- 1Scientific Institute, IRCCS Eugenio Medea, Lecco, Italy
- 2Department of Psychology, University of Milano – Bicocca, Milan, Italy
- 3Department of Clinical Neurosciences, Hermanas Hospitalarias, FoRiPsi, Albese con Cassano, Italy
- 4Department of Neurosciences and Mental Health, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, University of Milan, Milan, Italy
- 5Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center at Houston, Houston, TX, USA
Although the Autism Spectrum Disorder (ASD) is renowned to be a connectivity disorder and a condition characterized by cerebellar involvement, the connectivity between the cerebellum and other cortical brain regions is particularly underexamined. Indeed, converging evidence has recently suggested that the cerebellum could play a key role in the etiopathogenesis of ASD, since cerebellar anomalies have been consistently reported in ASD from the molecular to the behavioral level, and damage to the cerebellum early in development has been linked with signs of autistic features. In addition, current data have shown that the cerebellum is a key structure not only for sensory-motor control, but also for “higher functions,” such as social cognition and emotion, through its extensive connections with cortical areas. The disruption of these circuits could be implicated in the wide range of autistic symptoms that the term “spectrum” connotes. In this review, we present and discuss the recent findings from imaging studies that investigated cortico-cerebellar connectivity in people with ASD. The literature is still too limited to allow for definitive conclusions; however, this brief review reveals substantial areas for future studies, underlining currently unmet research perspectives.
Introduction
Autism spectrum disorder (ASD) is a multifaceted neurodevelopmental disorder characterized by persistent social impairment, communication abnormalities, and restricted and repetitive behaviors (DSM-5) (1, 2). ASD is a complex condition with an average prevalence of about 1% worldwide (3), one in 68 U.S. children (4). Although high heritability estimates suggest a critical role for genetic factors (5), its etiology is generally considered multifactorial. It has been hypothesized that the heterogeneous phenotype of ASD could implicate a greater likelihood of abnormalities in the connectivity between different neural networks rather than alterations in a specific cerebral area (6). Over the last decade, the claim that ASD is a disorder of connectivity has been reliably supported by evidence from neuroimaging studies (7, 8), even though with mixed findings. On one hand, some studies have provided initial evidence of underconnectivity in ASD (9–11); on the other hand, another line of research has indicated overconnectivity in ASD, arguing for an increased local and short-distance connectivity within the frontal cortex, with respect to reduced long-range connectivity between frontal lobes and posterior brain regions (12–14).
Two recent studies (15, 16) that analyzed the database of fMRI resting-state scans from the Autism Brain Imaging Data Exchange have revealed the occurrence of underconnectivity and overconnectivity in ASD, although with different topographical distributions. More precisely, overconnectivity seems to be primarily associated with subcortical regions, whereas hypoconnectivity appears to characterize the pattern of cortico-cortical and interhemispheric functional connectivity.
However, the connectivity between different brain areas with the cerebellum is still a particularly under-considered issue in ASD research. Although it was traditionally believed that the cerebellum was exclusively a motor structure (17), converging evidence suggested a role for the cerebellum in other “higher” functions, including language and cognition, as well as emotion (18–20). Indeed, neuroanatomical findings have clearly shown that the cerebellum can influence a number of neocortical areas, including premotor, prefrontal, and posterior parietal areas of the cerebral cortex, through polysynaptic circuits via thalamus and basal ganglia. These pathways subserved specifically different functions, such as movement, cognition, and social skills (21–24). On the basis of decades of anatomical and imaging data [see Ref. (25, 26, 27–30) for reviews], it has been suggested that the cerebellum could be primarily implicated in ASD (31, 32), with cognitive and behavioral effects beyond the difficulties in the motor domain (33, 34). In fact, a cerebellar dysfunction early in development has been associated with deficits in executive functions, visual-spatial processing, linguistic function, and affective regulation (35, 36), and even with social difficulties, such as avoidance of physical contact or gaze aversion, within a diagnosis of ASD (37). Moreover, Wang et al. (36) recently proposed that cerebellar damage in childhood may perturb the maturation of distant neocortical circuits during developmental sensitive periods through a “developmental diaschisis,” increasing the risk for developing ASD.
Despite its connections with several brain areas and the well-known involvement of this structure in the disorder, few imaging studies have investigated the role of cerebro-cerebellar connections in ASD and correlations between cerebro-cerebellar connectivity and clinical measures. Considering this lack of evidence, we briefly summarized the recent imaging findings on cortico-cerebellar connectivity in ASD in order to (a) address strengths and pitfalls of previous studies and (b) explore potential strategies for future research. In addition, we aimed to understand whether disruptions of specific cortico-cerebellar circuits could be associated with specific difficulties in ASD or different phenotypes within the disorder. Publications for this review were identified from a PubMed search in November 2015 using terms related to autism, connectivity, magnetic resonance (MR) imaging, and cerebellum. This search was supplemented with other publications from the reference lists of all included citations, and from the personal reference databases of the authors.
Diffusion Imaging Studies
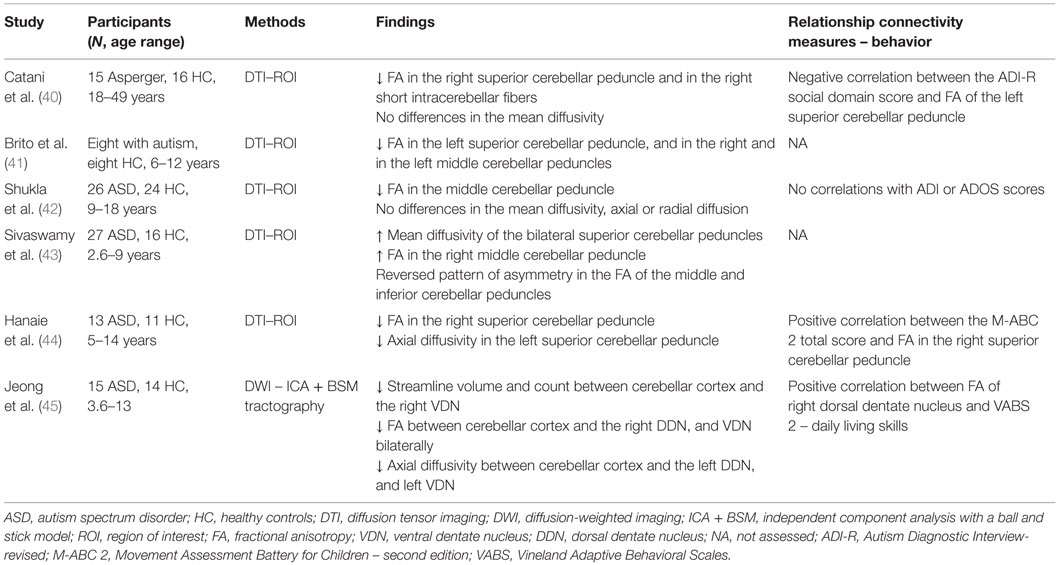
Structural connectivity can be assessed in vivo using MR techniques like diffusion-weighted imaging (DWI) or Diffusion Tensor Imaging (DTI). These non-invasive techniques provide indirect quantitative measures of white matter integrity, such as fractional anisotropy (FA), mean diffusivity, axial diffusivity, and radial diffusivity, by measuring water diffusion in the underlying tissue microstructure (38). Mean diffusivity is the average of the diffusion in the different directions of the space, and its values are related to the presence of barriers or obstacles, like cellular membranes and axons, which can interfere with the free water displacement within a voxel. When diffusion of water molecules is not the same along the three axes of the space (as in axons), it is called anisotropic, which means it has a preferential direction of displacement. Axial and radial diffusivity measure the entity of displacement along the principal and its perpendicular axis. FA values, which range between 0 and 1, are also a measure of anisotropy that seem to be related with myelination, axon diameter, and fiber coherence (39). High FA values denote well organized and normally myelinated axons that provide natural barriers to water movement within tissue. Lower FA values, in contrast, may reflect axonal loss and/or demyelination (39) as well as areas of crossing fibers. DWI allows for quantification of FA at voxel levels, whereas DTI, using different tracking algorithms, enables reconstruction of structural connections. An overview of the studies on structural connectivity between the cerebellum and different cerebral areas in ASD can be found in Table 1 (40–45). When using the terms overconnectivity or underconnectivity in diffusion imaging studies, we refer here to connectivity disruption in terms of tissue organization.
The majority of results from diffusion imaging studies showed a weaker structural connectivity in participants with ASD, as indicated by decreased FA both in the superior cerebellar peduncles (40, 41, 44) and in intracerebellar circuitries (45). However, findings in the middle cerebellar peduncles did not yield consistent evidence. Shukla et al. (42) revealed reduced values of FA in adolescents with ASD; conversely, Sivaswamy et al. (43) found increased values of FA in the right middle cerebellar peduncle, although within a reverse asymmetry pattern in FA of the middle and inferior cerebellar peduncles. A quantitative tractography study (45), in which a newly developed method for DWI called the “independent component analysis with a ball and stick model” was used to reveal abnormally reduced volume and number of fibers between the cerebellar cortex and right ventral dentate nucleus, accompanied by decreased FA between the cerebellar cortex, right dorsal dentate nucleus, and bilateral ventral dentate nucleus. Alterations of FA in cerebellar structures are mainly, but not always, reported to occur in association with reduced axial diffusivity [Ref. (44, 45); but not Ref. (42)], in absence of abnormalities of mean or radial diffusion.
In respect to the relationship between white matter integrity, behavior, and ASD symptoms, Catani et al. (40) found a negative correlation between FA values in the right superior cerebellar peduncle and in the right short intracerebellar fibers, in addition to social difficulties as reported by a caregiver using ADI-R (46), a “gold standard” diagnostic interview tool for clinical diagnosis. Moreover, Jeong et al. (45) depicted a relation between lower FA values between the cerebellar cortex and the right dorsal dentate nucleus, and between the ventral dentate nucleus bilaterally and poor daily living skills measured by Vineland Adaptive Behavioral Scales (47). Lastly, the motor abilities measured at Movement ABC-2 (48) were found to be positively correlated to FA in the right superior cerebellar peduncle (44). However, Shukla et al. (42) reported no relationship between DTI measures and clinical symptoms of ASD.
In sum, findings from diffusion imaging studies indicate underconnectivity – in reference to a different white matter integrity and coherence between participants – between the cerebellar main outflow pathways (i.e., the superior cerebellar peduncles) and the neocortex, and in intracerebellar circuitries that involve the dentate nucleus. Results for the middle and inferior cerebellar peduncles are not so consistent, with mixed reports of reduced and increased structural connectivity. Nevertheless, these findings altogether seem to suggest a possible abnormal connectivity between the cortical areas and the main afferent fibers of the cerebellum. Finally, the findings from the reviewed studies suggested some preliminary evidence of a relationship between the structural connectivity of the cerebellum and manifestations of ASD.
Task-Related Functional Imaging Studies
Functional brain connectivity can be effectively quantified during both task performance and rest by correlating variations of the blood-oxygen-level-dependent (BOLD) signal over time (49, 50). Different neuroanatomical regions are assumed to be functionally connected when the time courses of the BOLD fluctuations in these regions have synchronized patterns of activation (51). To the best of our knowledge, only three studies investigated the functional connectivity between cerebellum and cortical areas during task performance in ASD. Mostofsky et al. (52) assessed activation during a sequential finger tapping task in 13 children with high-functioning autism aged 8–12 years and in 13 age-matched typically developing peers, using functional magnetic resonance imaging (fMRI). The authors found activations in motor circuits across participants, which include contralateral pre/postcentral gyrus, ipsilateral anterior cerebellum (lobules IV/V), bilateral activation in the superior medial wall (BA6), and contralateral activation in the thalamus. However, children with typical development showed recruitment of cerebellar structure, i.e., the anterior lobe of the contralateral cerebellum (lobules IV/V) and ipsilateral anterior cerebellum, that is absent in autistic children. Conversely, the clinical group showed an increased activation of the supplementary motor area. In addition, a reduced functional connectivity within the motor circuits including premotor areas and the cerebellum was observed in autistic children, suggesting alterations in long-range connections in the fronto-cerebello-thalamo-frontal network.
Jack and Morris (53) directly investigated the relationship between functional connectivity and ASD features in an fMRI study on the neural bases of perception and use of human actions in imitation. Using psychophysiological interaction (PPI) analysis, the authors indicated an involvement of the network between posterior superior temporal sulcus (pSTS) – neocerebellum (i.e., Crus I) in social cognition in both adolescents with and without ASD. Although PPI data did not differ between groups, the authors showed that functional coactivation of pSTS and Crus I could predict the social deficits in ASD, as rated by parents on a questionnaire assessing “mentalizing skills,” (54) i.e., the ability to attribute mental states to others.
Recently, Kana et al. (55) examined the neural network underlying theory of mind, including the cerebellum, in high-functioning children and adolescents with autism while they were decoding the interactions between animated figures. The authors found a reduced cerebellar activation, particularly in Crus I, in participants with ASD in the theory of mind condition. Furthermore, they outlined reduced functional connectivity in ASD between the cerebellum and medial regions (i.e., medial prefrontal cortex and posterior cingulate cortex).
Resting-State Imaging Studies
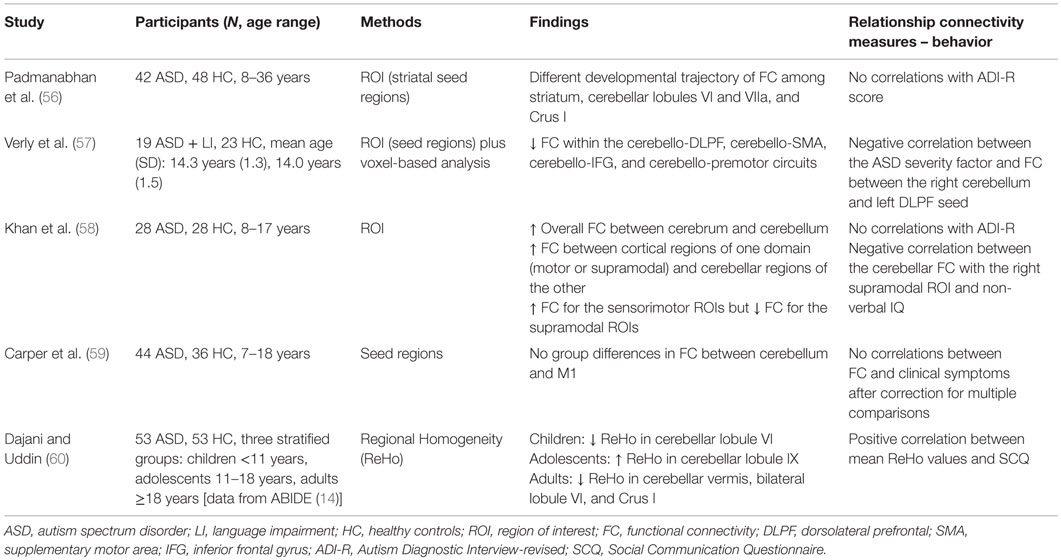
Five recent studies that use resting state to assess cerebro-cerebellar connectivity were included in the present review (see Table 2 for an overview).
Verly et al. (57) investigated the role of the cerebellum and its functional connectivity in the classic areas of the language network in children with ASD and language impairment. To do this, a verb generation fMRI task was first used to define language areas commonly active in participants with and without ASD. Afterward, the selected regions were used as seeds for resting-state analysis, in addition to the traditional voxel-based analysis. Results from both the seed-based and voxel-wise maps indicated a significantly reduced functional connectivity in ASD among cerebellum, Broca’s, and Wernicke’s areas. The authors interpreted this dissociation of cerebral and cerebellar language regions as a possible index of altered cerebellar modulation of language functioning.
The study by Khan et al. (58) is, to date, the first work that aimed to directly assess the cerebro-cerebellar connectivity in ASD. The authors used resting-state MRI to measure the functional connectivity between the cerebellum and seven bilateral cortical regions of interest (ROIs) in 28 children and adolescents with ASD compared to their typically developing peers. Cerebral regions were grouped in sensorimotor ROIs (premotor and primary motor cortices, somatosensory superior temporal cortex, and occipital lobe) and in supramodal ROIs (prefrontal cortex, posterior parietal cortex, and inferior and middle temporal gyri). Overall, the authors found a general cerebro-cerebellar overconnectivity in the ASD group. In addition, the analysis of the connections’ domain-specificities revealed an increase in non-canonical links, i.e., in the connections between cortical regions of one domain (sensorimotor or supramodal) and cerebellar regions of the other. Furthermore, an increased cerebro-cerebellar connectivity was also found in sensorimotor circuitries at the expense of connectivity in supramodal “cognitive” networks (reduced in ASD).
Carper et al. (59) have recently investigated the anatomical and functional connectivity of the motor control system in children and adolescents with ASD compared to healthy controls. With regard to the connectivity between the cerebellum and M1, the authors did not find any group differences in functional connectivity.
Finally, the other two resting-state studies reviewed here aimed to assess the functional connectivity of the cerebellum across development (56, 60). Indeed, both studies were cross-sectional and recruited participants of different ages, from childhood to adulthood. Findings from these works consistently indicated abnormal developmental trajectories of functional connectivity. Specifically, Padmanabhan et al. (56) found an increase of connectivity over development between cerebellar and subcortical regions (i.e., the striatum nucleus) in people with ASD, but a decrease in healthy controls. Dajani and Uddin (60) used regional homogeneity (ReHo) analysis to individuate local patterns of connectivity within the cerebellum. The authors were able to describe an age-specific pattern of short-range connectivity in ASD, with children and adults having lower ReHo in the cerebellum than controls, while adolescents exhibited an increased cerebellar local connectivity.
In respect to the relationship between functional connectivity and ASD features, findings from the study analyzed here are not entirely consistent. Indeed, no links between connectivity and “gold standard” clinical measures of ASD were observed (56, 58), although reduced connectivity seem to be accompanied by an increase in the severity of the disorder (57, 60), as assessed by the Social Communication Questionnaire [SCQ, Ref. (61)]. Lastly, lower values of connectivity between cerebellum and supramodal “cognitive” areas have been observed to be linked to higher non-verbal IQ (58).
Summary and Future Directions
In the present work, we aimed to provide an up-to-date overview of current findings on cortico-cerebellar connectivity in ASD. This issue represents an emerging field of interest for ASD research, following the hypothesis that ASD is a connectivity disorder associated with cerebellar dysfunctions. The cerebellum has been recently indicated as a key structure not only for sensory-motor control, but also for language, social cognition, and emotion, via its extensive connections with cortical areas (33–37). Although the literature is at a very early stage and more work on cortico-cerebellar connectivity is urgently needed, some preliminary suggestions can be drawn from the reviewed studies. Findings from task-related imaging studies showed a pattern of underconnectivity between the cerebellar outflow pathways and the neocortex, and in long-range fronto-cerebello-thalamo connections. Results from diffusion imaging studies are partly in line with these conclusions, although it is worth noting that this technique does not provide any direct measure of connectivity but is solely an index of fibers coherence and integrity. Significantly reduced long-range functional connectivity, among cerebral and cerebellar language regions, was also found in a resting-state study. However, results from afferent fibers of the cerebellum and from other resting-state studies indicated more complex, or even opposite, patterns of findings, disallowing any firm conclusion at this time. The causes of this discrepancy might be various, as the studies differed in many important methodological aspects. As clearly shown by Nair et al. (62), factors such as the type of analysis, the choice of seed placement, and the type of dataset can have a dramatic impact on results of functional connectivity studies in ASD. Keeping this in mind, a possible theoretical explanation could be a concurrence of under connectivity and overconnectivity between cortical areas and cerebellum. This suggestion seems to be supported by findings from a study that, for the first time, explicitly assessed cortico-cerebellar connectivity in ASD (58). Another explication of the partly conflicting reports may be the developmental changes in functional connectivity (63). Theoretically, this opinion is based on the account of ASD as a “developmental disconnection syndrome,” first proposed by Geschwind and Levitt, and more recently, by Wang et al. (36, 64). Given the developmental nature of the disorder, the connectivity abnormalities in ASD could vary in direction and in degrees of alteration through different stages of development as a result of neural plasticity. This hypothesis has received empirical support from diffusion imaging studies (65), resting-state studies using fMRI (66), and near-infrared spectroscopy (67). Abnormal developmental trajectories in ASD have been also found for the cortico-cerebellar connectivity (56, 60), as discussed above. Cross-sectional and longitudinal studies are warranted to control for the impact of studies’ differences in age ranges of participants on findings. In order to better understand possible developmental abnormalities of cortico-cerebellum connectivity, animal models can also provide useful insights into how a damage in cortico-cerebellar connections at a specific age could result in abnormal autistic-like behaviors (68).
Another area of concern raised by the evidence reviewed here is the lack of a specific relationship between connectivity and behavioral/diagnostic measures of ASD. This might be due, at least in part, to the well-known heterogeneity of people with ASD. Thus, with respect to the aim of our work, it is not possible at this stage to draw a direct link between the disruption of a specific cerebro-cerebellar circuit and a restricted behavioral phenotype of patients. Future research could overcome this limitation by including subsets of patients defined on the basis of different quantifiable measures of ASD phenotype, such as motor impairments, stereotyped behaviors, or language difficulties, in order to understand the relationship between these “proxy markers” of the disorder and the cortico-cerebellar connectivity. Within this context, more neuroimaging observations are also needed to localize ASD abnormalities in connectivity to specific areas of the cerebellum. To do this, it could be useful to couple both structural and functional imaging with experimental neurobehavioral paradigms that encompass the role of the cerebellum in movement, language, and social cognition.
Author Contributions
AC and PB conceived, designed, acquired background material, and drafted this work; GD, SBC, MN, and FA interpreted the background material, critically revised, and approved the final version of and agreed to be accountable for this work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by grant from the Italian Ministry of Health to Prof. Brambilla (Ricerca Corrente 2015, VirtAut) and to Crippa (GR-2011-02348929).
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association (2013).
2. Zoccante L, Viviani A, Ferro A, Cerini R, Cerruti S, Rambaldelli G, et al. Increased left parietal volumes relate to delayed language development in autism: a structural MRI study. Funct Neurol (2010) 25(4):217–21.
3. Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res (2012) 5(3):160–79. doi: 10.1002/aur.239
4. Wingate M, Kirby RS, Pettygrove S, Cunniff S, Schulz E, Ghosh T, et al. Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ (2014) 63(2):1–21.
5. Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol (2014) 10(2):74–81. doi:10.1038/nrneurol.2013.278
6. Maximo JO, Cadena EJ, Kana RK. The implications of brain connectivity in the neuropsychology of autism. Neuropsychol Rev (2014) 24(1):16–31. doi:10.1007/s11065-014-9250-0
7. Kana RK, Uddin LQ, Kenet T, Chugani D, Müller RA. Brain connectivity in autism. Front Hum Neurosci (2014) 8:1–4. doi:10.3389/fnhum.2014.00349
8. Mengotti P, D’Agostini S, Terlevic R, De Colle C, Biasizzo E, Londero D, et al. Altered white matter integrity and development in children with autism: a combined voxel-based morphometry and diffusion imaging study. Brain Res Bull (2011) 84(2):189–95. doi:10.1016/j.brainresbull.2010.12.002
9. Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism: intercorrelations of regional glucose utilization. Arch Neurol (1988) 45(7):749–55. doi:10.1001/archneur.1988.00520310055018
10. Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain (2002) 125(8):1839–49. doi:10.1093/brain/awf189
11. Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain (2004) 127(8):1811–21. doi:10.1093/brain/awh199
12. Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol (2005) 15(2):225–30. doi:10.1016/j.conb.2005.03.001
13. Courchesne E, Redcay E, Morgan JT, Kennedy DP. Autism at the beginning: microstructural and growth abnormalities underlying the cognitive and behavioral phenotype of autism. Dev Psychopathol (2005) 17(3):577–97. doi:10.1017/S0954579405050285
14. Marlies VE, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev (2012) 36(1):604–25. doi:10.1016/j.neubiorev.2011.09.003
15. Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry (2014) 19(6):659–67. doi:10.1038/mp.2013.78
16. Avital H, Behrman M, Malach R. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat Neurosci (2015) 18(2):302–9. doi:10.1038/nn.3919
17. Manni E, Petrosini L. A century of cerebellar somatotopy: a debated representation. Nat Rev Neurosci (2004) 5(3):241–9. doi:10.1038/nrn1347
18. Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behav Neurosci (1986) 100(4):443–54. doi:10.1037/0735-7044.100.4.443
19. Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron (2013) 80(3):807–15. doi:10.1016/j.neuron.2013.10.044
20. Arrigoni F, Romaniello R, Nordio A, Gagliardi C, Borgatti R. Learning to live without cerebellum. Neuroreport (2015) 26(14):809–13. doi:10.1097/WNR.0000000000000428
21. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex (2010) 46(7):831–44. doi:10.1016/j.cortex.2009.11.008
22. Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci (2013) 17(5):241–54. doi:10.1016/j.tics.2013.03.003
23. Balsters JH, Laird AR, Fox PT, Eickhoff SB. Bridging the gap between functional and anatomical features of cortico-cerebellar circuits using meta-analytic connectivity modeling. Hum Brain Mapp (2014) 35(7):3152–69. doi:10.1002/hbm.22392
24. Palesi F, Tournier JD, Calamante F, Muhlert N, Castellazzi G, Chard D, et al. Contralateral cerebello-thalamo-cortical pathways with prominent involvement of associative areas in humans in vivo. Brain Struct Funct (2015) 220(6):3369–84. doi:10.1007/s00429-014-0861-2
25. Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology (1985) 35(6):866. doi:10.1212/WNL.35.6.866
26. Courchesne E, Yeung-Courchesne R, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med (1988) 318(21):1349–54. doi:10.1056/NEJM198805263182102
27. Becker EB, Stoodley CJ. Autism spectrum disorder and the cerebellum. Int Rev Neurobiol (2013) 113:1–34. doi:10.1016/B978-0-12-418700-9.00001-0
28. Calderoni S, Bellani M, Hardan AY, Muratori F, Brambilla P. Basal ganglia and restricted and repetitive behaviours in Autism Spectrum Disorders: current status and future perspectives. Epidemiol Psychiatr Sci (2014) 23(3):235–8. doi:10.1017/S2045796014000171
29. Bellani M, Calderoni S, Muratori F, Brambilla P. Brain anatomy of autism spectrum disorders II. Focus on amygdala. Epidemiol Psychiatr Sci (2013) 22(4):309–12. doi:10.1017/S2045796013000346
30. Bellani M, Calderoni S, Muratori F, Brambilla P. Brain anatomy of autism spectrum disorders I. Focus on corpus callosum. Epidemiol Psychiatr Sci (2013) 22(3):217–21. doi:10.1017/S2045796013000139
31. Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ. Consensus paper: pathological role of the cerebellum in autism. Cerebellum (2012) 11(3):777–807. doi:10.1007/s12311-012-0355-9
32. Rogers TD, McKimm E, Dickson PE, Goldowitz D, Blaha CD, Mittleman G. Is autism a disease of the cerebellum? An integration of clinical and pre-clinical research. Front Syst Neurosci (2013) 7:1–16. doi:10.3389/fnsys.2013.00015
33. Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain (2007) 130:2646–60. doi:10.1093/brain/awm201
34. Steinlin M. Cerebellar disorders in childhood: cognitive problems. Cerebellum (2008) 7(4):607–10. doi:10.1007/s12311-008-0083-3
35. Tavano A, Borgatti R. Evidence for a link among cognition, language and emotion in cerebellar malformations. Cortex (2010) 46(7):907–18. doi:10.1016/j.cortex.2009.07.017
36. Wang SSH, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron (2014) 83(3):518–32. doi:10.1016/j.neuron.2014.07.016
37. Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain (2000) 123(5):1051–61. doi:10.1093/brain/123.5.1051
38. Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med (1996) 36(6):893–906. doi:10.1002/mrm.1910360612
39. Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed (2002) 15:435–55. doi:10.1002/nbm.782
40. Catani M, Jones DK, Daly E, Embiricos N, Pugliese L, Deeley Q, et al. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage (2008) 41(4):1184–91. doi:10.1016/j.neuroimage.2008.03.041
41. Brito AR, Vasconcelos MM, Domingues RC, Celso L, da Cruz H, Rodrigues LS, et al. Diffusion tensor imaging findings in school-aged autistic children. J Neuroimaging (2009) 19(4):337–43. doi:10.1111/j.1552-6569.2009.00366.x
42. Shukla DK, Keehn B, Lincoln AJ, Müller RA. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry (2010) 49(12):1269–78. doi:10.1016/j.jaac.2010.08.018
43. Sivaswamy L, Kumar A, Rajan D, Behen M, Muzik O, Chugani D, et al. A diffusion tensor imaging study of the cerebellar pathways in children with autism spectrum disorder. J Child Neurol (2010) 25:1223–31. doi:10.1177/0883073809358765
44. Hanaie R, Mohri I, Kagitani-Shimono K, Tachibana M, Azuma J, Matsuzaki J, et al. Altered microstructural connectivity of the superior cerebellar peduncle is related to motor dysfunction in children with autistic spectrum disorders. Cerebellum (2013) 12(5):645–56. doi:10.1007/s12311-013-0475-x
45. Jeong W, Tiwari VN, Behen ME, Chugani H, Chugani D. In vivo detection of reduced Purkinje cell fibers with diffusion MRI tractography in children with autistic spectrum disorders. Front Hum Neurosci (2014) 8:1.11. doi:10.3389/fnhum.2014.00110
46. Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord (1994) 24(5):659–85. doi:10.1007/BF02172145
47. Sparrow SS, Cicchetti D. Vineland Adaptive Behavior Scales: Survey form Manual. Circle Pines, MN: American Guidance Service (1984).
48. Henderson S, Sugden D, Barnett AL. The Movement Assessment Battery for Children. 2nd ed. London: The Psychological Corporation (2007).
49. Ogawa S, Lee TM, Kay AR, Tank DE. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A (1990) 87(24):9868–72. doi:10.1073/pnas.87.24.9868
50. Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature (2001) 412:150–7. doi:10.1038/35084005
51. Sporns O, Tononi G, Edelman GM. Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural Netw (2000) 13(8–9):909–22. doi:10.1016/S0893-6080(00)00053-8
52. Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain (2009) 132(9):2413–25. doi:10.1093/brain/awp088
53. Jack A, Morris JP. Neocerebellar contributions to social perception in adolescents with autism spectrum disorder. Dev Cogn Neurosci (2014) 10:77–92. doi:10.1016/j.dcn.2014.08.001
54. Hutchins TL, Bonazinga LA, Prelock PA, Taylor RS. Beyond false beliefs: the development and psychometric evaluation of the Perceptions of Children’s Theory of Mind Measure – Experimental Version (PCToMM-E). J Autism Dev Disord (2008) 38(1):143–55. doi:10.1007/s10803-007-0377-1
55. Kana RK, Maximo JO, Williams DL, Keller TA, Schipul SE, Cherkassky VL, et al. Aberrant functioning of the theory-of-mind network in children and adolescents with autism. Mol Autism (2015) 6:1–12. doi:10.1186/s13229-015-0052-x
56. Padmanabhan A, Lynn A, Foran W, Luna B, O’Hearn K. Age related changes in striatal resting state functional connectivity in autism. Front Hum Neurosci (2013) 7:1–16. doi:10.3389/fnhum.2013.00814
57. Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, et al. Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. Neuroimage Clin (2014) 4:374–82. doi:10.1016/j.nicl.2014.01.008
58. Khan AJ, Nair A, Keown CL, Datko MC, Lincoln AJ, Müller RA. Cerebro-cerebellar resting-state functional connectivity in children and adolescents with autism spectrum disorder. Biol Psychiatry (2015) 78(9):625–34. doi:10.1016/j.biopsych.2015.03.024
59. Carper RA, Solders S, Treiber JM, Fishman I, Müller RA. Corticospinal tract anatomy and functional connectivity of primary motor cortex in autism. J Am Acad Child Adolesc Psychiatry (2015) 54(10):859–67. doi:10.1016/j.jaac.2015.07.007
60. Dajani DR, Uddin LQ. Local brain connectivity across development in autism spectrum disorder: a cross-sectional investigation. Autism Res (2016) 9(1):43–54. doi:10.1002/aur.1494
61. Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ). Los Angeles, CA: Western Psychological Services (2003).
62. Nair A, Keown CL, Datko M, Shih P, Keehn B, Müller RA. Impact of methodological variables on functional connectivity findings in autism spectrum disorders. Hum Brain Mapp (2014) 35(8):4035–48. doi:10.1002/hbm.22456
63. Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci (2013) 7:1–11. doi:10.3389/fnhum.2013.00458
64. Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol (2007) 17(1):103–11. doi:10.1016/j.conb.2007.01.009
65. Mengotti P, D’Agostini S, Terlevic R, De Colle C, Biasizzo E, Londero D, et al. Altered white matter integrity and development in children with autism: a combined voxel-based morphometry and diffusion imaging study. Brain Res Bull (2011) 84(2):189–95. doi:10.1016/j.brainresbull.2010.12.002
66. Wiggins JL, Peltier SJ, Ashinoff S, Weng SJ, Carrasco M, Welsh RC, et al. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Res (2011) 1380:187–97. doi:10.1016/j.brainres.2010.10.102
67. Keehn B, Wagner JB, Tager-Flusberg H, Nelson CA. Functional connectivity in the first year of life in infants at-risk for autism: a preliminary near-infrared spectroscopy study. Front Hum Neurosci (2013) 7:1–11. doi:10.3389/fnhum.2013.00444
Keywords: cortico-cerebellar connectivity, autism spectrum disorders, autism, DTI, fMRI, resting-state fMRI
Citation: Crippa A, Del Vecchio G, Busti Ceccarelli S, Nobile M, Arrigoni F and Brambilla P (2016) Cortico-Cerebellar Connectivity in Autism Spectrum Disorder: What Do We Know So Far? Front. Psychiatry 7:20. doi: 10.3389/fpsyt.2016.00020
Received: 01 December 2015; Accepted: 09 February 2016;
Published: 23 February 2016
Edited by:
Stefan Borgwardt, University of Basel, SwitzerlandReviewed by:
Richard Eugene Frye, Children’s Hospital Boston and Harvard University, USAMegha Sharda, University of Montreal, Canada
Copyright: © 2016 Crippa, Del Vecchio, Busti Ceccarelli, Nobile, Arrigoni and Brambilla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Crippa, alessandro.crippa@bp.lnf.it
 Alessandro Crippa
Alessandro Crippa Giuseppe Del Vecchio
Giuseppe Del Vecchio Silvia Busti Ceccarelli1
Silvia Busti Ceccarelli1 Paolo Brambilla
Paolo Brambilla