- 1Psychopharmacology, Drug Misuse and Novel Psychoactive Substances Research Unit, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom
- 2Neomesia Mental Health, Villa Jolanda Hospital, Jesi, Italy
- 3Polyedra, Teramo, Italy
- 4NHS, Department of Mental Health, Psychiatric Service of Diagnosis and Treatment, Hospital “G. Mazzini,” Teramo, Italy
- 5Department of Neuroscience, Imaging and Clinical Science, Chair of Psychiatry, University “G. D’Annunzio,” Chieti, Italy
Hallucinogen-persisting perception disorder (HPPD) is a syndrome characterized by prolonged or reoccurring perceptual symptoms, reminiscent of acute hallucinogen effects. HPPD was associated with a broader range of LSD (lysergic acid diethylamide)-like substances, cannabis, methylenedioxymethamphetamine (MDMA), psilocybin, mescaline, and psychostimulants. The recent emergence of novel psychoactive substances (NPS) posed a critical concern regarding the new onset of psychiatric symptoms/syndromes, including cases of HPPD. Symptomatology mainly comprises visual disorders (i.e., geometric pseudo-hallucinations, haloes, flashes of colors/lights, motion-perception deficits, afterimages, micropsia, more acute awareness of floaters, etc.), even though depressive symptoms and thought disorders may be comorbidly present. Although HPPD was first described in 1954, it was just established as a fully syndrome in 2000, with the revised fourth version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). HPPD neural substrates, risk factors, and aetiopathogenesys still largely remain unknown and under investigation, and many questions about its pharmacological targets remain unanswered too. A critical mini review on psychopathological bases, etiological hypothesis, and psychopharmacological approaches toward HPPD, including the association with some novel substances, are provided here, by means of a literature search on PubMed/Medline, Google Scholar, and Scopus databases without time restrictions, by using a specific set of keywords. Pharmacological and clinical issues are considered, and practical psychopharmacological recommendations and clinical guidelines are suggested.
Introduction
Hallucinogen-persisting perception disorder (HPPD) is a long-lasting and potentially permanent syndrome characterized by a spontaneous recurrence of perceptual/visual disturbances which are reminiscent of those generated while a subject was intoxicated with hallucinogens. According to the Fifth Version of Diagnostic and Statistical Manual of Mental Disorders (DSM-5), HPPD is defined as the following criteria (1):
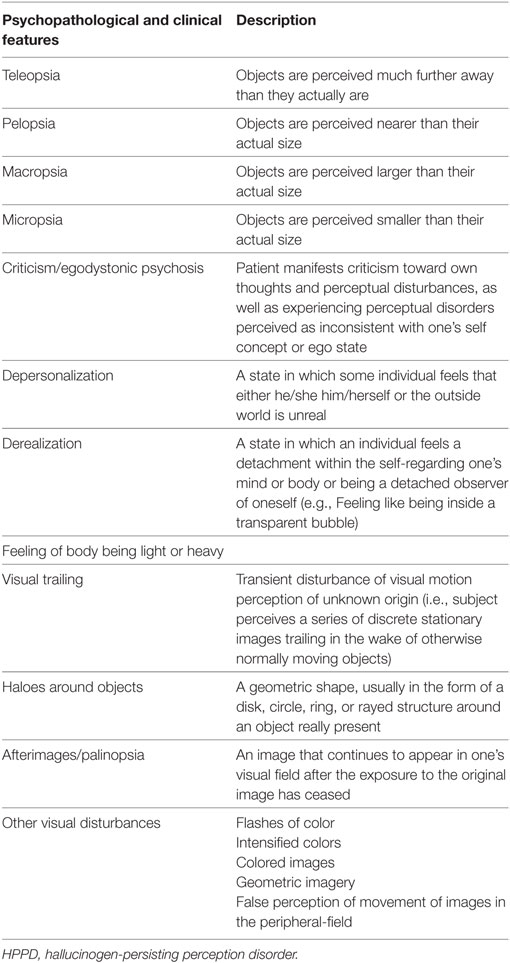
(A) following cessation of use of a hallucinogen, the reexperiencing of one or more of the perceptual symptoms that were experienced while intoxicated with the hallucinogens (e.g., geometric hallucinations, false perceptions of movement in the peripheral visual fields, flashes of color, intensified colors, trial images of moving objects, positive after images, haloes around objects, macropsia, and micropsia);
(B) the symptoms in criterion (A) cause clinically significant distress or impairment in social, occupational, or other important areas of functioning;
(C) the symptoms are not due to a general medical condition (e.g., anatomical lesions and infections of the brain, visual epilepsies) and are not better accounted for by another mental disorder (e.g., delirium, dementia, schizophrenia) or hypnopompic hallucinations.
Before diagnosing an HPPD, post-traumatic stress disorder, depersonalization, derealization, and hallucinogen-induced psychotic mood or anxiety disorders should be excluded (2). Moreover, other causes of visual disturbances should be investigated and excluded, such as anatomical lesions, brain infections, epilepsy, schizophrenia, delirium state, or hypnopompic hallucinations (2). Furthermore, an association between the first intake, frequency, and quantity of drug taken and the likelihood of developing an HPPD has not been demonstrated, as has been the onset of the disorder following a single hallucinogenic experience (3).
Epidemiology
Overall, prevalence of HPPD has been generally considered low (2). However, limited publications suggested that chronic visual disturbances may be relatively common among hallucinogens’ users. It has often been assumed that HPPD may be a severe clinical manifestation of the drug-induced visual changes (3–5). While the probability of flashbacks occurring in the wake of hallucinogen use may vary from 5 to 50% among hallucinogens’ users (6, 7), the probability of an HPPD being manifested is lower (3).
Historical Background
Hallucinogen-persisting perception disorder was first described in 1954 (8). Subsequent observations have been then described (3, 4, 8–12). Horowitz (10) first introduced the term flashbacks, referring to recurrent and spontaneous perceptual distortions and unbidden images. When these “flashbacks” present as recurrent, but without a current acute, or chronic hallucinogen intake, the disturbance is referred to as HPPD. Horowitz (10) classified also three types of visual flashbacks: (a) perceptual distortions (e.g., seeing haloes around objects); (b) heightened imagery (e.g., visual experiences as much more vivid and dominant in one’s thoughts); and (c) recurrent unbidden images (e.g., subjects see objects that are not there). HPPD has been introduced under the diagnosis of Post- hallucinogen Perception Disorder in 1987 within the DSM-III-R (13). Subsequently, the DSM-IV-TR (14) recognized the syndrome as Hallucinogen-Persisting Perception Disorder (Flashbacks) (code 292.89) (15). The disorder was confirmed as nosological entity as well in the DSM-5 (1).
Phenomenology
Hallucinogen-persisting perception disorder is characterized by a plethora of visual disturbances (e.g., geometric imagery, afterimages, false perceptions of movement in the peripheral fields, flashes of light, etc.) (3) (Table 1), including pseudohallucinations. It has been also associated with a LSD (lysergic acid diethylamide)-like dysphoria, panic attacks, and depressive symptomatology. Visual disturbances may be episodic, stress or substance-induced, or persistent. However, the episodes may last for 5 years or more and the symptomatology is disliked by patients (10). While a flashback is usually reported to be infrequent and episodic, HPPD is usually persisting and long-lasting. Moreover, some HPPD subjects report that adaptation to the dark takes significantly longer compared with the general population (16). Moreover, HPPD has been associated with abnormal results for tests of visual function, suggesting disinhibition in the processing of visual information (4, 16). The subject does not develop any paranoid misinterpretation related to and does not believe that their own visual hallucinogenic experiences currently occur (3). Therefore, it has been supposed that there is involvement of the primary visual cortex, the first cortical area responsible for geometric processing of visual input (17). Further data coming from clinical, psychological, and neurophysiological sources may suggest specific physiological changes in the visual system function implicated in the onset of hallucinations after the intake of psychedelics/hallucinogens (5). Table 1 summarizes all clinical and psychopathological characteristics associated with an HPPD.
Aetiopathogenesys
The pathogenesis of HPPD is currently unknown, even though it has been frequently reported to be associated with the intake of LSD (4, 16, 41). However, HPPD has also been reported following the consumption of all substances with hallucinogenic properties which possess pharmacological and clinical effects resembling those experienced with LSD by serotoninergic 5-HT2A (42), such as cannabis (18, 43, 44), 3,4-methylenedioxymethamphetamine (MDMA, aka “ecstasy”) (45–47), and the recently marketed novel psychoactive substances (NPS). In fact, the advent of NPS facilitated the onset of new psychopathologies and new clinical manifestations, including cases of HPPD, particularly following the intake of synthetic cannabinoids (SCs) and other new synthetic psychedelics and hallucinogens, which facilitated the reoccurrence of this disorder, by posing a new clinical concern to clinicians (19, 20, 48, 49).
Types of HPPD
Two types of HPPD have been proposed here, accordingly to Lev-Ran (40). Type-1 HPPD, consistent with the definition of flashback provided by the ICD-10 (50), is characterized by brief reexperiences of altered perception, mood, and/or consciousness, as previously experienced during a hallucinogenic intoxication. Symptomatology may be pleasurable and even controllable. They may appear days to months after the hallucinogen-induced experience. The subject is usually aware of the unreality of their own experience. The perception of time may be altered. Visual perceptions usually comprise perceived increased color intensity, dimensionality, vibrancy, illusory changes, and movements of a perceived object. Type-1 HPPD comprises the flashbacks definition, while type-2 HPPD has been used to indicate the HPPD definition elaborated by Abraham (3) as well proposed in the DSM-5. The symptoms usually include palinopsia (afterimages effects), the occurrence of haloes, trails, akinetopsia, visual snows, etc. Sounds and other perceptions are usually not affected. Visual phenomena have been reported to be uncontrollable and disturbing. Symptomatology may be accompanied by depersonalization, derealization, anxiety, and depression (3).
The present systematic mini review aims at providing an overview of HPPD, by specifically focusing on both clinical manifestations and psychopharmacological approaches, in general, and among NPS users.
Materials and Methods
Search Sources and Strategies
A critical mini review was conducted, following the methods recommended by the Cochrane Collaboration (51) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (52). Searches were carried out by using PubMed/Medline, Google Scholar, and Scopus. We combined the search strategy of free text terms and exploded MESH headings for the topics of HPPD and NPS as follows: ((Hallucinogen Persisting Perception Disorder [Title/Abstract] OR HPPD [Title/Abstract])) OR ((Hallucinogen Persisting Perception Disorder [Title/Abstract] OR HPPD [Title/Abstract])) and ((novel psychoactive substances [Title/Abstract]) OR (NPS[Title/Abstract])). All articles through August 15, 2017 without time restriction were selected. In addition, the authors performed further secondary searches by using the reference listing of all eligible papers.
Study Selection
We considered studies about HPPD, and whenever available, evaluating the relationship between HPPD and NPS. The authors examined all titles/abstracts and obtained full texts of potentially relevant papers. Two reviewers (LO and DP), independently and in duplicate, read the papers and selected papers according to the inclusion criteria. Duplicate publications were excluded. All the articles identified by the data sources, reporting original data related to HPPD in general and, more specifically, among NPS users, were considered in the present review. All experimental and observational study designs, including case reports and case series, were included as limited data have been published so far. Narrative and systematic reviews, letters to the editor, and book chapters were excluded, even though they were used for retrieving further secondary searches. To be included in the present review, studies were required to meet the following criteria: (a) empirical and peer-reviewed study; (b) at least an abstract with estimates and/or full results available/complete; (c) investigate HPPD in general and more specifically among NPS users; (d) human studies; and (e) provide data on psychopathological features and/or psychopharmacological treatments in these cases.
Data Extraction and Management
LO and DP independently extracted the data on participant characteristics, intervention details, and outcomes measures. Disagreements were resolved by discussion and consensus with a third member of the team (DDB). Data were collected using an ad hoc developed data extraction spreadsheet.
Characteristics of Included Studies
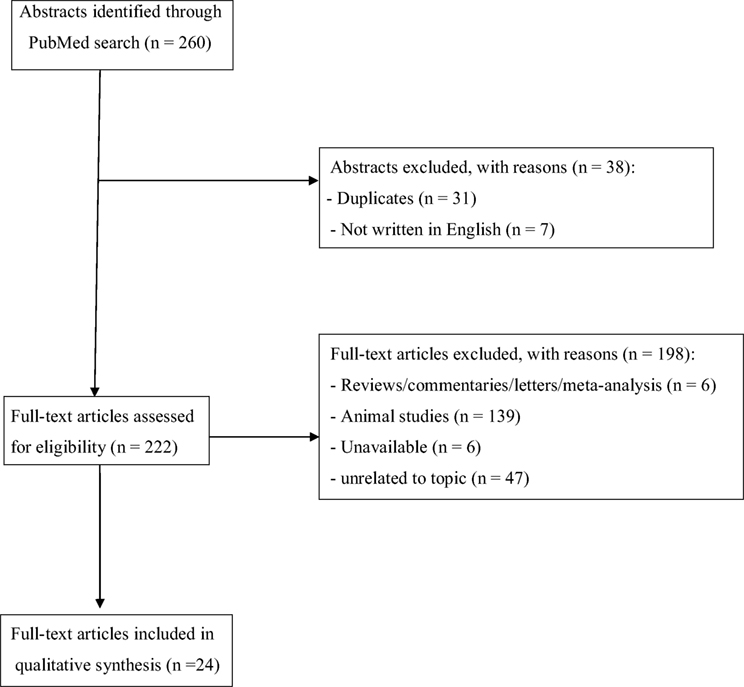
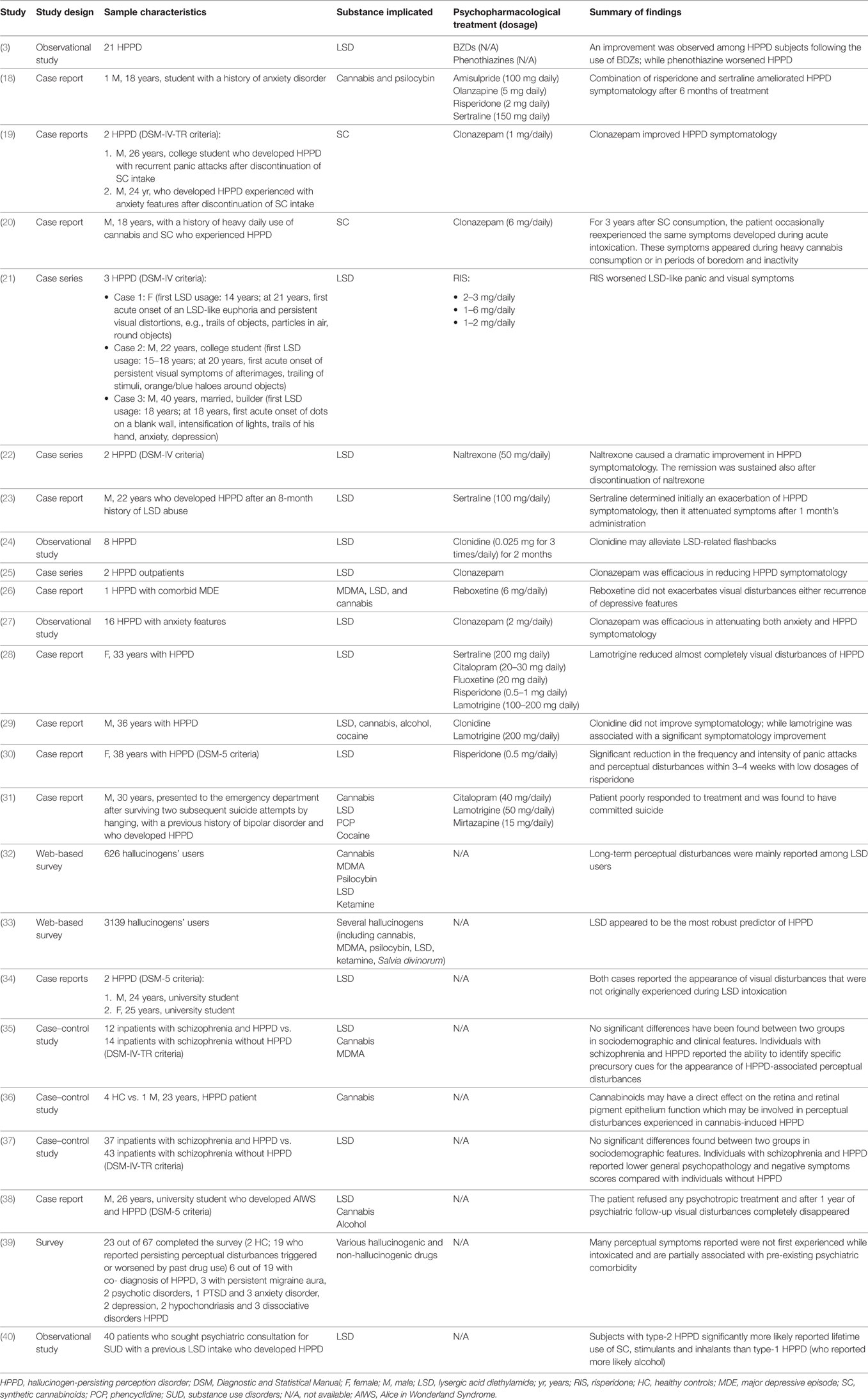
The set of keywords initially generated 260 results (Figure 1). A total of 31 papers were excluded because of duplicates; 7 papers were excluded because they did not provide relevant data useful for the aims of our papers (due to the lack of an English abstract). Of the remaining 222 studies, further 186 studies were excluded because they did not meet the inclusion criteria or because they were non-human studies. Of the remaining 36 papers, 6 papers were excluded because they were reviews, letters to editors, or metanalyses; however, 6 papers were not included here due to the lack of an available full text or an abstract useful for collecting relevant data. Finally, a total of 24 papers were included and accounted for in our analysis. Table 2 shows the main characteristics (study design, sample size, main outcomes, and findings) of all studies reviewed here.
Results
Studies on Psychopharmacotherapy of HPPD
An observational study recruited 21 HPPD subjects who were treated with benzodiazepines and/or phenothiazines (3). Among subjects receiving benzodiazepines, eight out of nine reported a reduction in intensity/frequency of visual disorders. Most (11 out of 12) phenothiazine-treated subjects described an exacerbation of HPPD (3). A case series reported three cases of HPPD, treated with risperidone, who presented a worsening of visual perceptions and panic symptomatology (21). Lerner et al. (22) described two LSD-induced HPPD male subjects who reported an improvement of symptomatology following naltrexone (50 mg daily) treatment (22). Another case report described a 22-old-year male who developed HPPD after 8-month discontinuation of LSD (22). The subject significantly improved after sertraline (100 mg/daily) treatment (23). An open-label pilot study recruited eight HPPD drug-free patients who were consecutively treated with 0.025 mg of clonidine, three times a day, for 2 months, in order to evaluate the efficacy of drug in treating persistent visual disturbances associated with the intake of LSD (24). LSD-related flashbacks demonstrated a good response to clonidine (24). A study described two HPPD outpatients who efficaciously responded to clonazepam (25). An HPPD patient with a comorbid depressive symptomatology and a prior history of cannabis, ecstasy and LSD abuse, clinically responded to 6 mg daily of reboxetine (26). An open-label study recruited 16 drug-free patients affected with HPPD with anxiety features for at least 3 months who received 2 mg daily of clonazepam for 2 months (27). Subjects reported a significant relief of anxiety and HPPD symptomatology with only mild symptomatology during the clonazepam treatment, suggesting the efficacy of clonazepam in these cases (27). Espiard et al. (18) presented a case report of an HPPD patient with a previous mixed intoxication with psilocybin and cannabis. Perceptual disorders appeared after a single psilocybin consumption. The subject re-experienced the symptomatology the following day during another cannabis snort. Moreover, symptomatology was recurrent daily with an attenuation after discontinuation until 6 months after he had stopped cannabis. Initially, he received amisulpride (100 mg/daily) treatment, subsequently stopped due to sedative effects. Then he started with olanzapine (5 mg/daily) which caused an exacerbation of symptomatology. Finally, he was treated with risperidone (2 mg/daily). Then, it was coadministered with sertraline (150 mg/daily) for the treatment of persisting dysphoric mood and recurrent anxiety-like symptoms. After 6 months of combination risperidone and sertraline, HPPD disappeared (18). A 33-year-old female developed an HPPD following the recreational use/abuse of LSD for a year at the age of 18 (28). Approximately, 2–3 weeks after the last drug intake, the subject developed persistent visual disturbances (a sort of attenuated flashbacks), like those experienced during an acute LSD intoxication, which lasted for over 13 years, with a little change in intensity and frequency. Despite the patient receiving several psychopharmacological treatments (e.g., sertraline, citalopram, fluoxetine, risperidone, etc.), only a year-long trial of lamotrigine (100–200 mg/day) improved abnormal visual perceptions. In addition, no significant cognitive deficits (i.e., memory functioning, attention span, visual-construction, and frontal-executive functioning) were detected, but an underperformance in the phasic attention. A follow-up after 19 months showed a continued improvement in attention performance. Brain magnetic resonance imaging scans, electroencephalograms, median nerve somatosensory, and visual evoked potential tests were all reported normal (28). A case report described a 36-year-old HPPD subject who experienced persistent visual disturbances after recreational use of LSD, cannabis, alcohol, and cocaine (29). The patient was initially treated with a 3-month course of clonidine without any improvement; then, he was treated with lamotrigine (200 mg daily) for a 7-month period with a significant improvement of his visual disturbances and overall mental well-being (29). A case report described a woman who experienced panic attacks and persistent “re-experience phenomena” characterized with flashbacks to the first trip with LSD. She responded to 0.5 mg daily of risperidone (30). A case report described an HPPD patient with a history of bipolar disorder, a previous consumption of LSD, cannabis, cocaine, and phencyclidine (PCP) (30). The patient, even though treated with lamotrigine, citalopram, and mirtazapine, committed suicide, after the hospitalization (31).
Studies on the Clinical Manifestation and Psychopathology in HPPD
A web-based questionnaire study investigated users’ perceptions of the benefits/harms of hallucinogens’ intake, including the occurrence and prevalence of flashback phenomena and/or HPPD (32). Only 10% of the sample recruited reported long-term perceptual changes, which they would “rather not have but could live with,” while 1% reported changes that “drove them mad” (32). Of these, 39% reported them following the use of LSD, 11% after psilocybin, 9% after MDMA and cannabis, 4% ketamine, and 1% alcohol. Despite 22% of subjects reported having experienced a “flashback”, only 3% of subjects experienced a “negative” flashback (32). A web-based questionnaire investigated abnormal visual experiences among 3139 hallucinogens’ users (33). Each participant had their drug-use history (i.e., classical serotonergic hallucinogens, including LSD, psilocybin, dimethyltryptamine, etc.; NMDA antagonists, including ketamine and dextromethorphan; MDMA, anticholinergic-containing Datura plants; cannabis; Salvia Divinorum, etc.); psychiatric and neurological history; and their visual experiences investigated. Several drugs with hallucinogenic properties have been statistically associated with unusual visual experiences, even though LSD seemed to be the most frequently reported among the HPPD-like cases (33). Lerner et al. (19) reported two case reports with a prior history of LSD intake, who experienced new visual disturbances, not previously appeared during the first LSD intoxication, after totally stopping substance use (34). A pilot study recruited 26 inpatients affected with schizophrenia and concomitant self-reported past LSD use who presented with HPPD (n = 12) or without HPPD (n = 14) (35). No significant differences in demographic, clinical features, adverse effects, and response to medications have been reported between the two groups. Furthermore, the authors reported that patients with schizophrenia and concomitant HPPD were able to distinguish HPPD symptoms from hallucinations related to their own psychotic state (35). A case-control study recruited a cannabis-user who developed HPPD and four healthy controls in order to evaluate the differences in their ophthalmological state (36). Ophthalmological examination did not report clinically significant abnormal values for the HPPD patient or for any healthy controls, even though the HPPD subject reported a slightly reduced fast oscillation rate, a diminished standing potential of the slow oscillations, and a light peak within normal range resulting in higher Arden ratios (36). Another study by Lev-Ran et al. (37) compared the characteristics of schizophrenic patients with a prior LSD consumption who developed HPPD (n = 37) with those who did not develop HPPD (n = 43). No significant differences in sociodemographic characteristics were reported between the two groups. Individuals with schizophrenia and HPPD showed lower scores for negative symptoms (p < 0.001), general psychopathology (p = 0.02), and total symptoms (p < 0.001), as measured with Positive and Negative Symptomatology Scale (PANSS), as compared with those reported in the group of schizophrenics without HPPD (37). A case report described a patient with a prior history of cannabis, alcohol, and LSD sporadic recreational consumption, who developed LSD-induced “Alice in Wonderland Syndrome” and HPPD after discontinuation of all substances (38). A questionnaire specifically identified prevalence and characteristics of self-reported altered perception experiences in hallucinogens’ users (39). The survey concluded that HPPD may be due to a subtle over-activation of predominantly neural visual pathways which in turn may worsen anxiety after ingestion of arousal-altering drugs (39). A study explored triggers associated with type-1 and type-2 HPPD following the use of LSD, by recruiting 40 outpatients affected with HPPD (40). The findings reported differences in terms of visual disturbances and triggers between the two HPPD types. The most common types of visual disturbances were slow movement of still objects (among type-1 HPPD) and trailing phenomena (among type-2 HPPD). Furthermore, type-1 HPPD subjects were more likely to experience disturbances in a dark environment (40).
Studies on NPS and HPPD
A case series described two cases of HPPD induced by SCs (19). A recent case report described a patient with a previous history of heavy daily use of cannabis admitted to an Addiction Treatment Unit who reexperienced visual hallucinations and disturbances, like those who experienced during acute consumption of SC, in the days following SC consumption (20).
Discussion
Several case reports of reoccurring or prolonged persistent visual perceptual disturbances (HPPD) have been described occurring within a certain time frame after cessation of some hallucinogenic drugs (2, 53, 54).
The present critical mini review presents several limitations. First, given the paucity of double-blind, placebo-controlled/case-control studies, we included case reports and case series as well, and studies with small sample size which may greatly limit the generalizability of findings reviewed here. Second, methodological strategies (sample size, study design, diagnostic criteria, etc.) may vary greatly in several studies retrieved. Most studies included here investigated HPPD cases following the intake of LSD, even though other isolated cases described incidents following the intake of other serotonergic hallucinogens, and cannabis. Furthermore, most studies here retrieved do not specifically distinguish between the two types of HPPD, as previously discussed, by limiting the complete understanding of the clinical symptomatology and manifestation.
The recent wave of novel substances available on the market, includes several novel cannabimimetics, novel hallucinogens (e.g., NBOMe compounds), and new LSD derivatives (48, 49). With regard to these substances, the prevalence of HPPD among NPS users is difficult to detect, due to the limited number of published reports, mainly due to the lack of knowledge among the clinicians and the consequent difficulty in the diagnosis (48). However, the specific pharmacological profile of some psychostimulants and hallucinogens may likely determine an increasing number of NPS-induced HPPD cases (48, 49). In fact, despite several studies reporting a higher occurrence of HPPD following the consumption of LSD, HPPD has been associated with a broader range of substances, e.g., natural and synthetic serotonergic 5-HT2A receptor hallucinogens (44, 47, 55–57), including the most recent new synthetic psychostimulants and hallucinogens (19, 20,48, 49). In particular, the intake of SCs, which are CB1 receptor full agonists and possess indole-derived structures, which may itself facilitates the 5-HT2A receptor dysfunction (48, 58, 59), has been associated with the onset of perceptual disorders, even after a total discontinuation, as it has been supposed that SCs may provoke a partial/total reminiscence of the previous perceptual experience in predisposed and susceptible NPS users (20, 60, 61).
Furthermore, the pharmacotherapy of HPPD may be different from study to study, as only few studies have been published so far and any recommendations are based almost entirely on non-controlled studies of small patient populations or single case reports (3, 18–31). Risperidone is a highly potent antagonist of both postsynaptic 5-HT2 and dopamine D2 receptors (62). As 5-HT2 receptor antagonists are effective in treating hallucinations among schizophrenic patients, it has been previously supposed that risperidone would be efficacious in treating HPPD (18, 30). However, most studies reviewed here reported a worsening of symptomatology, particularly visual disturbances and an abrupt onset of panic attacks after the intake of risperidone among LSD users (21, 28). The symptomatology tended to return to baseline levels when risperidone was discontinued (21, 28). A case report described an improvement in HPPD symptomatology with a combination of sertraline and risperidone (18). Some studies described a mild efficacy of clonidine (24, 25) or completely ineffective (28). Several studies suggested prescribing clonazepam as an effective treatment in reducing persistent perceptual disturbances (19, 20, 25, 27). In fact, it has been hypothesized that high-potency benzodiazepines (e.g., clonazepam), which as well as possessing serotoninergic activity, may be superior to low-potency benzodiazepines (27). Moreover, a case report suggested that reboxetine made a good improvement both in visual disturbances and depressive symptomatology (26). Reboxetine is an α-2-adrenoceptor modulating the effect on both noradrenaline and serotonin release which may affect sympathetic activity, hence facilitating the improvement of HPPD symptomatology (26). Other studies suggest lamotrigine as efficacious in ameliorating HPPD symptomatology (28, 29). Lamotrigine acts by blocking sodium and voltage-gated calcium channels and inhibiting glutamate-mediated excitatory neurotransmission, thereby suggesting its potential use in the treatment of HPPD (28).
Serotonin neurotransmission has been hypothesized to be involved in the aetiopathogenesys of both acute and persisting LSD- and SC-induced perceptual disturbances (61). The main mechanism supposed to be implicated consists of a vulnerability/predisposition of psychedelics’ consumers to continue centrally processing visual imagery after the visualization has been totally eradicated from the visual field (23). Persisting visual disorders may be explained by a reversible (or irreversible) “dysfunction” in the cortical serotonergic inhibitory inter-neurons with GABA-ergic outputs (63). The anandamidergic system has been also implicated by involving the areas of visual information processing (64, 65).
Further studies should be implemented in order to better clarify the role of the NPS, particularly the new psychedelics and psychostimulants with LSD-like properties in the pathogenesis and etiology of new HPPD cases.
Author Contributions
LO and FS conceived the topic of the manuscript, while LO, AG and GP carried out the main analysis. JC and DB assisted in either screening of the studies or preparation of the attachments. FS served as study reviewer. FS served as senior study reviewer. All the coauthors substantially contributed to the present piece of work before approving it for final submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding
This paper was supported in part by grants of the European Commission (Drug Prevention and Information Programme 2014–16; contract no. JUST/2013/DPIP/AG/4823; EU-MADNESS project). Further financial support was provided by the EU Commission-targeted call on cross-border law enforcement cooperation in the field of drug trafficking—DG Justice/DG Migrations and Home Affairs (JUST/2013/ISEC/DRUGS/AG/6429) Project EPS/NPS (Enhancing Police Skills concerning novel psychoactive substances, NPS).
References
1. American Psychiatric Association. DSM 5. Diagnostic and Statistical Manual of Mental Disorders––Fifth Version. Arlington: American Psychiatric Association Publishing (2013).
2. Halpern JH, Pope HG Jr. Hallucinogen persisting perception disorder: what do we know after 50 years? Drug Alcohol Depend (2003) 69(2):109–19. doi:10.1016/S0376-8716(02)00306-X
3. Abraham HD. Visual phenomenology of the LSD flashback. Arch Gen Psychiatry (1983) 40:884–9. doi:10.1001/archpsyc.1983.01790070074009
4. Abraham HD. A chronic impairment of color vision in users of LSD. Br J Psychiatry (1982) 140:518–20. doi:10.1192/bjp.140.5.518
5. Abraham HD, Duffy FH. EEG coherence in post-LSD visual hallucinations. Psychiatry Res (2001) 107(3):151–63. doi:10.1016/S0925-4927(01)00098-1
6. Alarcon RD, Dickinson WA, Dohn HH. Flashback phenomena. Clinical and diagnostic dilemmas. J Nerv Ment Dis (1982) 170:217–23. doi:10.1097/00005053-198204000-00006
7. McGee R. Flashbacks and memory phenomena. A comment on “Flashback phenomena – clinical and diagnostic dilemmas”. J Nerv Ment Dis (1984) 172:273–8. doi:10.1097/00005053-198405000-00004
8. Sandison RA, Spencer AM, Whitelaw JD. The therapeutic value of lysergic acid diethylamide in mental illness. J Ment Sci (1954) 100:491–502.
9. Robbins E, Frosch WA, Stern M. Further observations on untoward reactions to LSD. Am J Psychiatry (1967) 124:393–5. doi:10.1176/ajp.124.3.393
10. Horowitz MJ. Flashbacks: recurrent intrusive images after the use of LSD. Am J Psychiatry (1969) 126:565–9. doi:10.1176/ajp.126.4.565
11. Shick JFE, Smith D. Analysis of the LSD flashback. J Psychedel Drugs (1970) 3:13–9. doi:10.1080/02791072.1970.10471357
12. Holsten F. Flashbacks: a personal follow-up. Arch Psychiatr Nervenkr (1976) 222:293–304. doi:10.1007/BF00343238
13. American Psychiatric Association. DSM III-R. Diagnostic and Statistical Manual of Mental Disorders––Third Revised Version. Arlington: American Psychiatric Association Publishing (1987).
14. American Psychiatric Association. DSM IV-TR. Diagnostic and Statistical Manual of Mental Disorders––Forth Text Revised Version. Arlington: American Psychiatric Association Publishing (2000).
15. Trull TJ, Vergés A, Wood PK, Jahng S, Sher KJ. The structure of diagnostic and statistical manual of mental disorders (4th edn) personality disorder symptoms in a large national sample. Personal Disord (2012) 3:355–69. doi:10.1037/a0027766
16. Abraham HD, Wolf E. Visual function in past users of LSD: psychophysical findings. J Abnorm Psychol (1988) 97(4):443–7. doi:10.1037/0021-843X.97.4.443
17. Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci (1977) 278(961):377–409. doi:10.1098/rstb.1977.0050
18. Espiard ML, Lecardeur L, Abadie P, Halbecq I, Dollfus S. Hallucinogen persisting perception disorder after psilocybin consumption: a case study. Eur Psychiatry (2005) 20(5–6):458–60. doi:10.1016/j.eurpsy.2005.04.008
19. Lerner A, Goodman C, Bor O, Lev-Ran S. Synthetic cannabis substances (SPS) use and hallucinogen persisting perception disorder (HPPD): two case reports. Isr J Psychiatry Relat Sci (2014) 51(4):277–80.
20. Coppola M, Mondola R. JWH-122 consumption adverse effects: a case of hallucinogen persisting perception disorder five-year follow-up. J Psychoactive Drugs (2017) 25:1–4. doi:10.1080/02791072.2017.1316431
21. Abraham HD, Mamen A. LSD-like panic from risperidone in post-LSD visual disorder. J Clin Psychopharmacol (1996) 16(3):238–41. doi:10.1097/00004714-199606000-00008
22. Lerner AG, Oyefe I, Isaacs G, Sigal M. Naltrexone treatment of hallucinogen persisting perception disorder. Am J Psychiatry (1997) 154(3):437. doi:10.1176/ajp.154.3.437a
23. Young CR. Sertraline treatment of hallucinogen persisting perception disorder. J Clin Psychiatry (1997) 58:85. doi:10.4088/JCP.v58n0206a
24. Lerner AG, Gelkopf M, Oyffe I, Finkel B, Katz S, Sigal M, et al. LSD-induced hallucinogen persisting perception disorder treatment with clonidine: an open pilot study. Int Clin Psychopharmacol (2000) 15(1):35–7. doi:10.1097/00004850-200015010-00005
25. Lerner AG, Skladman I, Kodesh A, Sigal M, Shufman E. LSD-induced hallucinogen persisting perception disorder treated with clonazepam: two case reports. Isr J Psychiatry Relat Sci (2001) 38(2):133–6.
26. Lerner AG, Shufman E, Kodesh A, Kretzmer G, Sigal M. LSD-induced hallucinogen persisting perception disorder with depressive features treated with reboxetine: case report. Isr J Psychiatry Relat Sci (2002) 39(2):100–3.
27. Lerner AG, Gelkopf M, Skladman I, Rudinski D, Nachshon H, Bleich A. Clonazepam treatment of lysergic acid diethylamide-induced hallucinogen persisting perception disorder with anxiety features. Int Clin Psychopharmacol (2003) 18(2):101–5. doi:10.1097/00004850-200303000-00007
28. Hermle L, Simon M, Ruchsow M, Geppert M. Hallucinogen-persisting perception disorder. Ther Adv Psychopharmacol (2012) 2(5):199–205. doi:10.1177/2045125312451270
29. Hermle L, Simon M, Ruchsow M, Batra A, Geppert M. Hallucinogen persisting perception disorder (HPPD) and flashback are they identical? J Alcohol Drug Depend (2013) 1:4. doi:10.4172/1000121
30. Subramanian N, Doran M. Improvement of hallucinogen persisting perception disorder (HPPD) with oral risperidone: case report. Ir J Psychol Med (2013) 31(1):47–9. doi:10.1017/ipm.2013.59
31. Brodrick J, Mitchell BG. Hallucinogen persisting perception disorder and risk of suicide. J Pharm Pract (2016) 29(4):431–4. doi:10.1177/0897190014566314
32. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use (2010) 15(4):283–300. doi:10.3109/14659890903271624
33. Baggott MJ, Coyle JR, Erowid E, Erowid F, Robertson LC. Abnormal visual experiences in individuals with histories of hallucinogen use: a web-based questionnaire. Drug Alcohol Depend (2011) 114:61–7. doi:10.1016/j.drugalcdep.2010.09.006
34. Lerner A, Goodman C, Rudinski D, Lev-Ran S. LSD flashbacks—the appearance of new visual imagery not experienced during initial intoxication: two case reports. Isr J Psychiatry Relat Sci (2014) 51(4):307–9.
35. Lev-Ran S, Feingold D, Frenkel A, Lerner AG. Clinical characteristics of individuals with schizophrenia and hallucinogen persisting perception disorder: a preliminary investigation. J Dual Diagn (2014) 10(2):79–83. doi:10.1080/15504263.2014.906155
36. Zobor D, Strasser T, Zobor G, Schober F, Messias A, Strauss O, et al. Ophthalmological assessment of cannabis-induced persisting perception disorder: is there a direct retinal effect? Doc Ophthalmol (2015) 130(2):121–30. doi:10.1007/s10633-015-9481-2
37. Lev-Ran S, Feingold D, Rudinski D, Katz S, Arturo LG. Schizophrenia and hallucinogen persisting perception disorder: a clinical investigation. Am J Addict (2015) 24(3):197–9. doi:10.1111/ajad.12204
38. Lerner A, Lev-Ran S. LSD-associated “Alice in Wonderland Syndrome”(AIWS): a hallucinogen persisting perception disorder (HPPD) case report. Isr J Psychiatry Relat Sci (2015) 52(1):67–8.
39. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. In: Current Topics in Behavioral Neurosciences. Berlin, Heidelberg: Springer (2016). doi:10.1007/7854_2016_457
40. Lev-Ran S, Feingold D, Goodman C, Lerner AG. Comparing triggers to visual disturbances among individuals with positive vs. negative experiences of hallucinogen-persisting perception disorder (HPPD) following LSD use. Am J Addict (2017) 26(6):568–71. doi:10.1111/ajad.12577
41. Abraham HD, Aldridge AM, Gogia P. The psychopharmacology of hallucinogens. Neuropsychopharmacology (1996) 14(4):285–98. doi:10.1016/0893-133X(95)00136-2
42. Nichols DE. Hallucinogens. Pharmacol Ther (2004) 101:131–81. doi:10.1016/j.pharmthera.2003.11.002
43. Annis HM, Smart RG. Adverse reactions and recurrences from marihuana use. Br J Addict Alcohol Other Drugs (1973) 68:315–9. doi:10.1111/j.1360-0443.1973.tb01263.x
44. Gaillard MC, Borruat FX. Persisting visual hallucinations and illusions in previously drug-addicted patients. Klin Monbl Augenheilkd (2003) 220:176–8. doi:10.1055/s-2003-38173
45. Creighton FJ, Black DL, Hyde CE. ‘Ecstasy’ psychosis and flashbacks. Br J Psychiatry (1991) 159:713–5. doi:10.1192/bjp.159.5.713
46. McGuire P, Fahy T. Flashbacks following MDMA. Br J Psychiatry (1992) 160:276. doi:10.1192/bjp.160.2.276a
47. McGuire PK, Cope H, Fahy TA. Diversity of psychopathology associated with use of 3,4- methylenedioxymethamphetamine (‘Ecstasy’). Br J Psychiatry (1994) 165:391–5. doi:10.1192/bjp.165.3.391
48. Schifano F, Orsolini L, Papanti GD, Corkery JM. Novel psychoactive substances of interest for psychiatry. World Psychiatry (2015) 14(1):15–26. doi:10.1002/wps.20174
49. Schifano F, Papanti GD, Orsolini L, Corkery JM. Novel psychoactive substances: the pharmacology of stimulants and hallucinogens. Expert Rev Clin Pharmacol (2016) 9(7):943–54. doi:10.1586/17512433.2016.1167597
50. World Health Organization. ICD-10. International Statistical Classification of Diseases and Related Health Problems 10th Revision. Geneva: World Health Organization (1992).
51. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Book Series. New Jersey: Wiley-Blackwell (2008).
52. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700. doi:10.1136/bmj.b2700
54. Halpern JH, Sherwood AR, Hudson JI, Yurgelun-Todd D, Pope HG Jr. Psychological and cognitive effects of long-term peyote use among Native Americans. Biol Psychiatry (2005) 58(8):624–31. doi:10.1016/j.biopsych.2005.06.038
55. Weil AT. Adverse reactions to marihuana. Classification and suggested treatment. N Engl J Med (1970) 282:997–1000. doi:10.1056/NEJM197004302821803
56. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis (1984) 172:577–95. doi:10.1097/00005053-198410000-00001
57. Batzer W, Ditzler T, Brown C. LSD use and flashbacks in alcoholic patients. J Addict Dis (1999) 18:57–63. doi:10.1300/J069v18n02_06
58. Papanti D, Schifano F, Botteon G, Bertossi F, Mannix J, Vidoni D, et al. ‘Spiceophrenia’: a systematic overview of ‘spice’—related psychopathological issues and a case report. Hum Psychopharmacol (2013) 28:379–89. doi:10.1002/hup.2312
59. Papanti D, Orsolini L, Francesconi G, Schifano F. ‘Noids’ in a nutshell: everything you (don’t) want to know about synthetic cannabimimetics. Adv Dual Diagn (2014) 7:137–48. doi:10.1108/ADD-02-2014-0006
60. Benford DM, Caplan JP. Psychiatric sequelae of spice, K2, and synthetic cannabinoid receptor agonists. Psychosomatics (2011) 52:295. doi:10.1016/j.psym.2011.01.004
61. Lerner AG, Goodman C, Rudinski D, Bleich A. Benign and time-limited visual disturbances (flashbacks) in recent abstinent high-potency heavy smokers. Isr J Psychiatry Relat Sci (2011) 48:25–9.
62. Orsolini L, Tomasetti C, Valchera A, Vecchiotti R, Matarazzo I, Vellante F, et al. An update of safety of clinically used atypical antipsychotics. Expert Opin Drug Saf (2016) 15(10):1329–47. doi:10.1080/14740338.2016.1201475
63. Abraham HD. Hallucinogen related disorders. 7th ed. In: Kaplan H, Sadock B, editors. Comprehensive Textbook of Psychiatry. Philadelphia: Lippincott Williams & Wilkins (2000).
64. Leweke FM, Schneider U, Radwan M, Schmidt E, Emrich HM. Different effects of nabilone and cannabidiol on binocular depth inversion in man. Pharmacol Biochem Behav (2000) 66:175–81. doi:10.1016/S0091-3057(00)00201-X
Keywords: hallucinogen-persisting perception disorder, novel psychoactive substances, hallucinogens, hallucinations, flashbacks, palinopsia
Citation: Orsolini L, Papanti GD, De Berardis D, Guirguis A, Corkery JM and Schifano F (2017) The “Endless Trip” among the NPS Users: Psychopathology and Psychopharmacology in the Hallucinogen-Persisting Perception Disorder. A Systematic Review. Front. Psychiatry 8:240. doi: 10.3389/fpsyt.2017.00240
Received: 14 September 2017; Accepted: 03 November 2017;
Published: 20 November 2017
Edited by:
Liana Fattore, Consiglio Nazionale Delle Ricerche (CNR), ItalyReviewed by:
Oussama KEBIR, Institut national de la santé et de la recherche médicale, FranceMaurizio Coppola, Azienda Sanitaria Locale CN2, Italy
Copyright: © 2017 Orsolini, Papanti, De Berardis, Guirguis, Corkery and Schifano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Orsolini, laura.orsolini@hotmail.it
 Laura Orsolini
Laura Orsolini Gabriele Duccio Papanti1
Gabriele Duccio Papanti1 Domenico De Berardis
Domenico De Berardis Amira Guirguis
Amira Guirguis John Martin Corkery
John Martin Corkery Fabrizio Schifano
Fabrizio Schifano