- 1Department of Psychiatry, Chung-Ang University Hospital, Seoul, South Korea
- 2Industry Academic Cooperation Foundation, Chung-Ang University, Seoul, South Korea
Problematic Internet game play is often accompanied by major depressive disorder (MDD). Depression seems to be closely related to altered functional connectivity (FC) within (and between) the default mode network (DMN) and salience network. In addition, serotonergic neurotransmission may regulate the symptoms of depression, including impulsivity, potentially by modulating the DMN. We hypothesized that altered connectivity between the DMN and salience network could mediate an association between the 5HTTLPR genotype and impulsivity in patients with depression. A total of 54 participants with problematic Internet game play and MDD completed the research protocol. We genotyped for 5HTTLPR and assessed the DMN FC using resting-state functional magnetic resonance imaging. The severity of Internet game play, depressive symptoms, anxiety, attention and impulsivity, and behavioral inhibition and activation were assessed using the Young Internet Addiction Scale (YIAS), Beck Depressive Inventory, Beck Anxiety Inventory (BAI), Korean Attention Deficit Hyperactivity Disorder scale, and the Behavioral Inhibition and Activation Scales (BIS-BAS), respectively. The SS allele was associated with increased FC within the DMN, including the middle prefrontal cortex (MPFC) to the posterior cingulate cortex, and within the salience network, including the right supramarginal gyrus (SMG) to the right rostral prefrontal cortex (RPFC), right anterior insular (AInsular) to right SMG, anterior cingulate cortex (ACC) to left RPFC, and left AInsular to right RPFC, and between the DMN and salience network, including the MPFC to the ACC. In addition, the FC from the MPFC to ACC positively correlated with the BIS and YIAS scores in the SS allele group. The SS allele of 5HTTLPR might modulate the FC within and between the DMN and salience network, which may ultimately be a risk factor for impulsive Internet game play in patients with MDD.
Introduction
Several national studies have demonstrated the relationship between impulsive Internet game play and major depressive disorder (MDD) (1–3). The severity of Internet use was associated with the risk of major depression in a group of 3,449 Korean middle school students (2). In a Hong Kong community (aged 18–60 years), the severity of Internet gaming disorder (IGD) was moderately strong correlation with the severity of depressive symptoms (3). A cross-sectional study of an Australian teenage community demonstrated that excessive Internet game play was associated with depression, anxiety, and poor health status (1). In studies on the treatment of patients with MDD and IGD, an improvement in depressive symptoms was associated with a reduction in the severity of IGD (4, 5). A comparison between the effects of bupropion and escitalopram on impulsive Internet game play in patients with MDD reported that decreased depressive symptoms were associated with an improvement of IGD in both groups (4). Furthermore, 12 weeks of bupropion treatment of 50 Patients with MDD and IGD also improved the symptoms of depression and IGD (5).
Recent studies suggested that IGD is caused by system level alterations between networks, rather than by the functional deficit within isolated regions (4–6). Many functional brain studies have demonstrated that human cognitive processes are orchestrated by a set of coherent spatiotemporal Independent Component networks (topographically organized human brain areas) (7). Our previous two studies demonstrated that the DMN and salience network were frequently associated in patients with MDD and IGD (4, 5). Sixty patients with MDD and IGD showed a failure to suppress DMN during an attentionally demanding task (the Wisconsin card sorting test) (5). In addition, decreased functional connectivity (FC) between the salience network and the DMN was associated with improved IGD symptoms and impulsivity in patients with MDD and IGD after 12 weeks of bupropion treatment (4). The FC in patients with MDD is thought to decrease between anterior DMN and posterior DMN, and increase between the salience network and anterior DMN (8). The salience network, which consists of the frontoinsular cortex, anterior cingulate, amygdala, and temporal pole, has been implicated in switching between the DMN and executive network (9). Grodin et al. reported that decreased volume within the salience network, including in the anterior insular (AInsular) and anterior cingulate, was negatively correlated with self-report impulsivity, decisional impulsivity, and compulsive measures (10).
Several neuroimaging studies have suggested that the serotonin transporter polymorphic region (5HTTLPR) plays an important neuromodulatory role on the DMN. In a positron emission tomography study, Hahn et al. (11) demonstrated that the density of the serotonin 1A (5-HT1A) receptor was associated with the FC within DMN. David et al. (12) suggested that the 5-HT1A receptor density could be modulated by genetic variants of 5HTTLPR. In a study of the effects of 5HTTLPR variants on impulsivity in patients with MDD, Cha et al. suggested that the short allele of 5HTTLPR increases impulsivity by decreasing the FC between the DMN and superior frontal gyrus (SFG) (13). The 5HTTLPR in SLC6A4 was reported to lower the transcription of the gene encoding serotonin (14). Due to the regulation of serotonergic neurotransmission, the short allele of 5HTTLPR is thought to play a role in the symptoms including depressive mood, impulsivity, and neuroticism of patients with MDD (15). Furthermore, escitalopram and venlafaxine was reportedly less effective on patients with MDD, who had the short allele of 5HTTLPR, than on those with the long allele. In a study of venlafaxine treatment study, short allele of 5HTTLPR was less effective than long allele of 5HTTLPR in patients with MDD (16) In our previous study, Internet use in 166 high school students was more excessive in students who were homozygous for the short allelic variant of the serotonin transporter gene (ss-5HTTLPR), compared to healthy participants (17).
Therefore, we hypothesized that the 5HTLPR short allele was associated with increased FC within the DMN and salience network, as well as increased FC between the two networks, which may lead to impulsive Internet game play in patients with MDD.
Materials and Methods
Participants
A total of 60 patients with problematic Internet game play and MDD agreed to participate in the current research. All patients were diagnosed as MDD based on the Diagnostic Statistical Manual of Mental Disorder-V (DSM-V) (18). The criterion used to define IGD in the present study was the same as that used in our prior study (19). The criteria was as follows: (1) Internet game play time more than 4 h per day or 30 h per week, (2) Young Internet Addiction Scale (YIAS) score >50, (3) irritable, anxious, and aggressive behaviors upon request to stop Internet game play, (4) impaired behavior or distress, economic problems, and maladaptive life pattern as a result of problematic Internet game play, (5) disruptive diurnal rhythms (difficulty waking up during daytime hours due to reduced sleep at night related to Internet game play), and (6) loss of job or school truancy. The inclusion criteria were as follows: (1) diagnosed as MDD, (2) problematic Internet game play, (3) drug-naive, (4) over 18 years old, and (5) right-handed. The exclusion criteria included the following: (1) history or current episode of other psychiatric disorders, (2) IQ <80, (3) substance abuse history (except for alcohol and tobacco), (4) neurological or medical disorder, and (5) contraindication for magnetic resonance imaging (MRI) scanning. Of the 60 patients with MDD and IGD, 2 patients had low IQ (<80), 3 patients had a history of psychiatric medication, and 1 patient had a history of bipolar disorder. A total of 54 patients with MDD and IGD completed the final research protocol. The research protocol was approved by the Institutional Review Board of Chung Ang University Hospital. Written informed consent was provided by patients.
Clinical Scale
The severity of Internet game play was assessed using the YIAS. The YIAS is a self-reporting scale for the severity of Internet use, with an internal consistency ranging from 0.90 to 0.91 (20). Depressive symptoms and anxiety were assessed using the Beck Depressive Inventory (BDI), with an internal consistency from 0.75 to 0.85 (21), and the Beck Anxiety Inventory (BAI), with Cronbach’s α = 0.93 (22), respectively. Attention and impulsiveness were assessed with the Korean Attention Deficit Hyperactivity Disorder scale (K-ARS) and Behavioral Inhibition and Activation Scales (BIS-BAS), respectively, which had an internal consistency from 0.77 to 0.89 (23) and 0.78 to 0.79 (24), respectively.
MRI Acquisition and Preprocessing
Resting-state brain activity was assessed using 3T blood-oxygen-level dependent functional MRI (Philips Achieva 3.0 Tesla TX MRI scanner, TR = 3 s, 12-min scan, 240 volumes, 128 × 128 matrix, 40 slices at a 4.0-mm slice thickness). Preprocessing included despiking (AFNI: 3dDespike), motion correction (SPM 12b), coregistration to Magnetization Prepared RApid Gradient Echo image (SPM 12b), normalization, smoothing, temporal detrend (Matlab: detrend.m), bandpass filtering (Matlab: idealfilter.m), and voxelwise regression of identically bandpass filtered time series of six head motion parameters (realignment steps with six rigid-body parameters characterizing the estimated subject motion for each subject), degraded CSF, degraded white matter, and facial soft tissues (MATLAB), as previously described (25–27). All images were spatially normalized to the standard Montreal Neurological Institute space (SPM 12b), spatially smoothed with a 4-mm FWHM 3D Gaussian kernel to reduce spatial noise, linearly de-trended, and temporally filtered with a bandwidth of 0.01–0.08 Hz to reduce the effects of low-frequency drift and high-frequency noise, respectively. To address the possibility of micro-head movements affecting connectivity results (28, 29), time points with head motion >0.2 mm were censored, but no regression of the global signal was performed (30, 31). To assess the susceptibility of head motion, independent t-tests were performed to ensure that groups did not differ on rotation or translation parameter [translation: SS group = 0.039 ± 0.018, SL + LL group = 0.042 ± 0.016, p = 0.172; rotation: SS group = 0.0007 ± 0.00004, SL + LL group = 0.0008 ± 0.00003, p = 0.165]; average frame wise displacements were included as co-variates. The initial volumes (240 volumes) of each participant were used in the current study.
In group independent component analysis of the 54 participants in the current research, five brain circuits including the DMN, salience network, visual, dorsal attention network (DAT), and cerebellar network were best matched. Of the five regions, we selected two networks (DMN and salience). We extracted 11 regions of two brain networks [4 DMNs: middle prefrontal cortex (MPFC), right/left lateral parietal cortex, and posterior cingulate cortex (PCC); 7 salience networks: right/left AInsular, right/left supramarginal gyrus (SMG), right/left rostral prefrontal cortex (RPFC), and anterior cingulate cortex (ACC)] from rois toolbox folder (ver.15; www.Nitrc.org/projects/conn/rois). Fisher-transformed correlation coefficients were measured for each pair of regions of interest (ROI) in each participant. The FC was calculated between ROIs using the CONN-fMRI FC toolbox (ver.15; www.Nitrc.org/projects/conn). Between-group effects were considered significant with a cluster level false discovery rate (FDR; q < 0.05), considering the multiple comparison correction of 55 pairs of 11 regions.
Genotyping
Genotyping was performed at Labgenomics, Korea. Genomic DNA was extracted from blood (stored frozen) using a G-DEX™ II Genomic DNA Extraction Kit (Intron Biotechnology, Korea), according to the manufacturer’s protocol. The region encompassing 5HTTLPR polymorphisms was amplified with the primers FORWARD: 5′-GGCGTTGCCGCTCTGAATGC-3′ and REVERSE: 5′- GAGGGGACTGAGCTGGACAACCAC-3′ via a polymerase chain reaction in 2.5 mM 7-deaza dNTP mix (Roche, Germany). Amplicons were resolved on a 2% agarose gel (Solgent, Korea) and visualized under a UV transilluminator. Herein the 528- and 484-bp bands will be called the L and S alleles of 5HTTLPR, respectively.
Statistical Analyses
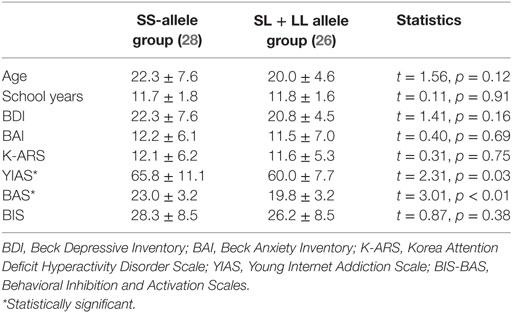
An independent t-test was performed to compare the mean differences of age, school years, BDI, BAI, K-ARS, YIAS, BAS, and BIS scores between the SS allele group and SL + LL allele group. Controlling for age, an ANCOVA was applied to measure differences in FC between the SS allele and SL + LL allele groups. Controlling for age, YIAS score, and BAS score, partial correlation was performed to assess the association between clinical scales, as well as between clinical scales and brain connectivity.
Results
Demographic and Clinical Characteristics
There were no significant differences in demographic data between SS allele group and SL + LL allele group, but impulsivity and severity of IGD were higher in SS allele group than those observed in SL + LL allele group. The sample in the current study consisted of 54 patients with MDD (all men) with a mean age of 21.7 ± 3.6 years (range: 18–28 years). The distribution of the current sample was as follows: SS allele (n = 28), SL allele (n = 21), and LL allele (n = 5). The current sample satisfied the Hardy–Weinberg equilibrium (χ2 = 0.13, df = 1, p = 0.71). There were no significant differences in age, school years, BDI, BAI, K-ARS, and BIS scores between the SS allele group and SL + LL allele group. However, the SS allele group had higher YIAS and BAS scores than SL + LL allele group (Table 1).
Comparing Brain FC of the DMN and Salience Network Between the SS and SL + LL Allele Groups
The FC within DMN and salience network, and between the networks, in the SS allele group (serotonin deficit) was higher than that in the SL + LL allele group. Compared to the SL + LL allele group, the SS allele group had greater FC within the DMN, including MPFC to PCC (t = 2.42, p = 0.02), and the salience network, including the right SMG to right RPFC (t = 2.02, p < 0.05), right AInsular to right SMG (t = 2.40, p = 0.02), ACC to left RPFC (t = 2.15, p = 0.04), and left AInsular to right RPFC (t = 2.42, p = 0.02), and between the DMN and salience network, including the MPFC to ACC (t = 2.61, p = 0.01) (Figure 1).
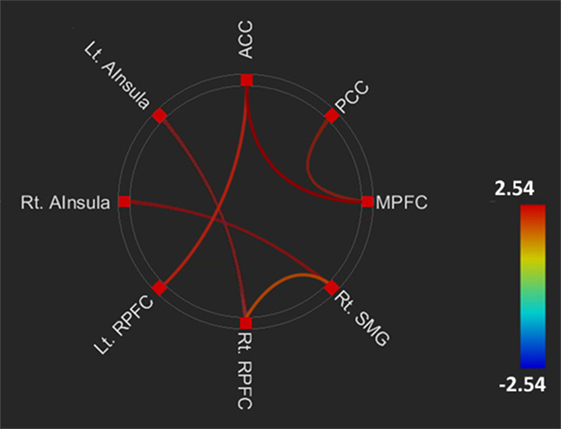
Figure 1. Comparing brain functional connectivity of brain areas between the SS and SL + LL allele groups. Default mode network.middle prefrontal cortex (DMN.MPFC) to posterior cingulate cortex (DMN.PCC): t = 2.42, FDRq = 0.02; left salience network.anterior insular (SAL.AInsula) to right rostral prefrontal cortex (SAL.RPFC): t = 2.42, FDRq = 0.02; right SAL.AInsula (SAL.AInsula) to right SAL supramarginal gyrus (SAL.SMG): t = 2.40, FDRq = 0.02; right SAL.SMG to right SAL.RPFC: t = 2.02, FDRq < 0.05; SAL.ACC to left SAL.RPFC: t = 2.15, p = 0.04; DMN.MPFC to anterior cingulate cortex (SAL.ACC): t = 2.61, FDRq = 0.01.
Correlation Between Clinical Scales and Brain Connectivity
Impulsivity and severity of IGD were associated with the FC between DMN and salience network in patients with MDD and IGD in the SS allele group alone.
In all patients with MDD and IGD, there was positive correlation between the BAS score and YIAS score (r = 0.63, p < 0.01). The BDI scores had no significant correlations with YIAS scores (r = 0.03, p = 0.79) and BAS scores (r = 0.07, p = 0.61). The YIAS scores (r = 0.58, p < 0.01) and BAS scores (r = 0.42, p < 0.01) were positively correlated with FC from the ACC to MPFC. Furthermore, the BDI score was positively correlated with the FC from the PCC to the MPFC (r = 0.71 p < 0.01) (Figure 2). The BDI scores were not correlated with the FC from the ACC to the MPFC (r = 0.11, p = 0.49). The YIAS scores (r = 0.28, p = 0.06) and BAS scores (r = 0.25, p = 0.08) were not correlated with the FC from the PCC to the MPFC.
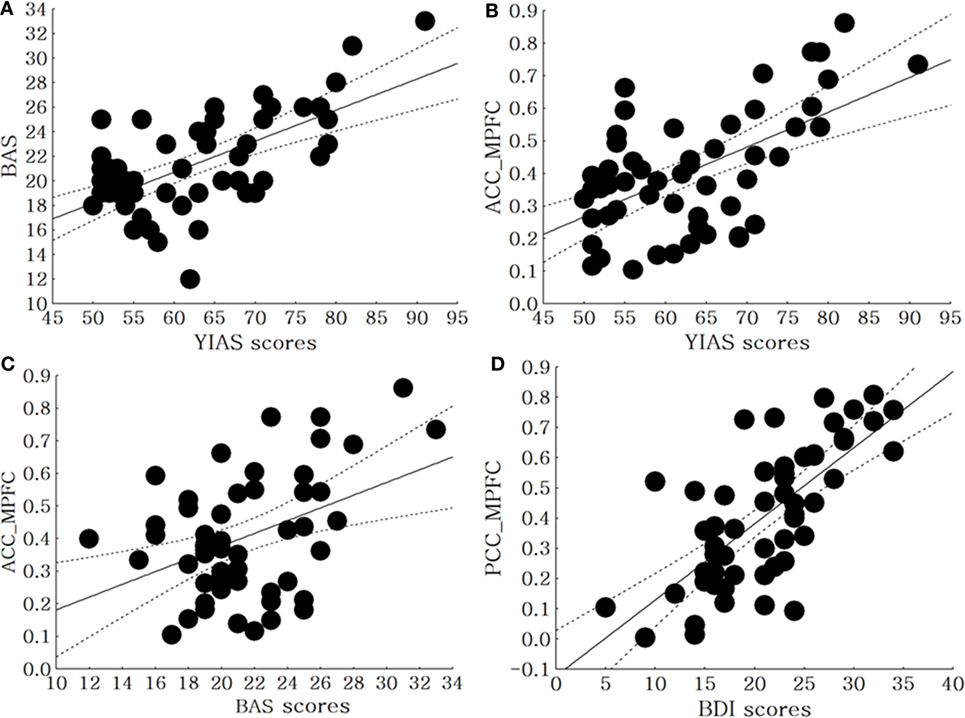
Figure 2. Correlation between clinical scales and brain connectivity in all patients with major depressive disorder (MDD) and Internet gaming disorder (IGD). (A) Correlation between the BAS scores and the Young Internet Addiction Scale (YIAS) scores in all patients with MDD with IGD (r = 0.63, p < 0.01), (B) correlation between the YIAS scores and FC between anterior cingulate cortex (ACC) to middle prefrontal cortex (MPFC) in all Patients with MDD with IGD (r = 0.58, p < 0.01), (C) correlation between the BAS scores and FC between ACC to MPFC in all patients with MDD with IGD (r = 0.42, p < 0.01), (D) correlation between the Beck Depressive Inventory (BDI) scores and FC between PCC to MPFC in all patients with MDD with IGD (r = 0.51, p < 0.01).
In the SS allele group, there was a positive correlation between the BAS score and YIAS score (r = 0.68, p < 0.01). Even after controlling for each of the two measures, the BAS score (r = 0.48, p = 0.01) and YIAS score (r = 0.64, p < 0.01) were positively correlated with the FC from the ACC to the MPFC (Figure 3). In the SL + LL allele group, there was no correlation between BAS score and YIAS score (r = 0.34, p = 0.09). After controlling for each of the two measures, the BAS scores (r = 0.21, p = 0.32) and YIAS score (r = 0.29, p = 0.06) were not correlated with the FC from the ACC to the MPFC.
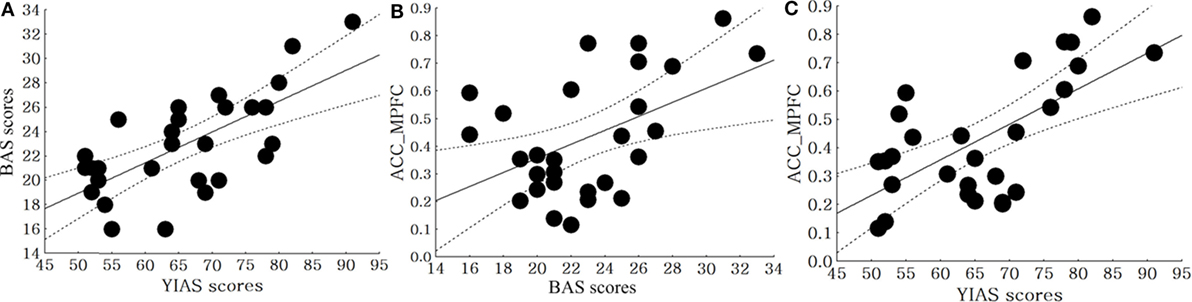
Figure 3. Correlation between clinical scales and brain connectivity in the SS allele and SL + LL allele groups. (A) Correlation between the BAS and Young Internet Addiction Scale (YIAS) scores in the SS allele group (r = 0.68, p < 0.01), (B) correlation between the BIS scores and FC from anterior cingulate cortex (ACC) to middle prefrontal cortex (MPFC) in SS allele group (r = 0.48, p = 0.01), (C) correlation between the YIAS scores and FC from ACC to MPFC in SS allele group (r = 0.64, p < 0.01).
Discussion
Comparison of Clinical Characteristics Between the SS and SL + LL Allele Groups
In the current study, there were no significant differences in depressive symptoms between the SS and SL + LL allele groups. The effects of the 5HTTLPR polymorphism on depressive symptoms in patients with MDD are controversial. In pharmacological studies, the S allele is thought to predict a bad response to medication in diseases associated with serotonergic system dysfunction (32, 33). A meta-analysis demonstrated that the 5HTTLPR polymorphism did not play a major role in predicting the progress of mood symptoms in Asian patients with MDD (34).
However, we found that the SS allele group had higher YIAS and BAS scores than the SL + LL allele group. In our previous study of 166 adolescent, adolescents with excessive Internet use had higher frequencies of the SS allele of 5HTTLPR and harm avoidance, compared to those in the SL + LL allele group (17). In addition, there was a positive correlation between BAS scores and YIAS scores. An association between impulsiveness and the 5HTTLP polymorphism has also been reported in other studies. When the effect of fluoxetine on reducing impulsiveness and irritability was assessed using the Modified Overt Aggression Scale, patients with L carrier (SL + LL alleles) borderline personality disorder showed a better response than S carriers (35). Taken together, we cautiously suggest that short allele of 5HTTLPR is associated with impulsive Internet game play in patients with MDD and IGD.
Comparing Brain Functional Connectivity of the DMN and Salience Network Between the SS and SL + LL Allele Groups
All patients with MDD and IGD in the present study showed a positive correlation between the BDI scores and FC within the DMN. Mulders et al. reported the increase in the FC within DMN and within salience network in patients with MDD, compared to healthy control participants (8). In addition, the FC between DMN and salience network was higher in patients with MDD than in healthy participants (8). Moreover, IGD symptoms in patients with MDD were reportedly associated with increased FC between the DMN and salience network (4, 5).
Considering the increased FC within (and between) the DMN and salience network in the SS allele group, our genetic neuroimaging findings suggest that the serotonergic system may play a role in impulsive Internet game play in patients with MDD. Previous reports already demonstrated that the deficit of serotonin neurotransmission in the DMN and salience network were associated with the severity of mood symptoms, chemical addictive symptoms, and impulsive Internet gaming symptoms (13, 36). Patients with MDD, who have the S allele, show microstructural white matter abnormalities within the frontolimbic networks and a lower remission rate, compared to patients with MDD who have the LL allele (36). Furthermore, Cha et al. reported that the S allele genotypes of 5HTTLPR (SS and SL) were associated with lower FC between the posterior DMN and SFG (13). In that study, path modeling analysis demonstrated that increased FC between the DMN and SFG would mediate impulsivity in patients with MDD (13). Increased FC within the DMN and salience network was also found in codeine-dependent patients; the FC within those areas was associated with impulsivity (37). Increased FC between the DMN and salience network was also reported in patients with IGD who had a childhood history of ADHD (38).
In our results, the SS allele group showed greater FC within the DMN and salience network, and between these networks, compared to the SL + LL allele group. In addition, BAS scores and YIAS scores were positively correlated with the FC between the DMN and salience network in SS allele group alone. Taken together, our results suggest that the short allele of 5HTTLPR may increase FC within the DMN and salience network, which may subsequently aggravate impulsive Internet game play in patients with MDD.
Limitations
A couple of limitations in the current study must be noted. First, the relatively small number of participants prevented the generalization of the current results. Second, there were no neurocognitive tests for assessing the function of the DMN or salience network. Thus, future studies should consider assessing a larger cohort of participants and applying a neurocognitive test.
Conclusion
The current results suggested that the SS allele of 5HTTLPR can be a risk factor for impulsiveness and excessive Internet game play in patients with MDD and IGD. In addition, the SS allele of 5HTTLPR may modulate FC, not only within the DMN and salience network but also between the networks.
Ethics Statement
The research protocol was approved by the Institutional Review Board of Chung Ang University Hospital. The study was conducted in accordance with the ethical standards of the Helsinki Declaration of 1964 and subsequent amendments or similar ethical standards. Written informed consent was obtained from all participants.
Author Contributions
JH, SK, and DH contributed to patient recruitment, and data collection and processing. JH, SB, and DH analyzed the data. All authors participated to drawing up the manuscript and were involved in the intellectual workup for the article. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was supported by a grant from the Korean Creative Content Agency (R2014040055).
References
1. Mathers M, Canterford L, Olds T, Hesketh K, Ridley K, Wake M. Electronic media use and adolescent health and well-being: cross-sectional community study. Acad Pediatr (2009) 9:307–14. doi:10.1016/j.acap.2009.04.003
2. Park S. The association between Internet use and depressive symptoms among South Korean adolescents. J Spec Pediatr Nurs (2009) 14:230–8. doi:10.1111/j.1744-6155.2009.00191.x
3. Sigerson L, Li AY, Cheung MW, Luk JW, Cheng C. Psychometric properties of the Chinese Internet Gaming Disorder Scale. Addict Behav (2017) 74:20–6. doi:10.1016/j.addbeh.2017.05.031
4. Nam B, Bae S, Kim SM, Hong JS, Han DH. Comparing the effects of bupropion and escitalopram on excessive Internet game play in patients with major depressive disorder. Clin Psychopharmacol Neurosci (2017) 15:361–8. doi:10.9758/cpn.2017.15.4.361
5. Han DH, Kim SM, Bae S, Renshaw PF, Anderson JS. A failure of suppression within the default mode network in depressed adolescents with compulsive Internet game play. J Affect Disord (2016) 194:57–64. doi:10.1016/j.jad.2016.01.013
6. Jin CW, Zhang T, Cai CX, Bi YZ, Li YD, Yu DH, et al. Abnormal prefrontal cortex resting state functional connectivity and severity of Internet gaming disorder. Brain Imaging Behav (2016) 10:719–29. doi:10.1007/s11682-015-9439-8
7. Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A (2006) 103:13848–53. doi:10.1073/pnas.0601417103
8. Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev (2015) 56:330–44. doi:10.1016/j.neubiorev.2015.07.014
9. Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage (2014) 99:180–90. doi:10.1016/j.neuroimage.2014.05.052
10. Grodin EN, Cortes CR, Spagnolo PA, Momenan R. Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug Alcohol Depend (2017) 179:100–8. doi:10.1016/j.drugalcdep.2017.06.014
11. Hahn A, Wadsak W, Windischberger C, Baldinger P, Hoflich AS, Losak J, et al. Differential modulation of the default mode network via serotonin-1A receptors. Proc Natl Acad Sci U S A (2012) 109:2619–24. doi:10.1073/pnas.1117104109
12. David SP, Murthy NV, Rabiner EA, Munafo MR, Johnstone EC, Jacob R, et al. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J Neurosci (2005) 25:2586–90. doi:10.1523/JNEUROSCI.3769-04.2005
13. Cha J, Guffanti G, Gingrich J, Talati A, Wickramaratne P, Weissman M, et al. Effects of serotonin transporter gene variation on impulsivity mediated by default mode network: a family study of depression. Cereb Cortex (2017):1–11. doi:10.1093/cercor/bhx097
14. Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (1996) 274:1527–31. doi:10.1126/science.274.5292.1527
15. Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet (1999) 88:83–7. doi:10.1002/(SICI)1096-8628(19990205)88:1<83::AID-AJMG15>3.0.CO;2-0
16. Lee SH, Choi TK, Lee E, Seok JH, Lee SH, Lee HS, et al. Serotonin transporter gene polymorphism associated with short-term treatment response to venlafaxine. Neuropsychobiology (2010) 62:198–206. doi:10.1159/000319362
17. Lee YS, Han DH, Yang KC, Daniels MA, Na C, Kee BS, et al. Depression like characteristics of 5HTTLPR polymorphism and temperament in excessive Internet users. J Affect Disord (2008) 109:165–9. doi:10.1016/j.jad.2007.10.020
18. APA. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Association Publishing (2013).
19. Hyun KJ, Han DH, Lee YS, Kang KD, Yoo SK, Chung U, et al. Risk factors associated with online game addiction: a hierarchical model. Comput Human Behav (2015) 48:706–13. doi:10.1016/j.chb.2015.02.008
20. Kim JW, Kim SY, Choi JW, Kim KM, Nam SH, Min KJ, et al. Differences in resting-state quantitative electroencephalography patterns in attention deficit/hyperactivity disorder with or without comorbid symptoms. Clin Psychopharmacol Neurosci (2017) 15:138–45. doi:10.9758/cpn.2017.15.2.138
21. Uh D, Jeong HG, Choi KY, Oh SY, Lee S, Kim SH, et al. Dehydroepiandrosterone sulfate level varies nonlinearly with symptom severity in major depressive disorder. Clin Psychopharmacol Neurosci (2017) 15:163–9. doi:10.9758/cpn.2017.15.2.163
22. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol (1988) 56:893–7. doi:10.1037/0022-006X.56.6.893
23. So YK, Noh JS, Kim YS, Ko SG, Koh YJ. The reliability and validity of Korean Parent and Teacher ADHD Rating Scale. J Korean Neuropsychiatr Assoc (2002) 41:283–9.
25. Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp (2011) 32:919–34. doi:10.1002/hbm.21079
26. Anderson JS, Nielsen JA, Ferguson MA, Burback MC, Cox ET, Dai L, et al. Abnormal brain synchrony in Down syndrome. Neuroimage Clin (2013) 2:703–15. doi:10.1016/j.nicl.2013.05.006
27. Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, et al. Functional connectivity magnetic resonance imaging classification of autism. Brain (2011) 134:3742–54. doi:10.1093/brain/awr263
28. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage (2012) 59:2142–54. doi:10.1016/j.neuroimage.2011.10.018
29. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage (2012) 59:431–8. doi:10.1016/j.neuroimage.2011.07.044
30. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage (2009) 44:893–905. doi:10.1016/j.neuroimage.2008.09.036
31. Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect (2012) 2:25–32. doi:10.1089/brain.2012.0080
32. Andre K, Kampman O, Illi A, Viikki M, Setala-Soikkeli E, Mononen N, et al. SERT and NET polymorphisms, temperament and antidepressant response. Nord J Psychiatry (2015) 69:531–8. doi:10.3109/08039488.2015.1012554
33. Huezo-Diaz P, Uher R, Smith R, Rietschel M, Henigsberg N, Marusic A, et al. Moderation of antidepressant response by the serotonin transporter gene. Br J Psychiatry (2009) 195:30–8. doi:10.1192/bjp.bp.108.062521
34. Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol (2012) 22:239–58. doi:10.1016/j.euroneuro.2011.10.003
35. Silva H, Iturra P, Solari A, Villarroel J, Jerez S, Vielma W, et al. Serotonin transporter polymorphism and fluoxetine effect on impulsiveness and aggression in borderline personality disorder. Actas Esp Psiquiatr (2007) 35:387–92.
36. Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Glatt CE, Latoussakis V, Kelly RE Jr, et al. Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord (2009) 119:132–41. doi:10.1016/j.jad.2009.03.004
37. Qiu YW, Su HH, Lv XF, Ma XF, Jiang GH, Tian JZ. Intrinsic brain network abnormalities in codeine-containing cough syrup-dependent male individuals revealed in resting-state fMRI. J Magn Reson Imaging (2017) 45:177–86. doi:10.1002/jmri.25352
Keywords: serotonin transporter gene, Internet gaming disorder, default mode network, salience network, major depressive disorder
Citation: Hong JS, Kim SM, Bae S and Han DH (2018) Impulsive Internet Game Play Is Associated With Increased Functional Connectivity Between the Default Mode and Salience Networks in Depressed Patients With Short Allele of Serotonin Transporter Gene. Front. Psychiatry 9:125. doi: 10.3389/fpsyt.2018.00125
Received: 05 January 2018; Accepted: 26 March 2018;
Published: 10 April 2018
Edited by:
Jung-Seok Choi, SMG-SNU Boramae Medical Center, South KoreaReviewed by:
Aviv M. Weinstein, Ariel University, IsraelHeejung Kim, Seoul National University, South Korea
Young-Chul Jung, Yonsei University, South Korea
Copyright: © 2018 Hong, Kim, Bae and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doug Hyun Han, hduk@yahoo.com
 Ji Sun Hong1
Ji Sun Hong1 Sujin Bae
Sujin Bae Doug Hyun Han
Doug Hyun Han