaining behaviour. Nature (2009) 463(7279):356–9. doi:10.1038/nature08711
226. Van Honk J, Montoya ER, Bos PA, van Vugt M, Terburg D. New evidence on testosterone and cooperation. Nature (2012) 485(7399):E4–5. doi:10.1038/nature11136
227. Mikulincer ME, Shaver PR. Prosocial Motives, Emotions, and Behavior: The Better Angels of Our Nature. Washington, DC: American Psychological Association (2010).
228. Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia (2007) 45(6):1270–9. doi:10.1016/j.neuropsychologia.2006.10.004
229. Spielberg JM, Forbes EE, Ladouceur CD, Worthman CM, Olino TM, Ryan ND, et al. Pubertal testosterone influences threat-related amygdala-orbitofrontal coupling. Soc Cogn Affect Neurosci (2014) 10:408–15. doi:10.1093/scan/nsu062
230. van Wingen G, Mattern C, Verkes RJ, Buitelaar J, Fernández G. Testosterone reduces amygdale – orbitofrontal cortex coupling. Psychoneuroendocrinology (2010) 35(1):105–13. doi:10.1093/scan/nsu062
Keywords: testosterone, cortisol, emotion, affect, reward, self-regulation, sex differences
Citation: Welker KM, Gruber J and Mehta PH (2015) A positive affective neuroendocrinology approach to reward and behavioral dysregulation. Front. Psychiatry 6:93. doi: 10.3389/fpsyt.2015.00093
Received: 14 March 2015; Accepted: 11 June 2015;
Published: 02 July 2015
Edited by:
Miriam Melis, University of Cagliari, ItalyReviewed by:
Liangsuo Ma, University of Texas Health Science Center at Houston, USAKesong Hu, Cornell University, USA
Copyright: © 2015 Welker, Gruber and Mehta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pranjal H. Mehta, Department of Psychology, 1227 University of Oregon, Eugene, OR 97403, USA, mehta@uoregon.edu
 Keith M. Welker
Keith M. Welker June Gruber1
June Gruber1 Pranjal H. Mehta
Pranjal H. Mehta