Hypergravity As a Tool for Cell Stimulation: Implications in Biomedicine
- 1Center for Micro-BioRobotics @SSSA, Istituto Italiano di Tecnologia, Pisa, Italy
- 2BioRobotics Institute, Scuola Superiore Sant'Anna, Pisa, Italy
- 3Department of Mechanical and Aerospace Engineering, Politecnico di Torino, Torino, Italy
Gravity deeply influences numerous biological events in living organisms. Variations in gravity values induce adaptive reactions that have been shown to play important roles, for instance in cell survival, growth, and spatial organization. In this paper, we summarize effects of gravity values higher than that one experienced by cells and tissues on Earth, i.e., hypergravity, with particular attention to the nervous and the musculoskeletal systems. Besides the biological consequences that hypergravity induces in the living matter, we will discuss the possibility of exploiting this augmented force in tissue engineering and regenerative medicine, and thus hypergravity significance as a new therapeutic approach both in vitro and in vivo.
Introduction
Physical stimuli significantly influence biological events, triggering biochemical signals involved in molecular cascades that result in altered cell migration, proliferation, and differentiation, and thus in variations in tissue/organ architecture and function.
Among physical stimuli, gravity deeply models land-based organisms, affecting in particular their musculoskeletal and nervous systems. Gravity is ubiquitous and influences tissue mechanical environment by affecting cell weight, extracellular hydrostatic pressure, and fluid convection. Since cell weight depends on the gravity force acting on cell mass, gravity variations can directly affect cell/substrate interactions (e.g., adhesion), cytoskeletal conformation, activation of stretch-activated receptors, transduction pathways and gene expression. Furthermore, indirect effects of altered gravity, such as those mediated by the hydrostatic pressure and fluid shear flow, strongly affect both in vitro and in vivo systems. An increasing number of researches has focused on the effects of gravity variations on physiological processes. By simulating the presence of intense gravitational vectors, for instance with the aid of large diameter centrifuges (Figures 1A,B), useful insight on cellular physiology can be gained and even exploited to elaborate novel therapies.
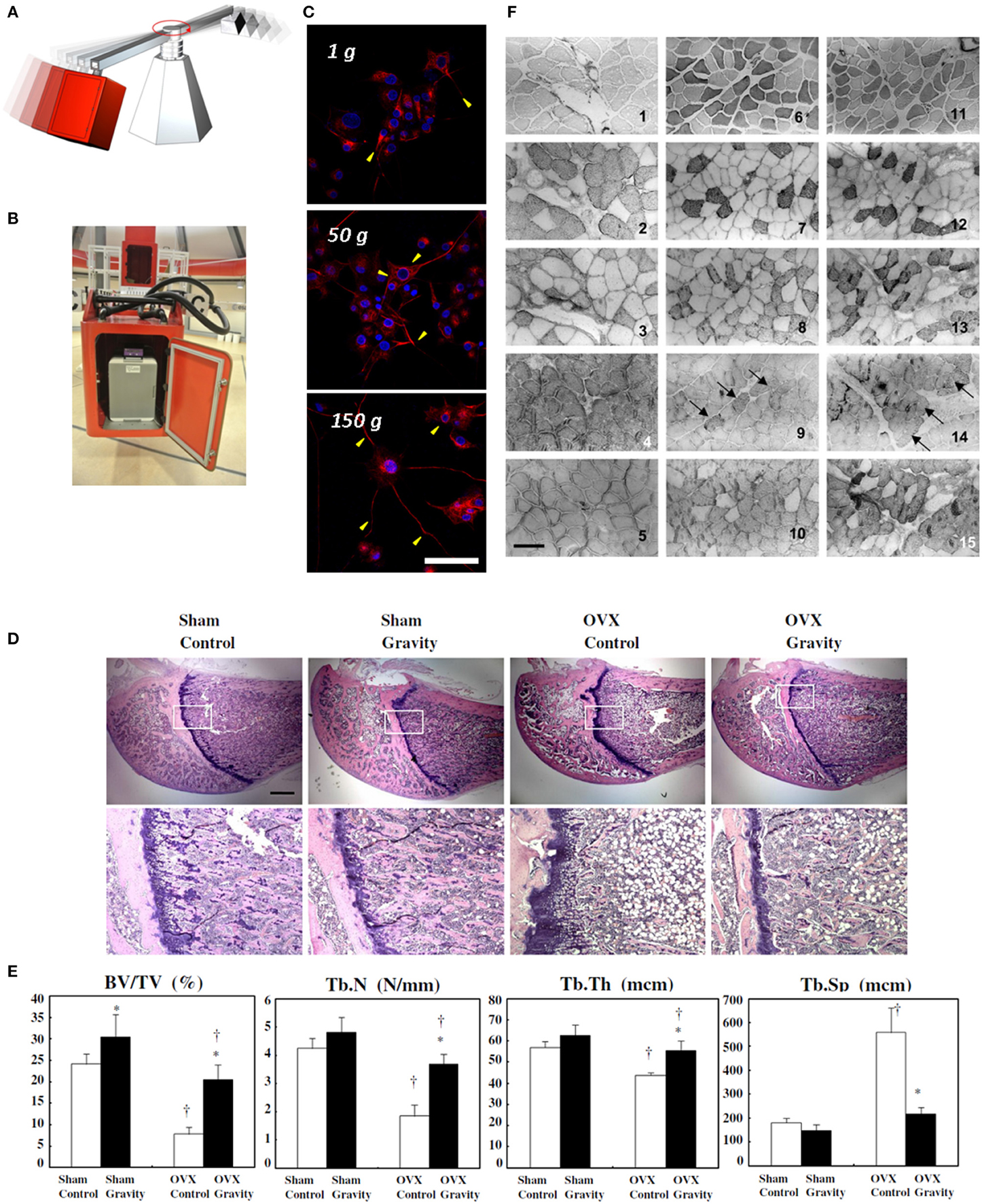
Figure 1. Hypergravity experiments on cell cultures. Pictorial representation of a large diameter centrifuge, exploited for hypergravity investigations (A) and a gondola of the large diameter centrifuge at the European Space Agency (B). Confocal fluorescence microscopy images of 72 h pre-differentiated PC12 neuron-like cells exposed to different gravity levels (1, 50, and 150 g for 1 h), and fixed 48 h post-stimulus, showing different localization of the differentiation marker (neurofilament 66, in red) within neurites and evidencing higher neurite length with increasing gravity level. In blue: nuclei. Scale bar is 50 μm. (C) Reproduced and adapted with permission from Hindawi (Genchi et al., 2015). Effect of hypergravity (2.9 g, 4 weeks) on bone structure of ovariectomized rats. Hematoxylin and eosin staining of sections of the proximal femurs. Scale bar is 1 mm. (D) Bone structure-related parameters in the bone histomorphometric analysis of the trabecular bone of the distal femur. Control group: white bar, hypergravity group: solid bar. BV/TV: bone volume/tissue volume, Tb.N: trabecular number, Tb.Th: trabecular thickness, Tb.Sp: trabecular separation. The data are presented as the average ± SD (n = 4). *p < 0.05 compared to control group, †p < 0.05 compared to sham group (E) reproduced with permission from Elsevier (Ikawa et al., 2011). Immunohistochemistry images of skeletal muscle sections from rats born and reared in hypergravity (2 g). 1–3 and 4–5, control and hypergravity (HG) soleus muscles, respectively; 6–10 and 11–15, control and HG plantaris muscles, respectively. Reactivity of the antibodies is presented as follows: anti-MHC I (2, 4, 7, and 12), anti-MHC IIA (3, 8, and 13), anti-MHC IIB (9 and 14; black arrows indicate positive fibers), and anti MHC II (5, 10, and 15); 1, 6, and 11 show the histochemical method that differentiates, by a dark color, fibers expressing MHC IIX. Scale bar is 140 μm. (F) Reproduced with permission from the American Physiological Society (Picquet et al., 2002).
Nervous System
Hypergravity has strongly different effects on nervous cell cultures compared to whole organisms. In intact rodents, hypergravity indeed over-activates the vestibular system, determines motion sickness, and decreases animal activity/vestibular phasic input (Uno et al., 1997; Santucci et al., 2000; Abe et al., 2010). Hypergravity also influences the autonomic nervous system (Hakeman and Sheriff, 2003) and the renal sympathetic nerve (Morita et al., 2001), while mitigates detrimental effects of microgravity in the autonomic cardiovascular control (Iwasaki et al., 2001).
Orthostatic intolerance and motion sickness are detected after parabolic/space-flights (Serrador et al., 2000; Schlegel et al., 2001) possibly depending on a plastic alteration of the vestibulo-cardiovascular reflex. Hypergravity-induced decrease in sensitivity of the vestibular-cardiovascular reflex however can efficiently be prevented with galvanic stimulation of the vestibular system (Abe et al., 2009).
Hypergravity (4 g, 48 h) also influences synaptic plasticity of the hippocampus, by inducing its long-term potentiation and thus possibly affecting memory in rats (Ishii et al., 2004).
In humans, hypergravity determines increased electroencephalography (EEG) activity in higher frequencies, resulting in loss of consciousness/EEG slowing due to hypoxia at 4 g (Marušič et al., 2014). Brain pre-frontal cortical activity and oxygenation are, respectively, increased and diminished by hypergravity exposure, with the former very likely related to psychological stress (Smith et al., 2013).
Behavioral alterations with gender and age specificity are found after hypergravity treatment in rats, mainly related to neurotrophin secretion variations (Francia et al., 2004a,b, 2006; Santucci et al., 2009).
Hypergravity acting on the vestibular system also induces an irregular monoaminergic innervations to the spinal cord during nervous system development (Giménez y Ribotta et al., 1998) and an increase of the central serotonin (5-HT) concentration, resulting in hypophagia (Abe et al., 2010).
Several attempts were done to decouple effects of hypergravity on neural systems from other organs/systems, such as the cardio-circulatory and the endocrine system. Many studies were thus performed in vitro and on simple animal models. Interestingly, Caenorhabditis elegans worms exposed to strong hypergravity (100 g, 3 h) show well retained muscle fiber organization/morphology, and functional integrity of the serotonergic/chemosensory neurons (Ren et al., 1996; Schackwitz et al., 1996; Kim et al., 2007). In C. elegans, feeding behavior is also preserved, demonstrating retained coordination of sensory and motor neurons with muscles.
Altered gravity can affect morphology and synaptogenesis of neuronal networks in vitro (Gruener and Hoeger, 1990, 1991; Mitsuhara et al., 2013). For instance, co-cultures of spinal neurons and myotomal myocytes demonstrate lack of synapse formation under simulated microgravity conditions (Gruener and Hoeger, 1990). Vice versa, we found that hypergravity (150 g for 1 h) significantly accelerates PC12 neuron-like cell differentiation and increases neurite extension (Figure 1C, Genchi et al., 2015). These results are in line with those achieved with human SH-SY5Y neuroblastoma cells (Rösner et al., 2006) and are particularly interesting concerning neural tissue engineering, where sustained neurite regeneration is required for a fast recovery of function (Rossi et al., 2007).
Bone and Other Connective Tissues
Mechanical loading is known to be the major stimulus influencing bone deposition and remodeling (Klein-Nulend et al., 2012). Recent literature demonstrates the beneficial effects of hypergravity on bone extracellular matrix (ECM) deposition and maintenance. Concerning organic ECM components, collagen I α2 (Col I α2) mRNA level and total collagen biosynthesis are for instance increased in human osteoblast-like cells by hypergravity (13 g, 24 h; Gebken et al., 1999).
In MC3T3-E1 osteoblasts, collagen-processing enzyme lysyl hydroxilase 2 is up-regulated at transcriptional and functional level by hypergravity (20–40 g, 72 h), increasing collagen I cross-linking. Collagen stabilizing lysyl oxidase transcription and enzyme activity is also up-regulated. Hypergravity thus enhances collagen immature and mature cross-linking, and the conversion rates of immature cross-linking into mature compounds in vitro (Saito et al., 2003).
In rhesus monkeys, hypergravity (2 g, 2 weeks) does not significantly affect urinary excretion of hydroxyproline (marker of total body collagen content). Degradation and excretion of mature collagen markers hydroxylysyl pyridinoline and lysylpyridinoline are instead increased during 1 g recovery. This suggests increased collagen maturation and possible ECM anabolism upon hypergravity stimulation in vivo (Martinez et al., 2008).
Long-term moderate hypergravity (2 g, 28 days) increases trabecular bone volume in ovariectomized adult rats by suppressing bone formation and resorption (Figures 1D,E), and by very likely inhibiting actin cortical ring formation in osteoclasts (Ikawa et al., 2011).
Highly relevant to tissue engineering purposes, hypergravity has also been show to promote stem cell osteo-differentiation. Rat bone marrow cells under osteo-differentiative medium treated at 12 g (24 h) indeed show enhanced gene transcription of bone gamma-carboxyglutamic acid-containing protein (Bglap), vitamin D receptor, Runt-related transcription factor 2 (Runx2) and alkaline phosphatase (Alpl), suggesting higher ECM mineralization (Morita et al., 2004). When exposed to 10 g (7 days), they also show significantly enhanced transcription of Runx2 and β-catenin, and of Bglap on a nanotextured substrate (Prodanov et al., 2013).
Synergistic effects of hypergravity and osteo-inductive nanoparticle administration are also reported for rat mesenchymal stem cells (MSCs): transcription of Runx2, Alpl, ColIα1 is significantly increased after 3 h of 20 g stimulation and 2 days of recovery under differentiation. Ras homolog gene family, member A (RhoA) transcription is also significantly increased under proliferation. Moreover, intracellular collagen I amount is increased, as well as area of calcium deposits indicating improved ECM deposition/mineralization (Rocca et al., 2015).
Overall, these results point to a higher and faster deposition of both organic and inorganic components in mature bone cells and in bone precursor cells, as well as to a limited bone resorption, upon exposure to hypergravity. These findings support the use of mild hypergravity as a physical stimulation for the achievement of tissue constructs in vitro to be transplanted in the case of bone defects (for instance due to trauma) and for direct stimulation of whole organisms.
Concerning other connective tissues, hypergravity promotes contrasting effects. Strong hypergravity (60 g obtained by vibration, 81 Hz, 2 days) indeed damages the Achilles tendon in rats, leading to fibroblast hypercellularity and prolonged secretive state (Hansson et al., 1988). Moderate hypergravity (2 g obtained by vibration, 30 Hz, 20 min/day, 5 days/week, 5 weeks) does not affect tensile/elastic properties of Achilles and patellar tendon. Number of fibroblasts and ColIα1 mRNA expression are instead beneficially increased (Keller et al., 2013). Increased expression of ECM components (collagen II and aggrecan) and of a transcriptional factor involved in ECM component synthesis (sex determining region Y-box 9) is also found in human chondrocytes exposed to 10 g for 10 min alternated to 1 g exposure for 10 min (Basile et al., 2009).
Based on these evidences, low hypergravity regimes seem to support connective tissue deposition both in vitro and in vivo.
Muscle Tissue
Hypergravity effects on heart muscle have been investigated from structural up to functional level. In C57BL6J mouse cardiomyocytes, hypergravity (2 g, 30 days) decreases the transverse stiffness by 16%, and decreases actin and α-actinin content in protein membranous fraction. After 12 h, α-actinin -1 content decreases in the membranous fraction (by 27%) and increases in the cytoplasmic fraction (by 28%) compared to the samples soon after treatment (Ogneva et al., 2015).
In Xenopus embryos at gastrulation stage, hypergravity (7 g, 96 h) increases ventricular cross-sectional area by >36%, pointing to a significant cardiac hypertrophy (Duchman and Wiens, 2012). In a mouse model, strong hypergravity (15 g, 5 min) negatively impacts on heart, but when associated to preconditioning (2 exposures at 15 g, 30 s), it seems to protect cardiac function, as assessed in terms of early diastolic and systolic myocardial velocity (Lu et al., 2008).
Concerning tissue engineering applications of hypergravity, 2 g (1 or 3 days) and 5-azacytidine treatment enhances mRNA and protein expression of cardiac muscle differentiation markers (in particular, troponin T) in MSCs (Huang et al., 2009). Further, it improves cardiac transcription factor expression/activity by translocation of histone deacetylase 5 from the nucleus. When treated cells are transplanted in a myocardial infarction model, functional recovery is improved and infarct site is reduced (Ling et al., 2011).
Final evidences on the safety of hypergravity treatment on heart muscle are still missing, however the possibility to exploit adaptive responses and stem cell potentialities encourage further studies.
Many evidences in the literature can be found on skeletal muscle, ranging from basic science studies to applications in regenerative medicine. Hypergravity mostly affects skeletal muscle contractile proteins. Moderate hypergravity (4 g, 6–24 h) does not perturb C2C12 myoblast growth, cell cycle and cyclin B/D expression (Damm et al., 2013). Higher hypergravity values (10 and 20 g, 2 h) evaluated after 24 h instead determine an increase in cell proliferation up to 3.5 times compared to normal gravity, and increase actin filament thickness (during proliferation) and myosin expression (during differentiation, Ciofani et al., 2012).
Significant modifications to myosin expression can also be found. Moderate hypergravity (2 g, 8 weeks) determines myosin heavy chain (MHC) compositional changes in rat muscle, increasing the slow MHC I isoform in the slow postural soleus muscle and MHC IIb in the fast agonist plantaris muscle (Fuller et al., 2006).
In rats exposed to 2 g hypergravity (19 days), the cross-sectional area of intrafusal fibers does not change. In B1 fibers, MHC I and α-cardiac MHC expression is significantly increased, whereas MHC IIa and MHC slow-tonic expression is decreased. In B2 fibers, MHC IIa (region A), slow-tonic (region A), and fast myosins (regions A–C) expression is significantly decreased. In chain fibers, MHC IIa and fast MHC expression is significantly reduced (Picquet et al., 2003).
The same hypergravity protocol determines a lower body growth than controls, but also an increase in the soleus muscle mass (15%) in rats. Distribution of MHC and troponin T isoforms is retained in both soleus and plantaris. The isoform expression pattern of troponin subunits I and C (TnI and TnC) is instead changed in a slow-to-fast manner (soleus only, Stevens et al., 2003).
When muscle features are assessed in rats exposed from conception to mature stage (100 days) to 2 g, muscle weight is found to be decreased, whereas fiber cross-sectional area/muscle weight, and relative maximal tension, is found to be increased compared to control animals. The soleus muscle changes into a slower type concerning contractile parameters and MHC content (only MHC I isoform is present). The plantaris muscle instead presents a faster contractile behavior, and shows a higher diversity of hybrid fiber types expressing multiple MHC isoforms (MHC IIB and MHC IIX, Figure 1F, Picquet et al., 2002).
Similar modifications to muscle weight and cross-sectional area are found in another study, showing that the specific force of soleus fibers is increased, and correlates with the elevation of Ca2+ affinity. Moreover, TnI and TnC isoforms undergo slow-to-slower transitions. TnT 3f and TnT 1f expression is, respectively, up- and down-regulated, whereas Mhc I and Mhc IIa mRNA transcription is, respectively, up- and down-regulated (Bozzo et al., 2004).
On the whole, hypergravity seems to induce a skeletal muscle transition to slow type fibers, with similar effects over different periods of exposure. This may prove useful in all those conditions affecting specific subsets of contractile proteins in skeletal muscle, as for instance sarcopenia related to aging or to spinal cord trauma (Ciciliot et al., 2013), with particular relevance to whole organism treatment protocols.
Immune System
Hypergravity effects on the immune system are controversial. Hypergravity for instance triggers metabolic pathways involved in cellular activation/cytokine secretion, and increases the expression of growth factor and immunoregulatory molecule receptors (Cogoli, 1993).
Concerning cell migration (crucial to continuous immune surveillance), hypergravity (10 g, 1–10 days) determines earlier human T lymphocyte motility on fibronectin-coated surfaces compared to normal gravity (Galimberti et al., 2006). Moderate hypergravity (1.8 g during parabolic flight), instead significantly inhibits human neutrophil migration, underlying possible effects on immunity of space crews (Lang et al., 2010). Concerning interplay among different cellular components of the immune system, hypergravity (10 g followed by 1 g recovery) enhances human dendritic cell ability of activating lymphocyte T proliferation and adhesion (over 85%) to human vascular smooth muscle cells (Bellik et al., 2009).
Hypergravity also enhances rat macrophage oxidative burst reaction in real/simulated conditions (Adrian et al., 2013), while increasing human polymorphonuclear leukocyte (PMN) number (Kaufmann et al., 2009) and sensitivity to adenosine (limiting PMN oxidative function) in parabolic flight participants (Kaufmann et al., 2011).
In a murine model of asthma, hypergravity (10 g, 4 h) causes symptom exacerbation by increasing serum interleukin-5 levels and by promoting pulmonary infiltration of inflammatory cells (Jang et al., 2014).
When hypergravity alternates to microgravity as in parabolic flights, the expression of important proteins involved in human T lymphocyte activation and signal transduction is altered (Tauber et al., 2015). Interestingly, simulated gravity profiles of Shuttle show that hormonal alterations triggering changes in leucocyte and lymphocyte subsets are mostly related to the hypergravity conditions of Shuttle launch and landing (Stowe et al., 2008).
Hypergravity (2 g, 3 weeks) also influences genetic rearrangements occurring during mouse embryonic/fetal development to generate the T cell receptor-beta chain (TCRβ) repertoire (used by T lymphocytes to bind and recognize antigens). Hypergravity acts on the transcription of genes involved in T lymphopoiesis resulting in a different TCRβ repertoire, and thus in a possible different ability to recognize different antigens than at 1 g (Ghislin et al., 2015).
Concerning its potential use as a therapeutic approach in pathological conditions due to infection, hypergravity (4 g) is reported to enhance survival of drosophila flies (wild type and rescued yuri mutant) infected with a pathogenic fungus (Beauveria bassiana). Experiments also demonstrate that hypergravity improves resistance to Toll-mediated fungal infections (Taylor et al., 2014). Of course, studies of hypergravity effects on different animal models under pathological conditions induced by bacterial infection are strongly needed prior to envision a realistic application of hypergravity as a therapeutic approach.
Other Bio-Molecular Processes
When exposed to hypergravity, many cells exhibit different proliferation and energy consumption than at 1 g. For instance, hypergravity (10 g, 48 h) improves cell proliferation by 20–30% in different cell types (Tschopp and Cogoli, 1983). Glucose consumption at 10 g is reduced with respect to 1 g, whereas the proliferation rate is enhanced. Hypergravity-induced enhancement of proliferation can be related to DNA polymerase α, which is crucial in eukayotic replication, and shows increased activity by stimulation up to 4 g for 1 h (Takemura and Yoshida, 2001). This result may prove particularly useful to the in vitro expansion of stem cells prior to commitment and following implantation in regenerative medicine protocols.
Other examples of biological adaptations to hypergravity are the remodeling of the ECM in dermal fibroblasts (20 g, 8 h; Gaubin et al., 1995), the increased production of cAMP in normal follicular thyroid cells treated with thyroid-stimulating hormone (9 g, 1 h; Meli et al., 1999), and the up-regulated heat shock protein 47 transcription in myoblasts culture and muscles (40 g, 2 h; Oguro et al., 2006). Moreover, mice exposed to moderate, short-term hypergravity (3 g, 4 h) show a greater cyclooxygenase 2 (Cox-2) transcription in the heart with respect to control animals (Oshima et al., 2005), while the marker expression is reduced by long-term hypergravity exposure (24 h). Since COX-2 has a cardio-protective role, the use of COX-2 inhibitors and other drugs enhances the risk of infarct and stroke under increased gravitational stress (Oshima et al., 2007).
Concerning liver functions, mice undergone a 3 g treatment for 12 h demonstrate a significantly higher transcription and expression of hepatic inducible nitric oxide synthase, suggesting negative impact on liver functions (Kim et al., 2014).
Hypergravity also affects melanocyte functions: human melanocytes stimulated for 24 h up to 5 g show a marked increment of cyclic guanosine monophosphate (GMP) efflux, which can be related to malignant conversions (Ivanova et al., 2003, 2004).
Conclusions
This mini-review summarizes recent results of cells/organisms exposure to hypergravity, with focus on cell transcriptional/translational events relevant to therapeutic approaches. The main objective of the review was reporting on the biological responses to hypergravity first in vitro then in vivo. This was obviously done in view of the application of hypergravity to the preparation on tissue constructs in vitro to be then implanted in vivo. This application requires deep knowledge of biological responses to hypergravity, which is to date largely deficitary, since the body of evidences in the literature comprises significantly different and hardly relatable hypergravity conditions, and since a deep comprehension of the adaptive responses to the return to normal gravity is missing.
Reporting on the biological responses of specific tissues in intact organisms to hypergravity is motivated by the possibility to expose whole bodies to hypergravity conditions in order to achieve therapeutic goals. This is another application of hypergravity which we deem extremely useful for several disease conditions (osteoporosis, muscular impairment etc.). In this concern, the understanding of the interplay among different anatomical systems during and after hypergravity exposure is though entirely insufficient and deserves future efforts from the scientific community for hypergravity to represent a really feasible therapeutic approach.
Author Contributions
GG took care of the paragraphs concerning bone, muscle, and other connective tissues; AR took care of the paragraph concerning molecular biology; AM took care of the paragraph concerning nervous system; AG took care of the paragraph concerning the immune system; VM took care of the conclusions and of the image arrangement; GC fixed the review structure, wrote the introduction section, and supervised the writing of the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abe, C., Tanaka, K., Awazu, C., and Morita, H. (2009). Galvanic vestibular stimulation counteracts hypergravity-induced plastic alteration of vestibulo-cardiovascular reflex in rats. J. Appl. Physiol. 107, 1089–1094. doi: 10.1152/japplphysiol.00400.2009
Abe, C., Tanaka, K., Iwata, C., and Morita, H. (2010). Vestibular-mediated increase in central serotonin plays an important role in hypergravity-induced hypophagia in rats. J. Appl. Physiol. 109, 1635–1643. doi: 10.1152/japplphysiol.00515.2010
Adrian, A., Schoppmann, K., Sromicki, J., Brungs, S., von der Wiesche, M., Hock, B., et al. (2013). The oxidative burst reaction in mammalian cells depends on gravity. Cell Commun. Signal. 11:98. doi: 10.1186/1478-811X-11-98
Basile, V., Romano, G., Fusi, F., and Monici, M. (2009). Comparison between the effects of hypergravity and photomechanical stress on cells producing ECM. Microgravity Sci. Technol. 21, 151–157. doi: 10.1007/s12217-008-9068-6
Bellik, L., Parenti, A., Ledda, F., Basile, V., Romano, G., Fusi, F., et al. (2009). Hypergravity effects on dendritic cells and vascular wall interactions. Microgravity Sci. Technol. 21, 145–150. doi: 10.1007/s12217-008-9054-z
Bozzo, C., Stevens, L., Bouet, V., Montel, V., Picquet, F., Falempin, M., et al. (2004). Hypergravity from conception to adult stage: effects on contractile properties and skeletal muscle phenotype. J. Exp. Biol. 207, 2793–2802. doi: 10.1242/jeb.01076
Ciciliot, S., Rossi, A. C., Dyar, K. A., Blaauw, B., and Schiaffino, S. (2013). Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell B 45, 2191–2199. doi: 10.1016/j.biocel.2013.05.016
Ciofani, G., Ricotti, L., Rigosa, J., Menciassi, A., Mattoli, V., and Monici, M. (2012). Hypergravity effects on myoblast proliferation and differentiation. J. Biosci. Bioeng. 113, 258–261. doi: 10.1016/j.jbiosc.2011.09.025
Cogoli, A. (1993). The effect of hypogravity and hypergravity on cells of the immune system. J. Leukoc. Biol. 54, 259–268.
Damm, T. B., Franco-Obregón, A., and Egli, M. (2013). Gravitational force modulates G2/ M phase exit in mechanically unloaded myoblasts. Cell Cycle 12, 3001–3012. doi: 10.4161/cc.26029
Duchman, B. J., and Wiens, D. (2012). The Effects of hypergravity on Xenopus embryo growth and cardiac hypertrophy. Am. J. Undergrad. Res. 11, 1–2.
Francia, N., Santucci, D., Chiarotti, F., and Alleva, E. (2004a). Cognitive and emotional alterations in periadolescent mice exposed to 2 g hypergravity field. Physiol. Behav. 83, 383–394. doi: 10.1016/j.physbeh.2004.08.011
Francia, N., Santucci, D., Aloe, L., and Alleva, E. (2004b). Neurobehavioral coping to altered gravity: Endogenous responses of neurotrophins. Prog. Brain Res. 146, 185–194. doi: 10.1016/S0079-6123(03)46013-5
Francia, N., Simeoni, M., Petruzzi, S., Santucci, D., Aloe, L., and Alleva, E. (2006). Repeated acute exposures to hypergravity during early development subtly affect CD-1 mouse neurobehavioural profile. Brain Res. Bull. 69, 560–572. doi: 10.1016/j.brainresbull.2006.02.019
Fuller, P. M., Baldwin, K. M., and Fuller, C. A. (2006). Parallel and divergent adaptations of rat soleus and plantaris to chronic exercise and hypergravity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R442–R448.
Galimberti, M., Tolić-Nørrelykke, I. M., Favillini, R., Mercatelli, R., Annunziato, F., Cosmi, L., et al. (2006). Hypergravity speeds up the development of T-lymphocyte motility. Eur. Biophys. J. 35, 393–400. doi: 10.1007/s00249-006-0046-x
Gaubin, Y., Pianezzi, B., Soleilhavoup, J. P., and Croute, F. (1995). Modulation by hypergravity of extracellular matrix macromolecules in in vitro human dermal fibroblasts. Biochim. Biophys. Acta. 1245, 173–180. doi: 10.1016/0304-4165(95)00088-S
Gebken, J., Ldders, B., Notbohm, H., Klein, H. H., Brinckmann, J., Müller, P. K., et al. (1999). Hypergravity stimulates collagen synthesis in human osteoblast-like cells: evidence for the involvement of p44/42 MAP-kinases (ERK 1/2). J. Biochem. 126, 676–682. doi: 10.1093/oxfordjournals.jbchem.a022502
Genchi, G. G., Cialdai, F., Monici, M., Mazzolai, B., Mattoli, V., and Ciofani, G. (2015). Hypergravity stimulation enhances PC12 neuron-like cell differentiation. BioMed. Res. Int. 2015:748121. doi: 10.1155/2015/748121
Ghislin, S., Ouzren-Zarhloul, N., Kaminski, S., and Frippiat, J. P. (2015). Hypergravity exposure during gestation modifies the TCRβ repertoire of newborn mice. Sci. Rep. 5:9318. doi: 10.1038/srep09318
Giménez y Ribotta, M., Sandillon, F., and Privat, A. (1998). Influence of hypergravity on the development of monoaminergic systems in the rat spinal cord. Dev. Brain Res. 111, 147–157. doi: 10.1016/S0165-3806(98)00132-1
Gruener, R., and Hoeger, G. (1990). Vector-free gravity disrupts synapse formation in cell culture. Am. J. Physiol. 258, C489–C494.
Gruener, R., and Hoeger, G. (1991). Vector-averaged gravity alters myocyte and neuron properties in cell culture. Aviat. Space Environ. Med. 62, 1159–1165.
Hakeman, A. L., and Sheriff, D. D. (2003). Role of the autonomic nervous system in push-pull gravitational stress in anesthetized rats. J. Appl. Physiol. 94, 709–715. doi: 10.1152/japplphysiol.00554.2002
Hansson, H. A., Dahlin, L. B., Lundborg, G., Lowenadler, B., Paleus, S., and Skottner, A. (1988). Transiently increased insulin-like growth factor I immunoreactivity in tendons after vibration trauma. An immunohistochemical study on rats. Scand. J. Plast. Recons. 22, 1–6. doi: 10.3109/02844318809097928
Huang, Y., Dai, Z.-Q., Ling, S.-K., Zhang, H.-Y., Wan, Y.-M., and Li, Y.-H. (2009). Gravity, a regulation factor in the differentiation of rat bone marrow mesenchymal stem cells. J. Biomed. Sci. 16:87. doi: 10.1186/1423-0127-16-87
Ikawa, T., Kawaguchi, A., Okabe, T., Ninomiya, T., Nakamichi, Y., Nakamura, M., et al. (2011). Hypergravity suppresses bone resorption in ovariectomized rats. Adv. Space Res. 47, 1214–1224. doi: 10.1016/j.asr.2010.12.004
Ishii, M., Tomizawa, K., Matsushita, M., and Matsui, H. (2004). Exposure of mouse to high gravitation forces induces long-term potentiation in the hippocampus. Acta Med. Okayama 58, 143–149.
Ivanova, K., Block, I., Das, P. K., and Gerzer, R. (2003). Role of cyclic GMP signaling in the melanocyte response to hypergravity. Signal Transduct. 6, 406–413. doi: 10.1002/sita.200600102
Ivanova, K., Zadeh, N. H., Block, I., Das, P. K., and Gerzer, R. (2004). Stimulation of cyclic GMP efflux in human melanocytes by hypergravity generated by centrifugal acceleration. Pigment Cell Res. 17, 471–479. doi: 10.1111/j.1600-0749.2004.00169.x
Iwasaki, K.-I., Sasaki, T., Hirayanagi, K., and Yajima, K. (2001). Usefulness of daily +2 Gz load as a countermeasure against physiological problems during weightlessness. Acta Astronaut. 49, 227–235. doi: 10.1016/S0094-5765(01)00101-1
Jang, T. Y., Kim, K.-S., Park, C.-S., Lim, J., Huh, K.-C., Heo, M.-J., et al. (2014). Exposure to hypergravity increases serum interleukin-5 and pulmonary infiltration in mice with allergic asthma. Centr. Eur. J. Immunol. 39, 434–440. doi: 10.5114/ceji.2014.47725
Kaufmann, I., Feuerecker, M., Salam, A., Thiel, M., and Choukèr, A. (2011). Adenosine A2A receptor modulates the oxidative stress response of primed polymorphonuclear leukocytes after parabolic flight. Hum. Immunol. 72, 547–552. doi: 10.1016/j.humimm.2011.03.021
Kaufmann, I., Schachtner, T., Feuerecker, M., Schelling, G., Thiel, M., and Choukèr, A. (2009). Parabolic flight primes cytotoxic capabilities of polymorphonuclear leucocytes in humans. Eur. J. Clin. Invest. 39, 723–728. doi: 10.1111/j.1365-2362.2009.02136.x
Keller, B. V., Davis, M. L., Thompson, W. R., Dahners, L. E., and Weinhold, P. S. (2013). Varying whole body vibration amplitude differentially affects tendon and ligament structural and material properties. J. Biomech. 46, 1496–1500. doi: 10.1016/j.jbiomech.2013.03.033
Kim, H. S., Jung, Y. Y., and Do, S. I. (2014). Hepatic inducible nitric oxide synthase expression increases upon exposure to hypergravity. Braz. J. Med. Biol. Res. 47, 940–946. doi: 10.1590/1414-431X20143834
Kim, N., Dempsey, C. M., Kuan, C.-J., Zoval, J. V., O'Rourke, E., Ruvkun, G., et al. (2007). Gravity force transduced by the MEC-4/MEC-10 DEG/ENaC channel modulates DAF-16/FoxO activity in Caenorhabditis elegans. Genetics 177, 835–845. doi: 10.1534/genetics.107.076901
Klein-Nulend, J., Bacabac, R. G., and Bakker, A. D. (2012). Mechanical loading and how it affects bone cells: the role of the osteocyte cytoskeleton in maintaining our skeleton. Eur. Cells Mater. 24, 278–291.
Lang, K., Strell, C., Niggemann, B., Kurt, K. S., Hilliger, A., Engelmann, F., et al. (2010). Real-time video-microscopy of migrating immune cells in altered gravity during parabolic flights. Microgravity Sci. Technol. 22, 63–69. doi: 10.1007/s12217-009-9107-y
Ling, S.-K., Wang, R., Dai, Z.-Q., Nie, J.-L., Wang, H.-H., Tan, Y.-J., et al. (2011). Pretreatment of rat bone marrow mesenchymal stem cells with a combination of hypergravity and 5-azacytidine enhances therapeutic efficacy for myocardial infarction. Biotechnol. Prog. 27, 473–482. doi: 10.1002/btpr.558
Lu, W.-H., Hsieh, K.-S., Li, M.-H., Ho, C.-W., Wu, Y.-C., Ger, L.-P., et al. (2008). Heart status following high G exposure in rats and the effect of brief preconditioning. Aviat. Space Environ. Med. 79, 1086–1090. doi: 10.3357/ASEM.2353.2008
Martinez, D. A., Patterson-Buckendahl, P. E., Lust, A., Shea-Rangel, K. M., Hoban-Higgins, T. M., Fuller, C. A., et al. (2008). A noninvasive analysis of urinary musculoskeletal collagen metabolism markers from rhesus monkeys subject to chronic hypergravity. J. Appl. Physiol. 105, 1255–1261. doi: 10.1152/japplphysiol.00573.2007
Marušič, U., Meeusen, R., Pišot, R., and Kavcic, V. (2014). The brain in micro- and hypergravity: the effects of changing gravity on the brain electrocortical activity. Eur. J. Sport Sci. 14, 813–822. doi: 10.1080/17461391.2014.908959
Meli, A., Perrella, G., Curcio, F., Hemmersbach, R., Neubert, J., and Impiombato, F. A. (1999). Response to thyrotropin of normal thyroid follicular cell strain FRTL5 in hypergravity. Biochimie 81, 281–285. doi: 10.1016/S0300-9084(99)80071-6
Mitsuhara, T., Takeda, M., Yamaguchi, S., Manabe, T., Matsumoto, M., Kawahara, Y., et al. (2013). Simulated microgravity facilitates cell migration and neuroprotection after bone marrow stromal cell transplantation in spinal cord injury. Stem Cell Res. Ther. 4, 35. doi: 10.1186/scrt184
Morita, H., Tanaka, K., Tsuchiya, Y., Miyahara, T., and Fujiki, N. (2001). Response of renal sympathetic nerve activity to parabolic flight-induced gravitational change in conscious rats. Neurosci. Lett. 310, 129–132. doi: 10.1016/S0304-3940(01)02099-7
Morita, S., Nakamura, H., Kumei, Y., Shimokawa, H., Ohya, K., and Shinomiya, K. (2004). Hypergravity stimulates osteoblast phenotype expression. A therapeutic hint for disuse bone atrophy. Ann. N.Y. Acad. Sci. 1030, 158–161. doi: 10.1196/annals.1329.020
Ogneva, I. V., Gnyubkin, V., Laroche, N., Maximova, M. V., Larina, I. M., and Vico, L. (2015). Structure of the cortical cytoskeleton in fibers of postural muscles and cardiomyocytes of mice after 30-day 2-g centrifugation. J. Appl. Physiol. 118, 613–623. doi: 10.1152/japplphysiol.00812.2014
Oguro, A., Sakurai, T., Fujita, Y., Lee, S., Kubota, H., Nagata, K., et al. (2006). The molecular chaperone HSP47 rapidly senses gravitational changes in myoblasts. Genes Cells 11, 1253–1265. doi: 10.1111/j.1365-2443.2006.01021.x
Oshima, M., Oshima, H., and Taketo, M. M. (2005). Hypergravity induces expression of cyclooxygenase-2 in the heart vessels. Biochem. Biophys. Res. Commun. 330, 928–933. doi: 10.1016/j.bbrc.2005.03.060
Oshima, M., Suzuki, H., Guo, X., and Oshima, H. (2007). Increased level of serum vascular endothelial growth factor by long-term exposure to hypergravity. Exp. Anim. 56, 309–313. doi: 10.1538/expanim.56.309
Picquet, F., Bouet, V., Canu, M. H., Stevens, L., Mounier, Y., Lacour, M., et al. (2002). Contractile properties and myosin expression in rats born and reared in hypergravity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R1687–R1695. doi: 10.1152/ajpregu.00643.2001
Picquet, F., De-Doncker, L., and Falempin, M. (2003). Expression of myosin heavy chain isoforms in rat soleus muscle spindles after 19 days of hypergravity. J. Histochem. Cytochem. 51, 1479–1489. doi: 10.1177/002215540305101108
Prodanov, L., van Loon, J. J., te Riet, J., Jansen, J. A., and Walboomers, X. F. (2013). Substrate nanotexture and hypergravity through centrifugation enhance initial osteoblastogenesis. Tissue Eng. A 19, 114–124. doi: 10.1089/ten.tea.2012.0267
Ren, P., Lim, C.-S., Johnsen, R., Albert, P. S., Pilgrim, D., and Riddle, D. L. (1996). Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science 274, 1389–1391. doi: 10.1126/science.274.5291.1389
Rocca, A., Marino, A., Rocca, V., Moscato, S., de Vito, G., Piazza, V., et al. (2015). Barium titanate nanoparticles and hypergravity stimulation improve differentiation of mesenchymal stem cells into osteoblasts. Int. J. Nanomed. 10, 433–445. doi: 10.2147/IJN.S76329
Rösner, H., Wassermann, T., Möller, W., and Hanke, W. (2006). Effects of altered gravity on the actin and microtubule cytoskeleton of human SH-SY5Y neuroblastoma cells. Protoplasma 229, 225–234. doi: 10.1007/s00709-006-0202-2
Rossi, F., Gianola, S., and Corvetti, L. (2007). Regulation of intrinsic neuronal properties for axon growth and regeneration. Prog. Neurobiol. 81, 1–28. doi: 10.1016/j.pneurobio.2006.12.001
Saito, M., Soshi, S., and Fujii, K. (2003). Effect of hyper- and microgravity on collagen post-translational controls of MC3T3-E1 osteoblasts. J. Bone Miner. Res. 18, 1695–1705. doi: 10.1359/jbmr.2003.18.9.1695
Santucci, D., Corazzi, G., Francia, N., Antonelli, A., Aloe, L., and Alleva, E. (2000). Neurobehavioural effects of hypergravity conditions in the adult mouse. Neuroreport 11, 3353–3356. doi: 10.1097/00001756-200010200-00018
Santucci, D., Francia, N., Trincia, V., Chiarotti, F., Aloe, L., and Alleva, E. (2009). A mouse model of neurobehavioural response to altered gravity conditions: an ontogenetical study. Behav. Brain Res. 197, 109–118. doi: 10.1016/j.bbr.2008.08.008
Schackwitz, W. S., Inoue, T., and Thomas, J. H. (1996). Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 17, 719–728. doi: 10.1016/S0896-6273(00)80203-2
Schlegel, T. T., Brown, T. E., Wood, S. J., Benavides, E. W., Bondar, R. L., Stein, F., et al. (2001). Orthostatic intolerance and motion sickness after parabolic flight. J. Appl. Physiol. 90, 67–82.
Serrador, J. M., Shoemaker, J. K., Brown, T. E., Kassam, M. S., Bondar, R. L., and Schlegel, T. T. (2000). Cerebral vasoconstriction precedes orthostatic intolerance after parabolic flight. Brain Res. Bull. 53, 113–120. doi: 10.1016/S0361-9230(00)00315-4
Smith, C., Goswami, N., Robinson, R., Von Der Wiesche, M., and Schneider, S. (2013). The relationship between brain cortical activity and brain oxygenation in the prefrontal cortex during hypergravity exposure. J. Appl. Physiol. 114, 905–910. doi: 10.1152/japplphysiol.01426.2012
Stevens, L., Bozzo, C., Nemirovskaya, T., Montel, V., Falempin, M., and Mounier, Y. (2003). Contractile properties of rat single muscle fibers and myosin and troponin isoform expression after hypergravity. J. Appl. Physiol. 94, 2398–2405. doi: 10.1152/japplphysiol.00808.2002
Stowe, R. P., Yetman, D. L., Storm, W. F., Sams, C. F., and Pierson, D. L. (2008). Neuroendocrine and immune responses to 16-day bed rest with realistic launch and landing G profiles. Aviat. Space Environ. Med. 79, 117–122. doi: 10.3357/ASEM.2205.200
Takemura, M., and Yoshida, S. (2001). Stimulation of DNA polymerase alpha by hypergravity generated by centrifugal acceleration. Biochem. Biophys. Res. Commun. 289, 345–349. doi: 10.1006/bbrc.2001.5986
Tauber, S., Hauschild, S., Paulsen, K., Gutewort, A., Raig, C., Hürlimann, E., et al. (2015). Signal transduction in primary human T lymphocytes in altered gravity during parabolic flight and clinostat experiments. Cell. Physiol. Biochem. 35, 1034–1105. doi: 10.1159/000373930
Taylor, K., Kleinhesselink, K., George, M. D., Morgan, R., Smallwood, T., Hammonds, A. S., et al. (2014). Toll mediated infection response is altered by gravity and spaceflight in Drosophila. PLoS ONE 9:e86485. doi: 10.1371/journal.pone.0086485
Tschopp, A., and Cogoli, A. (1983). Hypergravity promotes cell proliferation. Experientia 39, 1323–1329. doi: 10.1007/BF01990088
Keywords: hypergravity, muscle, skeleton, neuronal system, immune system, tissue engineering
Citation: Genchi GG, Rocca A, Marino A, Grillone A, Mattoli V and Ciofani G (2016) Hypergravity As a Tool for Cell Stimulation: Implications in Biomedicine. Front. Astron. Space Sci. 3:26. doi: 10.3389/fspas.2016.00026
Received: 19 October 2015; Accepted: 03 August 2016;
Published: 19 August 2016.
Edited by:
Jack Van Loon, Vrije Universiteit Amsterdam, NetherlandsReviewed by:
Vyacheslav Ivanovich Dokuchaev, Institute for Nuclear Research, RussiaZurab Silagadze, Budker Institute of Nuclear Physics, Russia
Ludmila Buravkova, Institute of Biomedical Problems, Russia
Eduardo Almeida, National Aeronautics and Space Administration, USA
Copyright © 2016 Genchi, Rocca, Marino, Grillone, Mattoli and Ciofani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giada G. Genchi, giada.genchi@iit.it
Gianni Ciofani, gianni.ciofani@iit.it; gianni.ciofani@polito.it
 Giada G. Genchi1*
Giada G. Genchi1*  Attilio Marino
Attilio Marino Agostina Grillone
Agostina Grillone Virgilio Mattoli
Virgilio Mattoli Gianni Ciofani
Gianni Ciofani