- 1Department of Psychiatry, Seoul National University Hospital, Seoul, South Korea
- 2Department of Medicine, Seoul National University College of Medicine, Seoul, South Korea
- 3Department of Neurology, Mayo Clinic, Rochester, MN, United States
- 4Department of Neurosurgery, Mayo Clinic, Rochester, MN, United States
- 5Department of Psychiatry, Institute of Human Behavioral Medicine in SNU-MRC, Seoul National University College of Medicine, Seoul, South Korea
Background: The amygdala plays a key role in emotional hyperreactivity in response to social threat in patients with social anxiety disorder (SAD). We investigated resting-state functional connectivity (rs-FCN) of the left and right amygdala with various brain regions and functional lateralization in patients with SAD.
Methods: A total of 36 patients with SAD and 42 matched healthy controls underwent functional magnetic resonance imaging (fMRI) at rest. Using the left and right amygdala as seed regions, we compared the strength of the rs-FCN in the patient and control groups. Furthermore, we investigated group differences in the hemispheric asymmetry of the functional connectivity maps of the left and right amygdala.
Results: Compared with healthy controls, the rs-FCN between the left amygdala and the dorsolateral prefrontal cortex was reduced in patients with SAD, whereas left amygdala connectivity with the fusiform gyrus, anterior insula, supramarginal gyrus, and precuneus was increased or positively deflected in the patient group. Additionally, the strength rs-FCN between the left amygdala and anterior insula was positively associated with the severity of the fear of negative evaluation in patients with SAD (r = 0.338, p = 0.044). The rs-FCN between the right amygdala and medial frontal gyrus was decreased in patients with SAD compared with healthy controls, whereas connectivity with the parahippocampal gyrus was greater in the patient group than in the control group. The hemispheric asymmetry patterns in the anterior insula, intraparietal sulcus (IPS), and inferior frontal gyrus of the patient group were opposite those of the control group, and functional lateralization of the connectivity between the amygdala and the IPS was associated with the severity of social anxiety symptoms (r = 0.365, p = 0.037).
Conclusion: Our findings suggest that in addition to impaired fronto-amygdala communication, the functional lateralization of amygdala function plays a central role in the pathophysiology of SAD.
Introduction
Social anxiety disorder (SAD) is characterized by abnormal fear in one or more social situations causing considerable distress, extensive disability, and reduced quality of life (1). Functional neuroimaging studies have demonstrated increased activity in the amygdala and insula of patients with SAD (2). The amygdala plays a central role in emotion recognition, which is essential for social interaction and communication (3). Amygdala damage is associated with impaired recognition of social emotions (4) and impairs eye contact during conversations (5). Previous studies have shown that cortical hubs are disrupted in the functional brain networks of patients with SAD (6) and the prefrontal networks of the amygdala and limbic system are altered (7–9). Amygdala dysfunction is thought to underlie the pathogenesis of SAD and cognitive-behavioral therapy has been shown to down-regulate the abnormally high connectivity of the prefrontal-amygdala network (10).
The left and right amygdala seem to have distinct roles in the emotion regulation process, such that the mediofrontal cortical functional connectivities of the left and right amygdala are differentially modulated by harm avoidance and can be a vulnerability marker for sensitivity to stress and pathologic anxiety (11). Furthermore, the left amygdala is reported to be activated more often than the right amygdala in emotional processing, regardless of stimulus type, task instructions, habituation rates, or elaborate processing (12). Wright et al. (13) found that the right amygdala showed greater habituation to emotional stimuli than the left; however, the left amygdala was significantly more activated than the right to the contrast of fear vs. happy emotions, suggesting that the right amygdala is “part of a dynamic emotional stimulus detection system,” whereas the left is specialized for “sustained stimulus evaluation.” However, little is known about the functional asymmetry of the right and left amygdala in SAD.
The hemispheric laterality of the brain function is beneficial to certain cognitive function (14, 15). Moreover, hemispheric differences in amygdala structure and function have shown clinical implications on fear processing (16–18). With such a fact, it may be demonstrated that bilateral asymmetry in the functional network of the left and right amygdala contributes to the pathogenesis of SAD. We investigated the resting-state functional connectivity (rs-FCN) of the left and right amygdala and hemispheric asymmetry in patients with SAD.
Materials and Methods
Participants and Measurements
Participants included 36 patients with SAD and 42 healthy controls recruited from the psychiatric outpatient clinic at Seoul National University Hospital and the community through an advertisement. Participants were screened using self-report questionnaires, including the Liebowitz Social Anxiety Scale (LSAS), a 24-item measure of fear and avoidance experienced in a range of social interaction and performance situations (19); the Social Interaction Anxiety Scale (SIAS), a 20-item questionnaire that measures the level of anxiety in interpersonal interactions (20); the Social Phobia Scale (SPS), which consists of 20 items that measure performance anxiety (20), the brief version of the Fear of Negative Evaluation scale (B-FNE), a 12-item scale measuring the apprehension of negative evaluation by others (21); and the Beck Depression Inventory (BDI), a 21-item measure of depressed mood (22). The inclusion scores were LSAS ≥ 30, SIAS ≥ 34, and/or SPS ≥ 24 for patients, and SIAS < 34, SPS < 24, B-FNE < 48, and BDI < 21 for control subjects, and ≥12 years of education for both groups. A cutoff score of 21 on the BDI was proposed for Korean population (23, 24). The exclusion criteria for both groups included any history of medical (such as cardiac, respiratory, and hematologic diseases), neurological (such as head trauma, seizure, brain tumor, and stroke), or psychiatric illness (other than SAD and related depressive disorder; such as severe suicidal ideation, substance abuse/dependence, major depressive and bipolar disorders, psychosis, and claustrophobia). Patients were diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (25) through a clinical interview with a psychiatrist (Choi SH). General anxiety symptoms were assessed using the Hamilton Anxiety Scale (HAS), a 14-item measure of psychic and physical anxiety (26). Four patients with SAD also diagnosed with comorbid depressive disorders. Nine patients were taking routine medications mainly with serotonergic antidepressants. Two of the patients were prescribed benzodiazepines and beta-blocker for as-needed medication; however, they did not take on the day of scanning.
Functional Magnetic Resonance Imaging Acquisition and Functional Localizer Run
Functional magnetic resonance imaging (fMRI) was performed on a 3.0-Tesla MR scanner (Magnetom TrioTrim; Siemens Medical Solutions, Erlangen, Germany). Whole-brain echo-planar images (EPI) were acquired (TR = 2,000 ms; TE = 30 ms; flip angle = 80°; 34 slices; 3.4 mm isotropic voxel resolution). Resting-state fMRI was acquired for 5 min. A high-resolution T1-weighted anatomical image was also acquired (flip angle = 9°; 208 slices; 1.0 mm isotropic voxel size).
EPI time series were acquired during a functional localizer run to determine the seed regions in the left and right amygdala. Participants were asked to look at the screen during the sequence of three conditions: a square checkerboard pattern, an angry face, and a neutral face. Each condition appeared for 10 s, with 10 s of fixation between conditions. The sequence was repeated eight times.
fMRI Data Preprocessing
Anatomical T1 images were coregistered to the first functional image using a local Pearson correlation cost function (27). Preprocessing of imaging data was performed using Analysis of Functional NeuroImages (AFNI; http://afni.nimh.nih.gov/afni) software (28). The first three volumes from each functional image were removed. We used Physiologic EStimation by Temporal ICA (PESTICA; http://www.nitrc.org/projects/pestica/) to determine and correct for physiological cardiac and respiratory noise in the resting-state fMRI data (29). After removing physiological noise using PESTICA, images were despiked, and then corrected for slice-time acquisition differences and head motion (30). The slice-timing and motion-corrected functional images were acquired using an anatomy-based image correction method known as ANATICOR (31). Hardware artifacts were modeled with one regressor for eroded local white matter signals and one averaged signal from eroded lateral ventricle masks. The regressors of no interest were (1) six parameters obtained by correction of head motion, (2) signal from the eroded large ventricle mask, and (3) signal from a region of the local white matter erosion mask (r = 15 mm). The residual time series were smoothed with a heat kernel that resulted in 4-mm full width-at-half-maximum (FWHM) resolution. At the ANATICOR stage, we performed motion censoring for head motion artifacts (32) using estimated translational and rotational displacement with respect to the x, y, and z axes. The threshold set was an estimated displacement of <0.3 mm for the Euclidean L2 norm of motion displacement between successive time series volumes. Head-coil artifacts were checked visually by observing the contribution of local white matter signals in the time series, and no strong artifact was detected across brain tissue types (31). Following these quality control steps, data from all subjects met our criteria and were included in this study (32, 33). All imaging data were then transformed to the N27 template space (34).
Functional Connectivity of the Amygdala Seed Locations and Hemispheric Asymmetry in the Functional Connectivity Maps of the Left and Right Amygdala
Using the amygdala anatomical masks from the FreeSurfer parcellation of the N27 brain template (35), we identified seed regions in the left [Talairach coordinates: (x = −17 mm, y = −7 mm, z = −8 mm)] and right [(19, −5, −10)] amygdala from the contrast map of the angry face vs. the square checkerboard pattern during the localizer run of the control group. For the functional connectivity map of the left amygdala in a single subject, the time series from each voxel within a given seed region of the left amygdala were averaged, a Pearson correlation coefficient was calculated between the averaged time series of the left amygdala and the time series of whole-brain vertices, and then the correlation coefficient was normalized using Fisher's transformation to enable statistical analysis. These procedures were applied to the data of all subjects. A two-sample t-test was used to compare the functional connectivity maps of the control and patient groups. The threshold of significance was set at family-wise error (FWE)-corrected p < 0.01 (36). The same procedures were used to compare the functional connectivity maps of the right amygdala in the patient and control groups.
To assess between-group differences in the hemispheric asymmetry of connectivity patterns across the left and right seed locations, we flipped the x-coordinate values of the individual right amygdala connectivity maps, subtracted those values from their contralateral pairs (left amygdala connectivity maps), and assessed differences using two-sample t-tests. The threshold of significance was set at FWE-corrected p < 0.01.
To exclude the effect of medication, the same analyses were carried out without nine patients taking medications.
Statistical Analyses
Two-sample t-tests and chi-squared tests were used to compare between-group differences in demographic and clinical characteristics. Pearson's correlation coefficients were calculated to assess the relationship between symptom severity and the strength of functional connectivity with the amygdala in patients with SAD. Pearson's correlation analyses were repeated without nine patients taking medications. In addition, we considered the potential influence of comorbid depressed mood by using partial correlation analyses with covariates of the BDI scores in the patient group. Hemispheric asymmetry of left and right amygdala rs-FCN was calculated as the difference in rs-FCN strength of the left vs. right amygdala. All statistical tests were two-tailed with a significance level of 0.05. Additionally, adjusted p-values for multiple correlations using a sequential Holm-Bonferroni procedure were considered.
Results
Participant Demographic and Clinical Characteristics
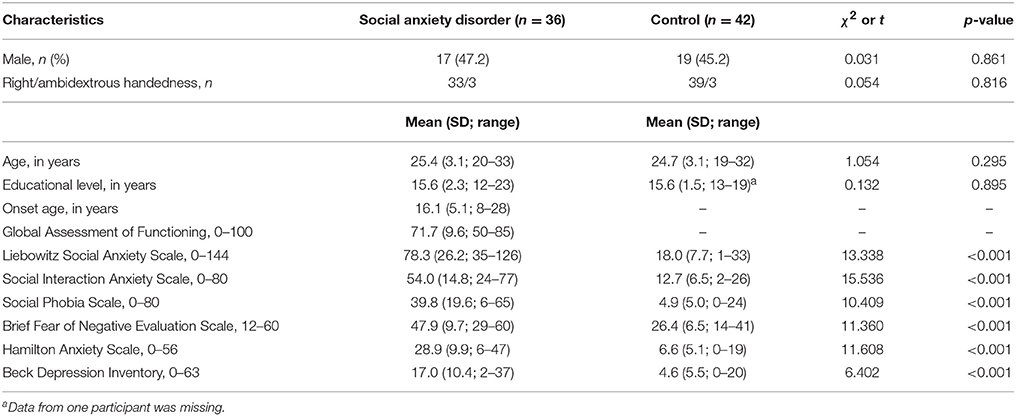
The demographic and clinical characteristics of the participants are shown in Table 1. Age, gender, and handedness were not significantly different between groups. The HAS, BDI, and social anxiety scores were higher in the patients than in the control group.
rs-FCN of the Left and Right Amygdala
Table 2 shows group differences in rs-FCNs of the left and right amygdala. The right dorsolateral prefrontal cortex was negatively connected with the left amygdala in both groups; however, the patient group showed less negative connectivity than the control group. In contrast, the left and right fusiform gyri were positively connected with the left amygdala in both groups; however, the rs-FCN was greater in the patients than in the control subjects. The rs-FCNs of the left amygdala with the right anterior insula, right supramarginal gyrus, left precuneus, and left cerebellum were positive in the patient group and negative in the control group.
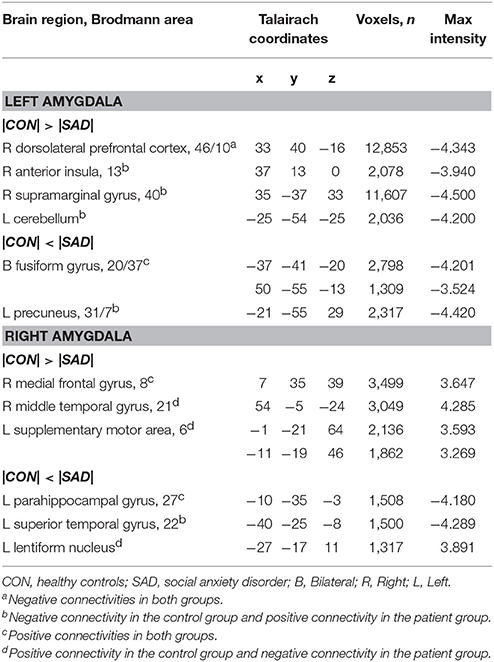
Table 2. Group differences in the resting-state functional connectivity of the left and right amygdala.
The middle frontal gyrus was positively connected with the right amygdala in both groups, although the strength of rs-FCN was reduced in the patient group compared with the control group. The right middle temporal gyrus, bilateral supplementary motor areas (SMA), and left lentiform nuclei were positively connected with the right amygdala in the control group, but negatively connected in the patient group. The positive connection between the right parahippocampal gyrus and right amygdala was greater in the patient group than in the control group. The left superior temporal gyrus showed negative connectivity with the right amygdala in the control group and positive connectivity in the patient group (Table 2).
The additional analyses for participants without medications revealed consistent results (Supplementary Table 1 in Supplementary Material).
Hemispheric Asymmetry of rs-FCN of the Left and Right Amygdala
We found several between-group differences in the hemispheric asymmetry of rs-FCN of the left and right amygdala (Figure 1). Left amygdala rs-FCNs with the right insula [(29, 21, 1), 16,194 voxels], right intraparietal sulcus [IPS, (32, −55, 42), 7,202 voxels], right inferior frontal gyrus [(54, 0, 23), 1,316 voxels], left occipitotemporal cortex [(−41, −65, 0), 4,138 voxels], and left SMA [(−13, −15, 62), 1,412 voxels] exhibited positive connections in the patient compared to negative connections in the control group. Furthermore, the rs-FCN patterns of the left and right amygdala with the insula, IPS, and inferior frontal gyrus in the patient group were opposite from those in the control group. The rs-FCNs of the left amygdala with the occipitotemporal cortex and SMA were dominant in the patient group, whereas the right amygdala was dominant in the control group. The results of additional analyses for participants without medications were presented in Supplementary Material (Supplementary Figure 1).
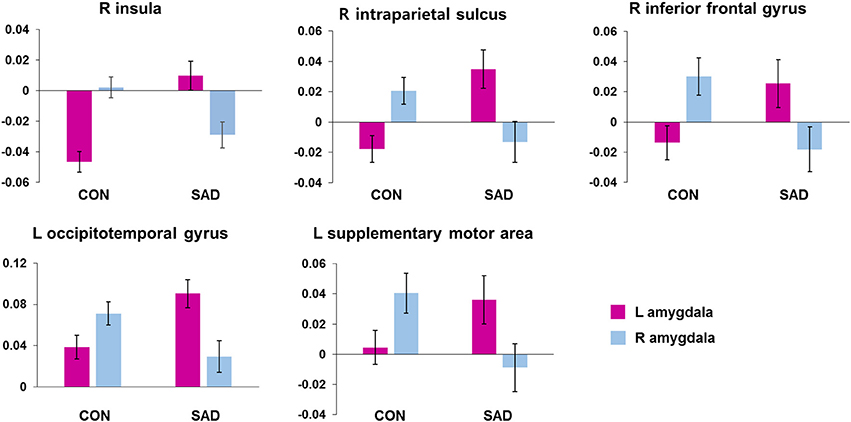
Figure 1. Brain regions showing between-group differences in hemispheric asymmetry in the resting-state functional connectivity of the left and right amygdala. The bar indicates mean ± standard error of z-scores of the functional connectivity in each cluster with the Left or Right amygdala. CON, healthy controls; SAD, social anxiety disorder; L, left; R, right.
Correlations Between Symptom Severity and rs-FCN Strength in the Left and Right Amygdala and Hemispheric Asymmetry in Patients With SAD
The correlation analyses revealed that the strength of the left amygdala connectivity with the right insula was positively correlated with the fear of negative evaluation (B-FNE score, r = 0.338, n = 36, p = 0.044; Figure 2A). The strengths of the rs-FCNs of the left amygdala with the supramarginal gyrus (r = 0.406, n = 36, p = 0.020) and precuneus (r = 0.416, n = 36, p = 0.017) were correlated with the level of general anxiety (HAS score). None of these correlations were significant after a sequential Holm-Bonferroni correction for multiple comparisons.
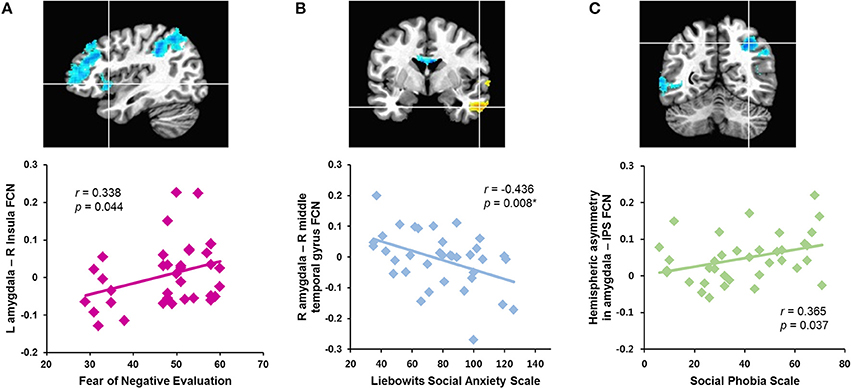
Figure 2. Correlations between symptom severity and rs-FCN strength of brain regions showing between-group differences in the functional connectivity with the left and right amygdala and hemispheric asymmetry in patients with social anxiety disorder. The right insula (A), right middle temporal gyrus (B), right intraparietal sulcus (C). *Significant finding after a sequential Holm-Bonferroni correction for multiple comparisons. L, left; R, right; FCN, functional connectivity; IPS, intraparietal sulcus.
The strength of functional connectivity between the right amygdala and middle temporal gyrus was inversely correlated with social anxiety symptom severity (LSAS scores, r = −0.436, n = 36, p = 0.008; SPS score, r = −0.439, n = 36, p = 0.007; Figure 2B). The strength of the rs-FCN between the right amygdala and superior temporal gyrus was positively correlated with level of depressed mood (BDI score, r = 0.488, n = 36, p = 0.003) and general anxiety (HAS score, r = 0.446, n = 36, p = 0.011). All of these correlations remained significant after a correction for multiple comparisons, except for the HAS.
Hemispheric asymmetry in the rs-FCN between the amygdala and the IPS was positively correlated with the social anxiety symptom severity (SPS score, r = 0.365, n = 36, p = 0.037; Figure 2C). This was not significant after a correction for multiple comparisons.
Most of above significant correlation findings were consistent in further analyses for participants without medications and the partial correlation with covariates of the BDI scores (Supplementary Material).
Discussion
Our findings indicate that communication between the cortical regions of the brain and the left and right amygdala is dysfunctional in patients with SAD, suggesting that the amygdala plays a distinct role in the pathophysiology of SAD. In particular, the positive functional connectivities of the left amygdala with the fusiform face area, insular limbic area, and components of the default mode network (DMN, supramarginal gyrus and precuneus) were greater in patients with SAD than in healthy controls. The insula, IPS, and inferior frontal gyrus showed different patterns of hemispheric asymmetry in rs-FCN of the left and right amygdala in patients with SAD compared with healthy controls. Additionally, our finding that the rs-FCNs between the left and right amygdala and frontal cortices, which play a key role in emotional regulation, were reduced in patients with SAD is consistent with previous studies (2, 37–39).
The fusiform gyrus, a hominoid-specific structure responsible for the ability to recognize faces, is critical for social interaction (40). Accordingly, the fusiform gyrus of patients with SAD has been shown to be pathologically reactive to socially threatening stimuli (41). Interestingly, the rs-FCN between the left amygdala and bilateral fusiform gyri was stronger in patients with SAD than in the healthy control group. Given that the fusiform gyrus is the largest macro-anatomical structure in the ventral temporal cortex and plays an essential role in social emotional processing (42), an exaggerated functional connection between the fusiform gyrus and left amygdala during the resting-state may contribute to the hypervigilant response to facial expressions indicating social threat observed in patients with SAD (42–44).
The anterior insula receives a direct projection from the central nucleus of the amygdala, and the anterior insula itself also projects to the amygdala (45). Emotional induction activates the amygdala, and the insula is involved in emotional processing (46). Given that individuals with major depression exhibit greater activation of the amygdala, insula, and ventrolateral prefrontal cortex in response to increasing social exclusion than controls (47), it may be that abnormal connectivity between the left amygdala and insula in patients with SAD contributes to their vulnerability to pathological social anxiety. A significant association between the fear of negative evaluation and rs-FCN between the left amygdala and insula suggests that this dysfunctional connection underlies the excessive fear of negative evaluation by others experienced by patients with SAD. Moreover, we found that hemispheric asymmetry in rs-FCN of the amygdala in the insula of patients with SAD was dissimilar to that of healthy controls.
Abnormal connectivity between the left amygdala and the precuneus and supramarginal gyrus was associated with the level of general anxiety in the patient group. Given that these regions are part of the DMN (48, 49), which processes introspective thoughts during the resting-state (50), it may be that abnormal positive connectivity between these regions and the left amygdala lead to enhanced emotional surveillance related to self-relevant information. A previous study found negative rs-FCN between the amygdala and precuneus in healthy adults and adolescents and positive rs-FCN between the amygdala and precuneus in adolescents with major depressive disorder (51). Furthermore, the authors reported that the rs-FCN between the left amygdala and precuneus was positively associated with the level of neuroticism in healthy participants. Thus, impaired negative rs-FCN between the left amygdala and DMN regions may underlie the disproportionate “emotional coloring” of self-relevant information processing in patients with SAD (51).
The functional connectivity between the right amygdala and middle temporal gyrus observed in healthy controls was lack in patients with SAD. The robust associations between the disconnection of these regions and symptom severity in the patient group suggest that this abnormality is specific for SAD. Moreover, we previously reported that impaired connectivity of the left middle temporal gyrus was proportional to the severity of functional impairment in patients with SAD (52). Our finding in the present study that the strength of the rs-FCN between the right amygdala and middle temporal gyrus was reduced in patients with SAD provides further evidence that the social-affective communication network is impaired in SAD.
In contrast, the functional connectivities of the right amygdala with the parahippocampal limbic area and superior temporal gyrus were increased in patients with SAD. Enhanced rs-FCN between the superior temporal gyrus and right amygdala was associated with depressed mood and general anxiety. The superior temporal gyrus has been implicated in social-emotional processing, along with other “social brain” regions, including the inferior frontal gyrus, posterior superior temporal sulcus, fusiform gyrus, ventromedial prefrontal cortex, and amygdala (42, 53). Exaggerated functional connectivity within these social brain regions, particularly in the right amygdala, may underlie the emotional susceptibility characteristic of SAD.
It is well known that cognitive capacities, such as language and visuospatial attention, are generally lateralized to one cerebral hemisphere (54, 55). Left-hemisphere regions show a preference for same hemisphere interactions, whereas the right-hemisphere regions interact in a more integrated fashion with both hemispheres. These two patterns of interaction are associated with left-lateralized functions (14). Previous studies have reported functional asymmetry in the processing of emotional stimuli in the bilateral amygdala (13, 56). Given that the left amygdala is associated with sustained stimulus processing, whereas the right amygdala shows greater habituation (13), it is likely that patients with SAD are more affected by left amygdala activity. Our finding that the left amygdala was dominant in rs-FCN with several cortical regions in patients with SAD supports this assumption. In particular, functional asymmetry between the amygdala and IPS, which has been implicated in visual attention and interpreting the intent of others, was correlated with symptom severity in patients with SAD. Given that the amygdala can modulate attention and help direct overt visuospatial attention in face gaze (57), the interconnectivity between the amygdala and IPS may play a critical role in the attentional bias toward negative social cues found in patients with SAD. Thus, increased aberrant connectivities between the left amygdala and the limbic area of the insula and social brain of the IPS and inferior frontal gyrus may underlie pathological social anxiety.
Our study has several limitations. First, as most participants were undergraduate students in their early-to-mid 20s, caution should be exercised in generalizing our findings to SAD patients in a wider age range. Second, there included four patients who have comorbid depressive disorders. Clinically, SAD and depressive symptoms often covary (58). Thus, we included these participants when depressive disorders were judged to have been accompanied by SAD. Additionally, partial correlation analysis with covariates of the level of depressed mood exhibited no significant influence of comorbid depressive symptoms on the original results. Third, there also included ambidextrous participants. Since the ambidextrous participants were < 10% in each group, the results were not considered to be affected significantly. In addition, it was reported that neurophysiological basis of language lateralization is different from that of handedness (59), and arcuate fasciculus asymmetry is independent of hand preference (60). Fourth, we used a liberal significance threshold in the correlation analyses, although a stricter significance threshold was required for individual comparisons to compensate for the number of inferences being made. Thus, the correlation analyses of our study has only an exploratory nature and should be limited in interpretation. However, because the direction of the correlations was consistent with the results of the two-sample t-tests of left and right amygdala rs-FCNs, we believe our findings have clinical implications.
In conclusion, we found dissociated functional connections between the left and right amygdala and cortical regions. Increased rs-FCN between the left amygdala and the fusiform face area and reduced rs-FCN between the right amygdala and the middle temporal gyrus were associated with symptom severity of social anxiety in patients with SAD. Moreover, the hemispheric asymmetry of the functional connections of the amygdala with the insula, IPS, and inferior frontal gyrus was altered in patients with SAD. In particular, the abnormal dominant left amygdala functional connectivity with the IPS in patients with SAD was related to symptom severity, which is consistent with the function of these regions in processing visual attention to social threat. We believe the association between hemispheric asymmetry in the functional network of the amygdala and the pathophysiology of anxiety disorders warrants further study in a manner similar to the investigation of hemispheric lateralization of cognitive abilities.
Ethics Statement
This study was approved by the Institutional Review Board of Seoul National University Hospital, and written informed consent was obtained from all participants. Our study was conducted in accordance with the Declaration of the World Medical Association.
Author Contributions
Y-HJ: analyzed the data and wrote the manuscript; JS and YL: contributed to data acquisition and processing; JJ: obtained funding and reviewed and commented on the first draft of the manuscript; HJ: contributed to study concept, formal analysis, study supervision, and writing the manuscript; S-HC: contributed to study concept and design, obtaining funding, and writing the manuscript and has responsibility for conduct of research and final approval.
Funding
This research was supported by the Brain Research Program and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning and the Ministry of Education (NRF-2016M3C7A1914449 and 2017R1D1A1B03036385, http://www.nrf.re.kr). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00164/full#supplementary-material
References
1. Stein MB, Kean YM. Disability and quality of life in social phobia: epidemiologicfindings. Am J Psychiatry (2000) 157:1606–13. doi: 10.1176/appi.ajp.157.10.1606
2. Stein MB, Stein DJ. Social anxiety disorder. Lancet (2008) 371:1115–25. doi: 10.1016/S0140-6736(08)60488-2
3. Habel U, Windischberger C, Derntl B, Robinson S, Kryspin-Exner I, Gur RC, et al. Amygdala activation and facial expressions: explicit emotion discrimination versus implicit emotion processing. Neuropsychologia (2007) 45:2369–77. doi: 10.1016/j.neuropsychologia.2007.01.023
4. Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. (2002) 14:1264–74. doi: 10.1162/089892902760807258
5. Spezio ML, Huang PY, Castelli F, Adolphs R. Amygdala damage impairs eye contact during conversations with real people. J Neurosci. (2007) 27:3994–7. doi: 10.1523/JNEUROSCI.3789-06.2007
6. Liu F, Zhu C, Wang Y, Guo W, Li M, Wang W, et al. Disrupted cortical hubs in functional brain networks in social anxiety disorder. Cling Neurophysiol. (2015) 126:1711–6. doi: 10.1016/j.clinph.2014.11.014
7. Hattingh CJ, Ipser J, Tromp SA, Syal S, Lochner C, Brooks SJ, et al. Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta-analysis. Front Hum Neurosci. (2013) 6:347. doi: 10.3389/fnhum.2012.00347
8. Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety (2013) 30:234–41. doi: 10.1002/da.22014
9. Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry (2002) 59:1027–34. doi: 10.1001/archpsyc.59.11.1027
10. Yuan M, Zhu H, Qiu C, Meng Y, Zhang Y, Shang J, et al. Group cognitive behavioral therapy modulates the resting-state functional connectivity of amygdala-related network in patients with generalized social anxiety disorder. BMC Psychiatry (2016) 16:198. doi: 10.1186/s12888-016-0904-8
11. Baeken C, Marinazzo D, Van Schuerbeek P, Wu GR, De Mey J, Luypaert R, et al. Left and right amygdala - mediofrontal cortical functional connectivity is differentially modulated by harm avoidance. PLoS ONE (2014) 9:e95740. doi: 10.1371/journal.pone.0095740
12. Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. (2004) 45:96–103. doi: 10.1016/j.brainresrev.2004.02.004
13. Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport (2001) 12:379–83. doi: 10.1097/00001756-200102120-00039
14. Gotts SJ, Jo HJ, Wallace GL, Saad ZS, Cox RW, Martin A. Two distinct forms of functional lateralization in the human brain. Proc Natl Acad Sci USA. (2013) 110:E3435–44. doi: 10.1073/pnas.1302581110
15. Esteves M, Marques P, Magalhães R, Castanho TC, Soares JM, Almeida A, et al. Structural laterality is associated with cognitive and mood outcomes: an assessment of 105 healthy aged volunteers. Neuroimage (2017) 153:86–96. doi: 10.1016/j.neuroimage.2017.03.040
16. Butler RK, Oliver EM, Fadel JR, Wilson MA. Hemispheric differences in the number of parvalbumin-positive neurons in subdivisions of the rat basolateral amygdala complex. Brain Res. (2018) 1678:214–9. doi: 10.1016/j.brainres.2017.10.028
17. Hardee JE, Thompson JC, Puce A. The left amygdala knows fear: laterality in the amygdala response to fearful eyes. Soc Cogn Affect Neurosci. (2008) 3:47–54. doi: 10.1093/scan/nsn001
18. Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci. (2004) 118:15–23. doi: 10.1037/0735-7044.118.1.15
19. Hahn HM, Yum TH, Shin YW, Kim KH, Yoon DJ, Chung KJ. A standardization study of Beck Depression Inventory in Korea. J Korean Neuropsychiatr Assoc. (1986) 25:487–500.
20. Choi SH, Shin JE, Ku J, Kim JJ. Looking at the self in front of others: neural correlates of attentional bias in social anxiety. J Psychiatr Res. (2016) 75:31–40. doi: 10.1016/j.jpsychires.2016.01.001
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA: American Psychiatric Publishing, Inc. (2013).
22. Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry (1987) 22:141–73. doi: 10.1159/000414022
23. Mattick RP, Clarke JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behav Res Ther. (1998) 36:455–70. doi: 10.1016/S0005-7967(97)10031-6
24. Leary MR. A brief version of the Fear of Negative Evaluation Scale. Pers Soc Psychol Bull. (1983) 9:371–5. doi: 10.1177/0146167283093007
25. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry (1961) 4:561–71. doi: 10.1001/archpsyc.1961.01710120031004
26. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
27. Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage (2009) 44:839–48. doi: 10.1016/j.neuroimage.2008.09.037
28. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. (1996) 29:162–73. doi: 10.1006/cbmr.1996.0014
29. Beall EB, Lowe MJ. Isolating physiologic noise sources with independently determined spatial measures. Neuroimage (2007) 37:1286–300. doi: 10.1016/j.neuroimage.2007.07.004
30. Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. (1999) 42:1014–8. doi: 10.1002/(SICI)1522-2594(199912)42:6<1014::AID-MRM4>3.0.CO;2-F
31. Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage (2010) 52:571–82. doi: 10.1016/j.neuroimage.2010.04.246
32. Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, et al. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. J Appl Math. (2013) 2013:935154. doi: 10.1155/2013/935154
33. Saad ZS, Reynolds RC, Jo HJ, Gotts SJ, Chen G, Martin A, et al. Correcting brain-wide correlation differences in resting-state FMRI. Brain Connect. (2013) 3:339–52. doi: 10.1089/brain.2013.0156
34. Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. (1998) 22:324–33. doi: 10.1097/00004728-199803000-00032
35. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron (2002) 33:341–55. doi: 10.1016/S0896-6273(02)00569-X
36. Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. fMRI clustering and false-positive rates. Proc Natl Acad Sci USA. (2017) 114:E3370–1. doi: 10.1073/pnas.1614961114
37. Demenescu LR, Renken R, Kortekaas R, van Tol MJ, Marsman JB, van Buchem MA, et al. Neural correlates of perception of emotional facial expressions in out-patients with mild-to-moderate depression and anxiety. a multicenter fMRI study. Psychol Med. (2011) 41:2253–64. doi: 10.1017/S0033291711000596
38. Boehme S, Ritter V, Tefikow S, Stangier U, Strauss B, Miltner WH, et al. Neural correlates of emotional interference in social anxiety disorder. PLoS ONE (2015) 10:e0128608. doi: 10.1371/journal.pone.0128608
39. Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. (1999) 3:11–21. doi: 10.1016/S1364-6613(98)01265-0
40. Gomez J, Barnett MA, Natu V, Mezer A, Palomero-Gallagher N, Weiner KS, et al. Microstructural proliferation in human cortex is coupled with the development of face processing. Science (2017) 355:68–71. doi: 10.1126/science.aag0311
41. Binelli C, Muñiz A, Subira S, Navines R, Blanco-Hinojo L, Perez-Garcia D, et al. Facial emotion processing in patients with social anxiety disorder and Williams-Beuren syndrome: an fMRI study. J Psychiatry Neurosci. (2016) 41:182–91. doi: 10.1503/jpn.140384
42. Mellem MS, Jasmin KM, Peng C, Martin A. Sentence processing in anterior superior temporal cortex shows a social-emotional bias. Neuropsychologia (2016) 89:217–24. doi: 10.1016/j.neuropsychologia.2016.06.019
43. Weiner KS, Grill-Spector K. The improbable simplicity of the fusiform face area. Trends Cogn Sci. (2012) 16:251–4. doi: 10.1016/j.tics.2012.03.003
44. Quaranta D, Piccininni C, Carlesimo GA, Luzzi S, Marra C, Papagno C, et al. Recognition disorders for famous faces and voices: a review of the literature and normative data of a new test battery. Neurol Sci. (2016) 37:345–52. doi: 10.1007/s10072-015-2437-1
45. Jakab A, Molnár PP, Bogner P, Béres M, Berényi EL. Connectivity-based parcellation reveals interhemispheric differences in the insula. Brain Topogr. (2012) 25:264–71. doi: 10.1007/s10548-011-0205-y
46. Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage (2002) 16:331–48. doi: 10.1006/nimg.2002.1087
47. Kumar P, Waiter GD, Dubois M, Milders M, Reid I, Steele JD. Increased neural response to social rejection in major depression. Depress Anxiety (2017) 34, 1049–56. doi: 10.1002/da.22665
48. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. (2003) 100:253–8. doi: 10.1073/pnas.0135058100
49. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. (2007) 8:700–11. doi: 10.1038/nrn2201
50. Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science (2007) 315:393–5. doi: 10.1126/science.1131295
51. Heeren A, Jones PJ, McNally RJ. Mapping network connectivity among symptoms of social anxiety and comorbid depression in people with social anxiety disorder. J Affect Disord. (2018) 228:75–82. doi: 10.1016/j.jad.2017.12.003
52. Ocklenburg S, Beste C, Arning L, Peterburs, 4, Güntürkün O. The ontogenesis of language lateralization and its relation to handedness. Neurosci Biobehav Rev. (2014) 43:191–8. doi: 10.1016/j.neubiorev.2014.04.008
53. Allendorfer JB, Hernando KA, Hossain S, Nenert R, Holland SK, Szaflarski JP. Arcuate fasciculus asymmetry has a hand in language function but not handedness. Hum Brain Mapp. (2016) 37:3297–309. doi: 10.1002/hbm.23241
54. Lanteaume L, Khalfa S, Régis J, Marquis P, Chauvel P, Bartolomei F. Emotion induction after direct intracerebral stimulations of human amygdala. Cereb Cortex (2007) 17:1307–13. doi: 10.1093/cercor/bhl041
55. Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Prog Brain Res. (2006) 156:363–78. doi: 10.1016/S0079-6123(06)56020-0
56. Yun JY, Kim JC, Ku J, Shin JE, Kim JJ, Choi SH. The left middle temporal gyrus in the middle of an impaired social-affective communication network in social anxiety disorder. J Affect Disord. (2017) 214:53–9. doi: 10.1016/j.jad.2017.01.043
57. Mazzola V, Arciero G, Fazio L, Lanciano T, Gelao B, Popolizio T, et al. What impact does an angry context have upon us? The effect of anger on functional connectivity of the right insula and superior temporal gyri. Front Behav Neurosci. (2016) 10:109. doi: 10.3389/fnbeh.2016.00109
58. Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect). Neurology (1980) 30:327–30. doi: 10.1212/WNL.30.3.327
59. Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, et al. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry (2014) 71:1138–47. doi: 10.1001/jamapsychiatry.2014.1087
Keywords: amygdala, resting-state functional connectivity, hemispheric asymmetry, insula, social anxiety disorder
Citation: Jung Y-H, Shin JE, Lee YI, Jang JH, Jo HJ and Choi S-H (2018) Altered Amygdala Resting-State Functional Connectivity and Hemispheric Asymmetry in Patients With Social Anxiety Disorder. Front. Psychiatry 9:164. doi: 10.3389/fpsyt.2018.00164
Received: 10 September 2017; Accepted: 11 April 2018;
Published: 26 April 2018.
Edited by:
Paul Stokes, King's College London, United KingdomReviewed by:
Beata Godlewska, University of Oxford, United KingdomStefan Borgwardt, Universität Basel, Switzerland
Copyright © 2018 Jung, Shin, Lee, Jang, Jo and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hang J. Jo, jo.hang@mayo.edu
Soo-Hee Choi, soohchoi@snu.ac.kr
†These authors have contributed equally to this work.
 Ye-Ha Jung
Ye-Ha Jung Jung E. Shin
Jung E. Shin Yoonji I. Lee
Yoonji I. Lee Joon H. Jang
Joon H. Jang Hang J. Jo
Hang J. Jo Soo-Hee Choi
Soo-Hee Choi