Unit of Anatomy and Embryology, Department of Anatomy and Animal Production, Faculty of Veterinary, University of Santiago de Compostela, Lugo, Spain
The sense of smell plays a crucial role in mammalian social and sexual behaviour, identification of food, and detection of predators. Nevertheless, mammals vary in their olfactory ability. One reason for this concerns the degree of development of their pars basalis rhinencephali, an anatomical feature that has been considered in classifying this group of animals as macrosmatic, microsmatic or anosmatic. In mammals, different structures are involved in detecting odours: the main olfactory system, the vomeronasal system (VNS), and two subsystems, namely the ganglion of Grüneberg and the septal organ. Here, we review and summarise some aspects of the comparative anatomy of the VNS and its putative relationship to other olfactory structures. Even in the macrosmatic group, morphological diversity is an important characteristic of the VNS, specifically of the vomeronasal organ and the accessory olfactory bulb. We conclude that it is a big mistake to extrapolate anatomical data of the VNS from species to species, even in the case of relatively close evolutionary proximity between them. We propose to study other mammalian VNS than those of rodents in depth as a way to clarify its exact role in olfaction. Our experience in this field leads us to hypothesise that the VNS, considered for all mammalian species, could be a system undergoing involution or regression, and could serve as one more integrated olfactory subsystem.
Most mammals have two mechanisms to detect odour. One is by means of the main olfactory system (MOS), with the main olfactory receptors (MOrs) located in the posterior part of the mucosa of the nasal cavity. The olfactory nerves project to the main olfactory bulb (MOB), from where the olfactory tracts transmit their inputs to specific areas of the brain: the anterior olfactory nucleus, piriform cortex, olfactory tubercle, anterior cortical amygdaloid nucleus, the lateral entorhinal cortex and the periamygdaloid cortex.
The other option is through the accessory olfactory system (AOS), commonly named the vomeronasal system (VNS). The accessory olfactory bulb (AOB) is the first relay station of the subsidiary system which is integrated by three different structures: the vomeronasal organ (VNO), the aforementioned AOB, and the vomeronasal amygdala (VNAg). Inside the VNO are the vomeronasal receptors (VNrs), which project through the vomeronasal nerves to the AOB. From here, the accessory olfactory tract conveys the corresponding information to the VNAg, in this case to the bed nucleus of the stria terminalis, the bed nucleus of the accessory olfactory tract, and the medial and postero-medial cortical amygdaloid nucleus (Halpern, 1987
; Halpern and Martinez-Marcos, 2003
).
In order to avoid any misinterpretation, this review will refer to the VNS consistent with the previous description. This means that a true VNS must have three structures with its corresponding nerves and connections. This anatomical definition is important because a critical point in the study of the VNS concerns its function (described furthering in subsequent sections), which historically takes into account the results and conclusions from studies conducted principally with mice, a species that possesses a very well defined VNS. Many authors, ourselves included, consider the mouse VNS as the model of reference among mammals.
As mentioned previously, the VNAg is an important VNS component, but it will not be discussed in the present manuscript. The amygdala is a very complex area (Swanson and Petrovich, 1998
) that has led to confusion and controversy in the study of the VNS (Meredith and Westberry, 2004
), although contemporary models have brought new clarity to this issue (Pro-Sistiaga et al., 2007
; Fan and Luo, 2009
; Kang et al., 2009
; Martinez-Marcos, 2009
).
Vomeronasal Organ
At the beginning of the nineteenth century the first detailed information was published about the VNO in mammals (Jacobson, 1813
), but the existence of the AOB – also in mammals – was not confirmed until several years later (Gudden, 1870
). The American anatomist Rollo McCotter (1912) demonstrated by micro-dissection that both structures are directly connected, and this was the first step in identifying the VNS. However, the modern description of the VNS was developed several decades later when the presumptive and definitive projection from the AOB to a particular area of the amygdala was identified (Winans and Scalia, 1970
; Scalia and Winans, 1975
). Subsequently, it was assumed that the majority of mammals have two different systems to identify chemical signals related to olfaction.
The existence of such an amazing accessory system attracted the attention of the scientific community, which sought to identify a physiological role for the VNS. Several pieces of information contributed to some early insights.
The first effort tried to determine whether the VNO was present in amphibians, reptiles and mammals, and to investigate whether it was absent in fish and birds. These studies resulted in an exhaustive revision of the definition of the VNO (Pearlman, 1934
), including theories related to its function. However, more relevant data as to VNS function came from experiments that dealt with the “physiological effects on reproduction”. It became clear that substances present in the urine of mice could modify sexual behaviour. Some substances present in female urine delayed the onset of puberty in prepuberal females and suppressed oestrous cyclicity in grouped females (Lee and Boot, 1955
), while substances present in the urine of males induced oestrous in anoestrous females (Whitten, 1956
), prevented pregnancy in recently mated females (Bruce, 1960
), and accelerated the onset of puberty (Vandenbergh, 1969
). Almost simultaneously, Karlson and Lüscher (1959)
coined the term “pheromones” to define a class of biologically active substances secreted by insects. It is certainly controversial whether the term pheromones applies to mammals, but the VNS has been seen as a specialised system for mediating pheromonal communication (Johnston, 1998
). The controversial theme of the mammalian pheromones has been discussed extensively in the literature (Beauchamp et al., 1976
; Luo and Katz, 2004
; Rodriguez, 2004
; Wysocki and Petri, 2004
; Brennan and Zufall, 2006
; Swaney and Keverne, 2009
; Tirindelli et al., 2009
).
As the relationship between the VNS and the sexual behaviour of mammals became evident (Wysocki, 1979
; Halpern, 1987
), interest in the field grew considerably. It is essential to emphasise that studies of laboratory mammals, especially mice, paved the way for our current working model of olfaction. This point is critical because form and structure dictate function in biological system. This principle implies that the ability of the VNS in rodents (mice) to detect chemical signals derives from an anatomical organization that allows this function. In a complete VNS, like that of the mouse, one of its peculiarities concerns VNrs that are not free, as in the case of the MOS, but instead are hidden inside the tubular structure of the vomeronasal duct (VNd), usually in the epithelium of its medial wall. In order to detect correctly chemical mediators picked up by the VNrs, there must be contraction/dilation of the VNO soft tissue. The conjunctive tissue and especially the vomeronasal vessels play a decisive role in this physiological phenomenon, known as a pumping mechanism (Meredith et al., 1980
). The number, size, distribution and morphological features of the vomeronasal arteries and veins suggest that these vessels may function similarly to erectile tissue (Salazar et al., 2008
).
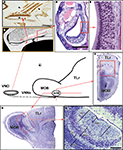
In summary, from a morphological point of view, the following components form part of the VNO: the VNd, whose medial wall is lined with a sensory epithelium that comprises of supporting, basal and sensory cells; the sensory cells, which are endowed with microvilli. Another anatomical peculiarity of the VNd is that its posterior part is blind while its anterior part ends in the nasal cavity. The duct is surrounded by soft tissue containing a considerable amount of glands, vessels and nerves, as well as connective tissue. Finally, a bone lamina encloses all of the elements (Figure 1
). Functionally, the VNO is closely related to the sexual behaviour of mammals (rodents).
Figure 1. Certain morphological characteristics of the VNO and AOB in mice. (A) Schematic representation of the arrival of the VNNs from the VNO to the AOB. (B) Drawing of the nasal cavity where the VNO is located, showing the orientation plane in (C) and (D). (C) Parasagittal section of the nasal septum stained by UEA-I. The VNNs (arrowed) and the neuroepithelium of the VNO (asterisk) bind to UEA-I. (D) Transverse section of the VNO stained with haematoxylin-eosin. (E) Magnification of the box in (D), showing the neuroepithelium of the VNO. (F,G) Nissl-stained sections of the anterior part of the brain showing the topography of the AOB (box) in the transverse (F) and parasagittal planes (G). (H) Nissl-stained sagittal section of the AOB. AOB, accessory olfactory bulb; GIL, glomerular layer; GrL, granule cells layer; LOT, lateral olfactory tract; M/TcL, mitral/tufted cells layer; MOB, main olfactory bulb; TLr, rostral telencephalum; VNNL, vomeronasal nerves layer; VNNs, vomeronasal nerves; VNO, vomeronasal organ. Scale bars = 500 μm (C), 250 μm (D,H), 50 μm (E), 1 mm (F,G).
Accessory Olfactory Bulb
At the beginning of the twentieth century, Santiago Ramón y Cajal (1902)
definitively clarified the structural characteristics of the AOB in mammals, and partially corroborated previous descriptions associated with different animals (Gudden, 1870
; Kölliker, 1877
; Ganser, 1882
; Gehuchten and Martin, 1891
; Herrick, 1892
). Once the first and most detailed description of the connection between the VNO and AOB (McCotter, 1912
) was broadly accepted, the AOB was considered as the first relay station mediating olfactory cues different from the MOS. Subsequently, the AOB has been studied for different reasons (Halpern, 1987
; Halpern and Martinez-Marcos, 2003
), although this review focuses only on its morphology. The most controversial issue regarding the morphology of the AOB is whether it can recognise mitral cells on it, or distinguish them from tufted cells. However, different studies have suggested that the AOB has a mixed population of neurones, namely the “mitral/tufted” cells (Mori, 1987
).
Recently, the characteristics of the different cells included on the AOB of the rat have been studied in depth (Larriva-Sahd, 2008
).
Essentially, the structure of the AOB is similar to the MOB: groups of cells organised into strata, albeit with some peculiarities. The nervous or vomeronasal, glomerular, and granular layers are readily distinguishable, while the mitral/tufted cells define an independent layer, which makes it difficult to identify the two plexiform layers (Figure 1
). Other morphological details are also apparent when the AOB is seen from a comparative anatomical point of view (discussed later in this paper). For example, the topographical disposition of the lateral olfactory tract, which includes the accessory olfactory tract, is either inside or outside the AOB.
The morphological variability found in the different structures which integrated the VNS is not surprising, among other reason, because of the big number of species included in the Mammalian Class, from Order such as Monotrema, Marsupialia and Insectivora to Scandentia, Tubulidenta and Cetacea, for example. Readers interested in the structural, phylogenetic, and species-specific variations in terms of AOB location, shape, and size and morphologic differentiation and development can consult some recent review (Meisami and Bhatnagar, 1998
). An unsuitable explored group of mammals, belonging to the Carvivora, Artiodactyla, and Perissodactyla Orders, shows morphological peculiarities which are worthwhile to comment in a separate section (see below, The Vomeronasal System in Domesticated Mammals).
It is interesting to stress that the diversity of the VNS is not only morphological but it is also physiological/behavioural, represented in that cases by the different expression of the corresponding receptor cells depending on the selected species (Halpern et al., 1998
; Salazar and Sánchez Quinteiro, 1998
).
Fortunately, the scientific literature about studies of the VNS in different mammalian species other than mice and rat is quite extensive, and informs us about interesting morphological and/or physiological data. As, obviously, it is not possible to comment and discuss all of them, we have opted to choose the case of one of the most amazing examples concerning the diversity of the VNS, the bats. In this Chiroptera mammals the AOB can either be perfectly developed or entirely absent, depending on the family. A well developed AOB is found in Phyllostomus hastatus, Glossophaga soricina, Artibeus jamaicensis and Desmodus rotundus, and its size parallels that of the VNO in all these cases in terms of the presence of vomeronasal nerves (Mann, 1961
). The same group of mammals was selected for comparison with primates, as an interesting extreme example of the VNO variability (Bhatnagar and Meisami, 1998
). According to this publication, in our opinion the study of the VNS in bats open the door to revising the characteristics of the VNS in humans.
The presence of a VNS in humans remains controversial. Two different strategies have been employed to investigate this question: the first involves analysing the morphology of the structures that constitute a true VNS in humans, while the second follows a molecular-genetic approach. The two strategies are not contradictory but instead are complementary.
Concerning the putative VNS in humans, there have been several interesting recent publications on the subject (e.g., Doving and Trotier, 1998
; Meisami and Bhatnagar, 1998
; Trotier et al., 2000
; Meredith, 2001
; Halpern and Martinez-Marcos, 2003
; Witt and Hummel, 2006
; Mast and Samuelsen, 2009
), but this article will focus on works dealing with certain concepts concerning the morphology of the VNO and AOB in humans.
About the Vomeronasal Organ
Before the publication of Jacobson (1813)
regarding the presence of the VNO in different mammalian species, the existence of a small bilateral canal was known, located on the anterior and lower area of the septum nasalis in humans (Ruysch, 1703
), and this was later corroborated (Sömmering, 1809
). Due to the close topographical relationship between both structures, there was a tendency, with some exceptions (Potiquet, 1891
), to consider both structures as a single structure (Dursy, 1869
; Kölliker, 1877
; Anton, 1895
). However, it is now clear that strict morphological criteria are required to distinguish the VNO, the VNd, the vomeronasal neuroepithelium (sensory epithelium), the VNrs, and the incisive duct. Several attempts have been made with varying degree of success (Cooper and Bhatnagar, 1976
; Bhatnagar and Meisami, 1998
; Takami, 2002
). In addition, the age of the subjects is another common source of error, because of results can vary considerably for samples of adult, young and foetal tissues.
The presumptive VNO described by previous anatomists in humans looks rather different to the VNO of mice. From a strict morphological point of view, it seems that humans do not have a true VNO, although this assumption is associated with several problems. What happens in mammalian species without a VNO? Are they unable to detect chemicals signals related to sex? Would sexual behaviour in humans be eliminated or dramatically diminished by the lack of such a structure? These and similar questions led us to revise our view of the human olfactory system, an effort initiated by clinicians (Kreutzer and Jafek, 1980
; Johnson et al., 1985
).
The resulting work can be classified into two schools of thought. The first is represented by those authors eager to demonstrate that humans have a true VNO, while the second includes those who believed in the need to describe only morphological facts as showcased by their research observations. Therefore, it is not surprising that from the first group (e.g., Garcia-Velasco and Mondragon, 1991
; Moran et al., 1991
; Stensaas et al., 1991
) identified the VNO in human in the 100% of the subjects whom they examined (reviewed by Monti-Bloch et al., 1998
). Other researchers have only noted the ages of the samples examined (embryos, foetuses, juvenile and adults), along with characteristics of the examined tissue: receptors (Boehm and Gasser, 1993
), vomeronasal cavity (Trotier et al., 2000
), putative VNO (Knecht et al., 2001
), VNd (Abolmaali et al., 2001
), vomeronasal epithelium (Witt et al., 2002
).
It is interesting to reflect on the opinions of some early anatomists. They indicated that “when the Jacobson’s organ cannot be found in a human embryo its absence is not the result of non development but due to early regressive changes” (Mihalkovics, 1898
); others that “the organ of man is without a doubt rudimentary” (Parker, 1922
); or that “Jacobson’s organ is regularly present toward the end of fetal life, it is not present constantly at birth, and it is very often absent after two years of age” (Richter, 1932
). These opinions, although expressed long ago, are still valid today. The views will be discussed later.
About the Accessory Olfactory Bulb
From early studies (Cajal, 1902
) to more recent ones (Meisami and Bhatnagar 1998
), the AOB in humans has not been identified. Nevertheless, there remains some controversy about the presence of the AOB in humans before birth. Certain studies (Bossy, 1980
; Chuah and Zheng, 1987
) have used an initial observation made by Humphrey (1940)
to verify the existence of the AOB during foetal development between 18 and 26 weeks gestational age. However, others researchers have failed to identify a separate AOB in either embryonic or foetal samples (Macchi, 1951
; Witt and Hummel, 2006
). The functional connection between a putative VNO and a presumptive AOB during development has been studied recently (Müller and O’Rahilly, 2004
). The main conclusion, accepted by the vast majority of researchers, is that the AOB in humans is a structure that, if present, regresses clearly before birth.
Thus, morphological data suggest that humans cannot detect chemical signals using the VNS. However, this does not mean that humans cannot recognise pheromones or similar substances that relate to sex (whether reproduction or sexual behaviour) (Savic et al., 2001
, 2009
; Knecht et al., 2003
). Knowing the identity and location of corresponding receptors remains a major challenge in the field.
Additionally, controversy persists regarding whether or not the VNS is a specific system that exclusively detects pheromones (Brennan, 2001
; Rodriguez, 2005
; Baxi et al., 2006
). The notion that the VNS does not mediate all pheromone effects and may mediate some nonpheromonal cues is largely accepted. Moreover, it has been shown that the MOS is also involved in the detection or identification of pheromones (Boehm et al., 2005
; Mandiyan et al., 2005
; Yoon et al., 2005
). As a result, both systems, namely the VNS and the MOS, are much better integrated than previously thought. Neither system is exclusively responsible for perceiving one class of chemical cues (Kelliher, 2007
; Wang et al., 2007
; Grus and Zhang, 2008
).
Modern anatomy suggests that the sense of smell must be considered as a whole and, in this regard, structures such as the septal organ (SO) and the ganglion of Grüneberg (GgG) must be taken into consideration and integrated as part of the olfactory system. These structures have olfactory receptors distributed throughout the respiratory epithelium of the nasal cavity, and are represented by small, compact and peculiar clusters of neurones first studied in detail by Rodolfo-Masera (1943) and Grüneberg (1973)
, respectively.
The SO is a par structure situated on the nasal septum, both posteriorly and superiorly to the VNO. Its epithelium contains basal, sustentacular, and ciliated and microvillar cells, with only two to three layers of sensory neurones. The characteristics of the odorant receptors expressed in the SO lead to the conclusion that it is a chemosensory system (Kaluza et al., 2004
; Tian and Ma, 2004
), while the projection pattern from the SO to the MOB (Levai and Strotmann, 2003
) organises a specific glomerular zone on the bulb (Ma et al., 2003
). Despite these insights, the exact role of the SO in olfaction remains enigmatic, although some authors suggest that it may represent a “general odour detector” (Ma, 2007
), and/or that it might function as a “mini-nose” that recognises food odours and also identifies social-sexual cues (Breer and Strotmann, 2005
).
In comparison with the SO, the GgG is a smaller structure with a population of cells estimated to number about 300–500 in adult mice, apparently without neither basal and sustentacular cells nor cilia (Fuss et al., 2005
). Each ganglion, situated on the roof of the nasal cavity close to the midline and adjacent to the nostrils, occupies an extension approximately 1 mm2, and is present both before birth and in adult mice (Roppolo et al., 2006
; Storan and Key, 2006
). Originally, the GgG was thought to be a non-sensory structure closely linked to the terminal nerve (Grüneberg, 1973
). Recently, its neurones have been shown to express the olfactory marker protein, considered the typical marker for mature chemosensory neurones. A cluster of its neurones projects to a concrete part of the MOB (Fuss et al., 2005
; Koos and Fraser, 2005
; Roppolo et al., 2006
; Storan and Key, 2006
). Both peculiarities would indicate that the GgG is a chemosensory system although, like the SO, its specific function is unknown. However, it may participate in the early identification of olfactory cues, or in detecting milk, or non-volatile molecules (Fuss et al., 2005
; Roppolo et al., 2006
; Storan and Key, 2006
). It may also be involved in temperature or pressure detection (Mamasuew et al., 2008
; Fleischer et al., 2009
), and even to detect pheromones (Brechbühl et al., 2008
).
In general, it is widely accepted the existence of different olfactory receptors independent of the MOS and VNS, the so called “olfactory subsystems” (Breer et al., 2006
; Ma, 2007
). It is evident that these novel structures have prompted debate regarding the concept of the sense of smell as a whole. The anatomical features imply that each structure must have a discrete functional role. Thus, it is plausible that division in the MOS and VNS could yield further insight into the organisation of the olfactory subsystem (Munger et al., 2009
).
After the discovery of the odorant receptor gene family (Buck and Axel, 1991
) it was established that the main olfactory epithelium was divided into four spatial zones according to the specificity of the corresponding MOrs (Ressler et al., 1993
; Vassar et al., 1993
). The subsequent MOr projections are transformed in the MOB into an organised and topographical map (Ressler et al., 1994
; Vassar et al., 1994
). Other orientation about olfactory mappings have been also employed (Rubin and Katz, 1999
; Uchida et al., 2000
; Oka et al., 2006
; Cleland et al., 2007
; Soucy et al., 2009
).
Soon after, the vomeronasal receptor gene family had been identified (Dulac and Axel, 1995
; Herrada and Dulac, 1997
; Matsunami and Buck, 1997
; Ryba and Tirindelli, 1997
), genetic experiments confirmed that the apical and basal vomeronasal axons projected onto the anterior and posterior part of the AOB.
This helped us to build the corresponding vomeronasal map (Belluscio et al., 1999
; Rodriguez et al., 1999
) that established that the segregated projections from the vomeronasal organ to the divisions of the accessory olfactory bulb express differentially both Gi2α and Go proteins and V1R and V2R vomeronasal receptors. Nevertheless, the enormous morphological heterogeneity of the vomeronasal system is very well exemplified in the sheep. This species is usually employed in behavioral studies concerning the main and accessory olfactory systems which show all structures which classically define the VNO but conversely it does not show the zone to zone projection from the apical/basal VNO to the anterior/posterior part of the AOB typical in rodents (Salazar et al., 2007
).
Both systems – namely MOS and VNS – have their olfactory/vomeronasal receptor area divided according to the nature of the chemical senses that they are able to detect. Accordingly, a zonal organisation of the mammalian MOS and VNS seems quite evident (Mori et al., 2000
). It remains unclear whether zonal organisation can be considered to map onto the subsystems themselves. Other subsystems will likely emerge with the availability of new molecular markers and detailed functional analyses (Ma, 2007
).
Certain morphological data concerning the olfactory subsystems, including those related to the MOS and VNS, are shown in Figures 2 and 3
.
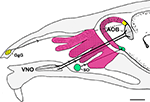
Figure 2. Schematic representation of a parasagittal section through part of the head of a mouse depicting the different olfactory receptors and their projections to the bulbs. Four zones of the main olfactory receptors-four zones of MOB (fuchsia). The GgG-MOB (yellow). The SO-MOB (green). Two zones of the vomeronasal receptors-two zones of the AOB (black). AOB, accessory olfactory bulb; GgG, ganglion of Grüneberg; SO, septal organ; VNO, vomeronasal organ. Scale bar: 1mm.
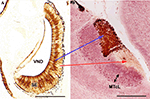
Figure 3. Immunolabeling demonstration of the zonal organisation in the vomeronasal system of mice, showing the correlation apical/SE-anterior AOB, basal/SE-posterior AOB. (A) (After Salazar and Sánchez Quinteiro, 2003
): Transverse section of the vomeronasal organ stained by the lectin LEA showing the differentiation between the basal and apical layers of the SE. (B) (After Salazar et al., 2006
): Parasagittal section of the AOB (left anterior, right posterior-orientated) double-labeled by LEA/MAP-2. Note the clear differentiation of the mitral/tufted cell layer. LEA, Lycopersicum esculentum agglutinin; MAP-2, microtubule-associated proteins 2; MTcL, mitral/tufted cells layer; SE, sensory epithelium; VND, vomeronasal duct. Scale bars = 250 μm.
The use of imaging, electrophysiology and molecular-genetic techniques, among others, will likely converge to give definitive answer regarding the general characteristics of the olfactory receptors. With that information, which will include morphological data such as the number and situation of olfactory receptors and their projections into the brain, it will become possible to understand how olfactory information is processed. Consequently, it will be relatively easy to classify these elements and link them to a specific olfactory system or subsystem.
It may be possible to glean additional information by exploring the VNS in mammals from a morphologically comparative point of view. An investigation of the VNO and the AOB in domesticated mammals (DMs), which do not constitute a separate zoological category, would certainly be interesting.
Although it is challenging to reach a consensus about which animals are DMs, we will follow the criteria of the Nomina Anatomica Veterinaria, an official and international guide for nomenclature (ICVGAN, 2005
), that takes into consideration the following species: cat (Felis catus), dog (Canis familiaris), pig (Sus scrofa domestica), cow (Bos Taurus), sheep (Ovies aries), goat (Capra hircus) and horse (Equus caballus). All these mammals are macrosmatic animals, i.e., they have a very well developed olfactory system.
According to the information available (Meisami and Bhatnagar, 1998
; Halpern and Martinez-Marcos, 2003
), all three of the structures that define a true VNS are present in macrosmatic mammals. Another common characteristic of DMs is that their brains exhibit a gyrencephalic structure, and from this point of view they are closer to humans than rodents. Furthermore, it is possible to establish an interesting relationship: in general, mammals with greater convoluted cerebral cortex convolution intensity exhibit a less pronounced rhinencephalon (Nieuwenhuys et al., 1998
), and, in this sense, DMs would occupy an intermediate position between rodents and primates.
Unfortunately, morphological studies are limited regarding the VNS in the context of DMs. The classical paper by Kratzing (1971)
, the publications of Adams (1992)
, revised by himself, and certain more recent reports (Dennis et al., 2003
) all deal with the VNO in DMs. Regarding the AOB, most studies are contributions from Japanese laboratories (Nakajima et al., 1998
; Takigami et al., 2004
). Although there exist certain discrepancies between the results and the conclusions obtained by these authors and our own work, we will turn our discussion to some other relevant issues. Of these, the most important requires establishing an anatomical comparison between the VNO and AOB of mice (revised by Salazar and Sánchez-Quinteiro, 2003
; Salazar et al., 2001
, 2006
) and of DM species. In this case, we would suggest following the information given by the authors from the previously mentioned groups, our publications (Salazar et al., 2000
, 2004
, 2007
) and our own collection of samples (unpublished data).
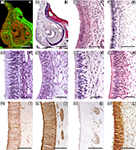
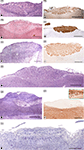
With regards to the VNO (Figure 4
), the more significant anatomical differences could be summarised as follow: in DMs, the lamina which envelops the whole VNO is composed of cartilage instead of bone; all of the structures that define the soft tissue of the organ in mice are similarly present in DMs, although the so called sinus venosus – typical in mice – is inconsistent across DM species. The anterior part of the VNd ends directly in the incisive duct in DMs, and its sensory epithelium exhibits inter-species variations, from less developed in pigs to the better conformed in sheep and cats, although the epithelium fails to reach the structural definition seen in mice. Four to six vomeronasal nerves are quite evident and are regularly seen on the nasal septum in mice. This finding is much less obvious in DMs and is variable, even within the same species. With micro-dissection, it is possible to observe, especially in carnivores, that some fibres of the vomeronasal nerves extend farther than the AOB.
Figure 4. The sensory epithelium of the VNO of certain domestic mammals. (A,B) The VNO of the dog (ca) is shown by autofluorescence imaging (A) and hematoxilin-eosin staining (B) in two adjacent sections of the same specimen. (C–H) Haematoxylin-eosin transverse sections of the VNO of the dog (ca) (C), horse (eq) (D), sheep (ov) (E), 141-days-old sheep (ov) fetus (F), pig (su) (G), and 110-days-old pig (su) fetus (H). (I–L) Cellular expression of the sensory epithelium against the lectins LEA in horse (eq) (I) and UEA-I in cat (fe) (J) and sheep (ov) (K), and against protein Gi2α in sheep (ov) (L). (Salazar and Sánchez-Quinteiro, 2003
; Salazar et al., 2000
, 2006
). Scale bars = 1mm (A,B), 100 μm (C,I–L), 50 μm (E–H).
In relation to the AOB (Figure 5
), we can make the following observations: in DMs, the vomeronasal nerve and glomerular layers are larger than in mice; there are fewer mitral/tufted cells, and they smaller, and scattered. The topographical location of the lateral olfactory tract, which encloses the accessory olfactory tract, is not uniform and may be species dependent. An interesting feature that we have tested in certain species, including pigs, is that the AOB reaches maturity several weeks before birth. This is in clear contrast with the situation in mice, where such maturation occurs during the first few days following birth.
Figure 5. The AOB of certain domestic mammals. Parasagittal sections of the AOB of dog (ca) (A), horse (eq) (B,G), pig (su) (C,H), 147-days-old sheep (ov) fetus (D), sheep (ov) (E,I,J), and 108-days-old pig (su) fetus (F) stained with haematoxylin-eosin (A,B), Nissl cresyl violet (C–F) and labeled by lectins against LEA (G) and UEA-I (H), and immunolabeled by Gi2α (I) and Go (J). The inset in (J) shows the labeling in the main bulb. LEA, Lycopersicum esculentum agglutinin; UEA-I Ulex europaeus agglutinin I. (Salazar and Sánchez-Quinteiro, 2003
; Salazar et al.,2000
, 2006
). Scale bars = 250 μm (A,C,F), 1 mm (B,E,G), 500 μm (D,H–J).
In our experience, lectins are excellent markers of olfactory systems, although their expression is far from uniform. Nevertheless, the behaviour of two lectins, namely Lycopersicum esculentum agglutinin (LEA) and Ulex europaeus agglutinin I (UEA-I), is similar not only among all DMs but also in mice. Interestingly, UEA-I may be a specific VNS marker. The exception to this general trend is represented by the sensory epithelium of the VNd and AOB in sheep and horses. These structures are not stained by UEA-I. Other markers usually employed in the study of the VNS, such as the proteins Go and Gi2α are present in both mice and DMs in the case of Gi2α, whereas G0 is positive in mice and negative in DMs. Nevertheless, it should be quoted out that according to the protocol that we usually follows, in DMs it is not possible to define the zonal organisation VNO-AOB typical in mice and in certain other mammals.
These and similar issues concerning the general characteristics of the VNO and AOB have been previously discussed elsewhere (Salazar and Sánchez-Quinteiro, 2003
; Salazar et al., 1992
, 2000
, 2001
, 2004
, 2006
, 2007
). On these papers is described the corresponding methodology to get most of the pictures includes in Figures 3
, 4 and 5
. The pictures belonging to the cat, horse and some of the dog (unpublished), have been obtained following an identical protocol.
Based on morphological evidence, we suggest caution in extrapolating data regarding the VNO and AOB from one mammalian species to other, and particularly when drawing correlations between humans and mice. Between these two extreme cases, there are intermediate models, including, for example those represented by DMs. The fact that the AOB is fully developed some time before birth in some species such sheep and pigs indicates several interesting evolutionary adaptations. Importantly, the AOB undergoes a process of regression or involution, and, in the course of evolution, the VNO and the VNrs started to project into the MOB instead of into AOB. This would suggest that the VNS is a true olfactory subsystem, similar to the SO and the GgG. In other words, all of the different populations of olfactory receptors distributed throughout the nasal cavity should project into the MOB. This is most likely the case with the VNrs of primates including humans, in which receptors are located at the same place in where a putative VNO was initially existed, namely an area that is located similarly to the VNO of mice.
This idea is corroborated by evidence that both systems, namely the MOS and VNS, can recognise chemical signals such as pheromones. Nevertheless, pheromones are species-specific signals, and it is risky to extrapolate pheromonal observations from mice to other mammals (Brennan and Zufall, 2006
). For example, such observations may not be universal because different species have different reproductive strategies (Keverne, 2005
).
To resolve problems in modern biology it is necessary to turn to a combination of the molecular anatomy, as suggested by Jacob (1981)
, and genetic methods. Recent publications have emphasised about the diversity of olfactory (Ache and Young, 2005
) and vomeronasal (Grus et al., 2005
) receptors. In certain mammals the vomeronasal receptors are encoded by the VR1 and VR2 genes superfamilies, which are expressed in vomeronasal sensory neurones in the apical and basal part of the epithelium respectively. Interestingly, cells related to VR1 express Gi2α, while those related to VR2 express Go.
Researchers have demonstrated that many species exhibit a large number of olfactory receptor pseudogenes, in addition to functional olfactory receptors. Many of these were once functional genes that may have since suffered inactivating mutations (Ache and Young, 2005
), similar to morphological structure regressions.
Finally, from an evolutionary point of view it is important to stress that while the VNS, as a morphological entity, is exclusively found in some tetrapodes, its genetic components are found in the sea lamprey genome (genes VR1 and Trpc2 expressed in the olfactory organ), and in the elephant shark genome (genes VR1, VR2 and Tpcr2). The first case offers very good evidence to accept that some of the genetic components of the VNS were present in a common ancestor of all extant vertebrates (Grus and Zhang, 2009
).
Increasing our knowledge of the genome of different species will make it easier to secure definitive answers about the evolutionary changes that may have occurred in olfactory systems. Other strategies – mainly electrophysiological recording techniques – will also continue to contribute to our knowledge of this intriguing system.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank N Vandenberghe for helpful comments regarding this manuscript, and to J Castiñeira for technical assistance. This work was supported in part by BFU2004-01004 and PGIDIT05PXIC26103PN. The comments of both reviewers greatly improved the clarity of the manuscript.