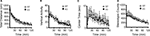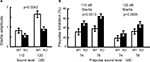1
Department of Biochemistry and Molecular Genetics, RINDG, Kyoto Prefectural University of Medicine, Kyoto, Japan
2
Department of Dermatology, Hyogo College of Medicine, Nishinomiya, Japan
3
Horizontal Medical Research Organization, Graduate School of Medicine, Kyoto University, Kyoto, Japan
4
Division of Systems Medical Science, Institute for Comprehensive Medical Science, Fujita Health University, Toyoake, Japan
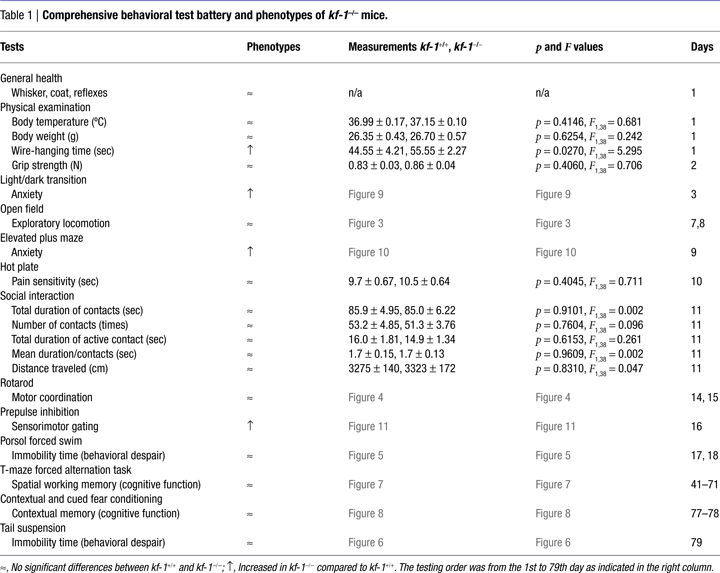
KF-1 was originally identified as a protein encoded by human gene with increased expression in the cerebral cortex of a patient with Alzheimer’s disease. In mouse brain, kf-1 mRNA is detected predominantly in the hippocampus and cerebellum, and kf-1 gene expression is elevated also in the frontal cortex of rats after chronic antidepressant treatments. KF-1 mediates E2-dependent ubiquitination and may modulate cellular protein levels as an E3 ubiquitin ligase, though its target proteins are not yet identified. To elucidate the role of kf-1 in the central nervous system, we generated kf-1 knockout mice by gene targeting, using Cre-lox recombination. The resulting kf-1−/− mice were normal and healthy in appearance. Behavioral analyses revealed that kf-1−/− mice showed significantly increased anxiety-like behavior compared with kf-1+/+ littermates in the light/dark transition and elevated plus maze tests; however, no significant differences were observed in exploratory locomotion using the open field test or in behavioral despair using the forced swim and tail suspension tests. These observations suggest that KF-1 suppresses selectively anxiety under physiological conditions probably through modulating protein levels of its unknown target(s). Interestingly, kf-1−/− mice exhibited significantly increased prepulse inhibition, which is usually reduced in human schizophrenic patients. Thus, the kf-1−/− mice provide a novel animal model for elucidating molecular mechanisms of psychiatric diseases such as anxiety/depression, and may be useful for screening novel anxiolytic/antidepressant compounds.
In the 1990s, a comprehensive search was conducted for the genes responsible for Alzheimer’s disease (AD). A number of dominantly inherited causative mutations were then discovered in the genes encoding β-amyloid precursor protein, presenilin-1 (PS1), and presenilin-2 (PS2) (for reviews, see Hashimoto-Gotoh et al., 2006
; Lundkvist and Näslund, 2007
). At the same time, we also sought to identify genes that were involved in AD pathogenesis, and we identified two genes whose transcripts were found more frequently in the cerebral cortex of an AD patient than in a non-AD subject. One of these genes, kf-1, was a novel gene that encoded a zinc-binding membrane protein with a RING-H2 finger domain at the C-terminus (Yasojima et al., 1997
). The kf-1 gene is conserved in chordates/vertebrates but not in invertebrates such as Drosophila melanogaster. Control of the half-life of kf-1 mRNA may be critical for its biological function, since nucleotide sequences in the 3′-untranslated region, consisting entirely of a stable two-fold-sheet structure, are highly conserved to an unusual extent between humans and mice. This may suggest that the level of KF-1 protein synthesis is regulated strictly in a cell. Northern blot analysis indicates that the kf-1 gene is expressed most prominently in the brain but more or less throughout the body, except in the liver (Yasojima et al., 1997
). KF-1 mediates E2-dependent ubiquitination, and thus may play a role in modulating cellular protein levels as an E3 ubiquitin ligase (Lorick et al., 1999
). Expression of the kf-1 gene is also elevated in the frontal cortex and hippocampus, but not hypothalamus, of rats after chronic antidepressant treatments such as the administration of a selective serotonin re-uptake inhibitor (SSRI) (Yamada et al., 2000
), electroconvulsive therapy (Nishioka et al., 2003
), and repetitive transcranial magnetic stimulation (Kudo et al., 2005
). Concerning a possible link of the kf-1 gene expression to other neurodegenerative or psychiatric diseases, we are not aware of any reports appearing in the NIH databases (PubMed and GEO profiles). It is known that depression can be a heralding symptom or prodrome rather than a separate and distinct disorder for AD or dementia (for a review, see Katz, 1998
). In fact, it has been reported that depression can occur early in the course of AD in ps1 mutation carriers unaware of their genetic status (Ringman et al., 2004
). Taken together, these observations suggest that KF-1 plays some role or roles in the regulation of mental disorders such as depression, which include anxiety and mood disorders as the core symptoms.
Anxiety is an instinct that may have been developed in vertebrates to promote adaptive survival by evasion of unnecessary danger. However, excessive anxiety is disadvantageous, because it reduces even behavioral activity that is necessary for adaptation. Thus, there must be underlying mechanisms that have developed as to appropriately regulate anxiety in response to environmental circumstances. For example, the amygdala is thought to be important in the expression of anxiety or fear, and the medial prefrontal cortex plays a key role for fear extinction by regulating amygdala-mediated expression of fear (for a review, see Bishop, 2007
).
All the antidepressant drugs developed so far are based on the monoamine hypothesis, which assumes that the pathogenesis of depression could be caused by depletion of monoamines like serotonin in the brain, although some recent approaches to the treatment of depression try to move beyond this hypothesis (Norman, 2006
). Nevertheless, there is no convincing evidence of a primary dysfunction of a specific monoamine system in depression; on the contrary, monoamine depletion does not worsen depression in patients and does not cause depression in healthy volunteers. Testing systems to measure behavioral despair have been developed in rats/mice for screening antidepressant compounds. These model systems include the forced swim test and the tail suspension test, and compounds that elevate monoamine levels in the brain have been found to reduce the immobility time of animals under these conditions of fear and despair (Porsolt et al., 1978
; Steru et al., 1985
). It is worth noting that in humans, approximately one-third of patients with depression do not respond to the drugs developed using these tests. To develop drugs to help patients who are unresponsive to currently available antidepressants, it is therefore desirable to have an animal model that mimics the core symptoms of depression, but that does not involve regulation of monoamine levels in the brain.
In the present study, we conducted a comprehensive behavioral test battery on the mice lacking KF-1 and found that kf-1−/− mice showed decreased stay time in and number of transitions to the light box in the light/dark transition test, decreased locomotor activity in the elevated plus maze test, and increased immediate freezing in the fear conditioning test, indicating overall increased anxiety-like behavior under stressful situations in these mutant mice. No significant cognitive abnormalities were found in the fear conditioning test and in the forced alternation test using the automatic T-maze. Based on the effect of KF-1 depletion on these anxiety-like behaviors, we propose that kf-1−/− mice may suppress selectively anxiety under physiological conditions, and be useful for both investigating the molecular mechanisms of anxiety/depression and screening novel anxiolytic/antidepressant compounds.
Targeting Vector
Mouse kf-1 genomic clones were isolated from a 129/Sv λ Fix III genomic DNA library (Stratagene) using mouse kf-1 cDNA as a hybridization probe. Gene fragments were subcloned into pKF4 and pHSG396 for further analysis and manipulation (Hashimoto-Gotoh, 1995
; Takeshita et al., 1987
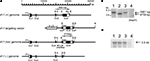
). The essential portion of the mouse kf-1 gene was sequenced, including all four exons (Ex) and three introns (In). The DNA segment used to construct the targeting vector was ∽14.7 kb long and was downstream of the AseI site at position 15,600 in In 3. The position numbers for restriction sites are based on the GenBank database (Accession no. AB012161). Schematic representations of the mouse kf-1 gene, targeting vector, and recombinant and deletion alleles are shown in Figure 1
A. To delete the critical coding region, i.e., Ex 4, a conditional knockout (KO) procedure using Cre-lox recombination was employed (Sakai and Miyazaki, 1997
). The resulting targeting DNA was cloned into pHSG396, yielding pHSG396mkf-1Ex4.
Figure 1. Schematic presentation of the mouse kf-1 gene structure, targeting vector, and floxed and knockout alleles. (A) For construction of the targeting vector, the diphtheria toxin A (DT-A) and neomycin resistance (neo) genes were inserted in reverse orientation at AseI and StuI sites at positions 15,600 and 17,515, respectively, and loxP cassettes were inserted using StuI and BglII sites at positions 17,515 in In 3 and 20,081 in Ex 4, respectively. Closed and open boxes indicate the approximate positions of coding and non-coding exons, respectively. P with an arrowhead indicates the 49-bp promoter region identified by the CAT assay (data not shown). BspHI restriction fragments, 5,861 and 4,759 bp in size, are indicated by open bidirectional-arrows above the kf-1 (+) and kf-1 (lox) genomes, respectively. The position corresponding to the 2,428-bp PCR product used to verify homologous recombination between the targeting vector and the kf-1 gene is shown below the kf-1 (lox) genome. Restriction sites for AseI, BspHI, BglII, and StuI were shown by A, B, Bg, and S, respectively. (B) Southern blot of BspHI-digested genomic DNA using the mouse kf-1 probe. The DNA bands indicated by arrows (5,861 and 4,759 bp) correspond to the BspHI-fragments shown in (A). Lanes 1, 2, 3, and 4 are kf-1+/+, kf-1lox/lox, kf-1+/−, and kf-1−/−, respectively. Note that the intensity of the DNA band in lane 3 is half as intense as in lane 1. (C) Northern blot analysis of kf-1 gene expression. Experimental procedures are as described in Sections ‘Targeting vector’ and ‘Selection of targeted ES clones and generation of kf-1−/− mice’. Lanes are as the same in (B).
Selection of Targeted ES Clones and Generation of kf-1 −/− Mice
The selection of targeted ES cells (line R1; Nagy et al., 1993
) and generation of kf-1−/− mice were done essentially as described previously (Matsuki et al., 1998
). Briefly, ten million ES cells were transfected by electroporation with 25 μg of NotI-linearized pHSG396mkf-1Ex4 DNA. After selection with 150 μg/ml of neomycin, 432 clones were analyzed, among which five clones were positive, giving a 2,428-bp PCR product using the forward primer: 5′-CCCTGTCTACCGTTTCTACCCCT-3′ and the reverse primer: 5′-GAATGGGCTGACGCTTCCTCGTG 3′ (Figure 1
A). One of the clones was expanded and microinjected into BDF1 blastocysts. Male chimeras derived from the clone were mated with C57BL/6N females. Germ-line transmission of the targeted recombination was verified by Southern blot analysis of tail DNA against 32P-labeled mouse kf-1 DNA of the Ex 4 coding region. The resulting kf-1+/lox male mice were mated with CAG (Cytomegalovirus immediate early enhancer + chicken β-Actin hybrid promoter + rabbit β-Globin polyA signal containing region) promoter-driven Cre gene containing transgenic female mice (Sakai and Miyazaki, 1997
). F1 heterozygotes, kf-1+/−, were then crossed to C57BL/6N, and the resulting F2 heterozygotes were backcrossed to C57BL/6N. This was repeated for eight generations, and then male and female F9 heterozygotes were intercrossed to generate a homozygous kf-1−/− line. The lack of kf-1 gene was verified by Southern hybridization of BspHI-digested tail DNA using the same probe as above (Figure 1
B). The absence of kf-1 mRNA was confirmed by Northern blot analysis using 10 μg of total RNA from the whole-brain against the same probe (Figure 1
C). The kf-1−/− mouse, called the Hicky mouse, may be obtained from the Riken BioResource Center, Japan (BRC No. 01916).
Animals and Experimental Design
Prior to behavioral studies, kf-1−/− males obtained from the crossing between male and female F9 heterozygotes were mated with C57BL/6N females (kf-1+/+). The resulting heterozygotes were then intercrossed to produce homozygous kf-1+/+ and kf-1−/− littermates. The behavioral tests were performed with male mice of this generation, which were 10 weeks old at the start of the behavioral studies (n = 20 for both groups). Mice were reorganized as soon as they were genotyped at the time of weaning, and group-housed with four mice per cage, two kf-1+/+ and two kf-1−/− littermates. Housing conditions included a 12-h light/dark cycle, with lights on at 7:00 a.m. and access to food and water ad libitum. Behavioral testing was performed between 9:00 a.m. and 6:00 p.m., excepting for that of T-maze forced alternation task that was performed between 7:00 p.m. and 5:00 a.m. After the tests, all apparatus were cleaned with super hypochlorous water to prevent a bias based on olfactory cues with the apparatus. The order of each testing is as indicated in Table 1
. All animal experiments, including the production and maintenance protocols, were reviewed and approved by the Animal Care and Use Committee of the Kyoto Prefectural University of Medicine. Behavioral studies were performed in accordance with the guidelines for animal experimentation of Graduate School of Medicine, Kyoto University.
General Health and Neurological Screen
To compare the physical characteristics of kf-1−/− mice and their kf-1+/+ littermates, we first conducted the neurological screening as previously described (Miyakawa et al., 2001
). The righting, whiskers touch, and ear twitch reflexes were evaluated, and a number of physical features including body weight and temperature, and the presence of whiskers or bald hair patches were recorded.
Neuromuscular Examination
The neuromuscular strength was examined by the grip strength and wire-hanging tests. The grip strength meter (O’Hara & Co.) was used to assess forelimb grip strength. Mice were lifted and held by their tail so that their forepaws could grasp a wire grid. Mice were then gently pulled backward by the tail with their posture parallel to the surface of the table until they released the grid. The peak force applied by mouse forelimbs was recorded in newtons (N). Each mouse was tested three times and the greatest value measured was used for statistical analysis. In the wire-hanging test, mice were placed on a wire mesh, which was then inverted and waved gently, so that the subject gripped the wire. Latency to fall was recorded, with a 60 s cut-off time.
Light/Dark Transition Test
The apparatus used for light/dark transition test consisted of a cage (21 cm × 42 cm × 25 cm) divided into two sections of equal size by a partition with door (O’Hara & Co., Tokyo). One chamber was brightly illuminated (390 lx), whereas the other chamber was dark (2 lx). Mice were placed into the dark side, and allowed to move freely between the two chambers with door open for 10 min. The total number of transitions, time spent in each compartment, first latency to light side, and distance traveled were recorded automatically. On-line material describing this method is available visually (Takao and Miyakawa, 2006
).
Open Field Test
Locomotor activity was measured using the open field test. Each subject was placed in the center of open field apparatus (40 cm × 40 cm × 30 cm; Accuscan Instruments, Columbus, OH, USA). Total distance traveled (centimeters), vertical activity (rearing measured by counting the number of photobeam interruptions), time spent in the center, the beam-break counts for stereotypic behaviors, and the number of fecal boli were recorded. Data were collected for 120 min. Photo beams were positioned 1 cm inside apart from walls at the edge of the open field. If any of these beams was not interrupted, mice were considered to be in the center area.
Elevated Plus Maze Test
The elevated plus-maze consisted of two open arms (25 cm × 5 cm) and two enclosed arms of the same size, with 15 cm high transparent walls. The arms and central square were made of white plastic plates, and were elevated to a height of 55 cm above the floor. In order to minimize the likelihood of animals falling from the apparatus, 3-mm high plexiglas ledges were provided for the open arms. Arms of the same type were arranged at opposite sides to each other. Each mouse was placed in the central square of the maze (5 cm × 5 cm), facing one of the closed arms. Mouse behavior was recorded during a 10 min test period. The number of entries into, and the time spent on open and enclosed arms were recorded. For data analysis, we employed the following four measures: the percentage of entries onto open arms, the stay time on open arms (seconds), the number of total entries, and total distance traveled (centimeters). Data acquisition and analysis were performed automatically, using Image EP software.
Hot Plate Test
The hot plate test was used to evaluate the nociception or the sensitivity to a painful stimulus. Mice were placed on a hot plate at 55.0 ± 0.3°C (Columbus Instruments, Columbus, OH, USA), and latency to the first hind-paw response was recorded. The hind-paw response was either a foot shake or a paw lick.
Social Interaction Test in a Novel Environment
Two mice of identical genotypes, which were previously housed in different cages, were placed into a box together (40 cm × 40 cm × 30 cm) and allowed to explore freely for 10 min. Social behavior was monitored by a CCD camera, which was connected to a Macintosh computer. Analysis was performed automatically using Image SI software. Number of contacts, duration of contacts, and total distance traveled were measured.
Rotarod Test
Motor coordination and balance were tested with the rotarod test. We performed the rotarod test using an accelerating rotarod (UGO Basile Accelerating Rotarod) by placing a mouse on a rotating drum (3 cm diameter) and measuring the time during which each animal was able to maintain its balance on the rod as latency time to fall (seconds). Speed of the rotarod was accelerated from 4 to 40 rpm over a 5-min period.
Startle Response/Prepulse Inhibition Test
A startle reflex measurement system was used (O’Hara & Co., Tokyo). A test session began by placing a mouse in a plexiglas cylinder where it was left undisturbed for 10 min. The duration of white noise that was used as the startle stimulus was 40 ms for all trial types. The startle response was recorded for 140 ms (measuring the response every 1 ms) starting with the onset of the prepulse stimulus. The background noise level in each chamber was 70 dB. The peak startle amplitude recorded during the 140 ms sampling window was used as the dependent variable. A test session consisted of six trial types [i.e., two types for ‘startle-stimulus-only’ trials, and four types for prepulse inhibition (PPI) trials]. The intensity of startle stimulus was 110 or 120 dB. The prepulse sound was presented 100 ms before the startle stimulus, and its intensity was 74 or 78 dB. Four combinations of prepulse and startle stimuli were employed (74–110, 78–110, 74–120, and 78–120 dB). Six blocks of the six trial types were presented in pseudo-random order such that each trial type was presented once within a block. The average inter-trial interval was 15 s (range: 10–20 s).
Porsolt Forced Swim Test
The Porsolt forced swim test was done essentially as described previously (Miyakawa et al., 2001
). The apparatus consisted of four plexiglas cylinders (20 cm height × 10 cm diameter). The cylinders were filled with water at 23°C, up to a height of 7.5 cm. Mice were placed into the cylinders, and their behavior was recorded over a 10-min test period (days 1 and 2). Data acquisition and analysis were performed automatically, using Image PS software (see Section ‘Image analysis’).
T-Maze Forced Alternation Task Test
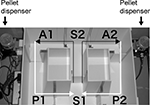
The forced alternation task test was conducted using an automatic T-maze (Figure 2
). It was constructed of white plastics runways with walls 25-cm high. The maze was partitioned off into six areas by sliding doors that can be opened downward. The stem of T was composed of area S2 (13 cm × 24 cm) and the arms of T were composed of area A1 and A2 (11.5 cm × 20.5 cm). Area P1 and P2 were the connecting passage way from the arm (area A1 or A2) to the start compartment (area S1). The end of each arm was equipped with a pellet dispenser that could provide food reward. The pellet sensors were able to record automatically pellet intake by the mice. One week before the pre-training, mice were deprived of food until their body weight was reduced to 80–85% of the initial level. Mice were kept on a maintenance diet throughout the course of all the T-maze experiments. Before the first trial, mice were subjected to three 10-min adaptation sessions, during which they were allowed to freely explore the T-maze with all doors open and both arms baited with food. On the day after the adaptation session, mice were subjected to a forced alternation protocol for 16 days (one session consisting of 10 trials per day; cutoff time, 50 min). Mice were given 10 pairs of training trials per day. On the first (sample) trial of each pair, the mouse was forced to choose one of the arms of the T (area A1 or A2), and received the reward at the end of the arm. Choosing the incorrect arm resulted in no reward and confinement to the arm for 10 s. After the mouse consumed the pellet or the mouse stayed >10 s without consuming the pellet, the door that separated the arm (area A1 or A2) and connecting passage way (area P1 or P2) would be opened and the mouse could return to the starting compartment (area S1), via connecting passage way. The mouse was then given 3 s delay there and a free choice between both T arms and rewarded for choosing the other arm that was not chosen on the first trial of the pair. The location of the sample arm (left or right) was varied pseudo-randomly across trials using Gellermann schedule so that mice received equal numbers of left and right presentations. A variety of fixed extra-maze cues surrounded the apparatus. On the 17th to 20th day, delay (10, 30, or 60 s) was applied after the sample trial.
Figure 2. Apparatus of T-maze forced alternation task test.
Contextual and Cued Fear Conditioning
Each mouse was placed in a test chamber (26 cm × 34 cm × 29 cm) inside a sound-attenuated chamber (O’Hara & Co., Tokyo) and allowed to explore freely for 2 min. A 60 dB white noise, which served as the conditioned stimulus (CS), was presented for 30 s, followed by a mild (2 s, 0.5 mA) foot shock, which served as the unconditioned stimulus (US). Two more CS–US pairings were presented with 2 min inter-stimulus interval. To examine shock sensitivity, we measured distance traveled when the foot shock were delivered sensitivity (from 2 s before the shock to 2 s after the shock, total 6 s). Context testing was conducted 1 day, 1 week, and 1 month after conditioning in the same chamber. Cued testing with altered context was conducted 1 day after conditioning using a triangular box (35 cm × 35 cm × 40 cm) made of white opaque plexiglas, which was located in a different room. Data acquisition, control of stimuli (i.e., tones and shocks), and data analysis were performed automatically, using Image FZ software. Images were captured at one frame per second. For each pair of successive frames, the amount of area (pixels) by which the mouse moved was measured. When this area was below a certain threshold (i.e., 20 pixels), the behavior was judged as ‘freezing’. When the amount of area equaled or exceeded the threshold, the behavior was considered as ‘non-freezing’. The optimal threshold (amount of pixels) to judge freezing was determined by adjusting it to the amount of freezing measured by human observation. ‘Freezing’ that lasted less than the defined time threshold (i.e., 2 s) was not included in the analysis.
Tail Suspension Test
The tail suspension test was performed for a 10-min test session according to the procedures described by Steru et al. (1985)
. Mice were suspended 30 cm above the floor in a visually isolated area by adhesive tape placed ∽1 cm from the tip of the tail, and their behavior was recorded over a 10-min test period. Data acquisition and analysis were performed automatically, using Image TS software (see Section ‘Image analysis’).
Image Analysis
The applications used for the behavioral studies (Image LD, Image EP, Image TM, Image FZ, Image SI, Image TS, and ImageHA) were based on the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/
) and ImageJ program (http://rsb.info.nih.gov/ij/
), which were modified for each test by Miyakawa (available through O’Hara & Co., Tokyo, Japan).
Statistical Analysis
Statistical analysis was conducted using Stat View (SAS institute). Data were analyzed by two-way ANOVA, or two-way repeated measures ANOVA, unless noted otherwise. Values in tables and graphs were expressed as mean ± SEM.
Structure of the Mouse kf-1 Gene and Generation of kf-1−/− Mice
Initially, we analyzed the human kf-1 gene for polymorphisms and found a G/A transition at the −13 position before the initiation codon in one of 10 subjects. Subsequently, we analyzed the mouse kf-1 gene using four independent mouse kf-1 genomic clones covering an ∽30.3-kb genomic region (see Materials and Methods). The mouse kf-1 gene lies within an ∽18 kb segment containing four exons. Ex 4, which codes for the 522 C-terminal amino acids of the 683 amino acid KF-1 protein, was chosen as a target for deletion (Figure 1
A). Ex 4 includes the coding sequences for the transmembrane regions, the ubiquitination site, and the zinc-binding RING-H2 finger domain. F1 heterozygotes, kf-1+/−, were obtained as described in Materials and Methods. After repeated backcrossing to C57BL/6N, the F9 heterozygotes kf-1+/− mice were intercrossed and kf-1+/+, kf-1+/−, and kf-1−/− mice were produced with the predicted Mendelian ratio, implying that there was no embryonic lethal effect due to the lack of KF-1 in mice. KF-1 was not detectable even in kf-1+/+ mice using immunohistochemistry or Western blotting due to the low level of KF-1 protein synthesis and also due to KF-1 ubiquitination, although the anti-mKF-1 antibodies we used could detect mouse KF-1 that was exogenously expressed in COS cells. Similar to PS1 and PS2, immunostaining showed that exogenous mouse KF-1 protein was localized to the endoplasmic reticulum membranes and to Golgi bodies (data not shown). For the success of immunohistochemistry against native KF-1 protein, one might need monoclonal antibodies with higher and/or more specific affinity.
Effect of the kf-1−/− Genotype on Somatic Parameters and General Behavior
KO mice lacking the kf-1 gene appeared normal and healthy; there were no evident abnormalities in the physical aspects of kf-1 KO mice, including reproductive capability. We then measured body temperature and weight, as well as grip strength and wire-hanging time. There were no significant differences in these measurements between kf-1+/+ and kf-1−/− littermate mice, excepting for wire-hanging time, namely the kf-1−/− mice exhibited significantly longer wire-hanging time than their kf-1+/+ littermates (Table 1
). Because there were no significant differences in grip strength and body weight between kf-1+/+ and kf-1−/− mice, differences in wire-hanging time did not seem to be rooted in physical differences; instead, this disparity could be a result of psychological differences. In addition, no significant differences were observed in locomotion (Figures 3
A–D), nociception (Table 1
), social behavior (Table 1
), motor coordination (Figure 4
), behavioral despair (Figures 5
A,B and 6
), or in spatial working memory (Figure 7
A). Concerning cognitive functions, no significant differences were found in the T-maze forced alternation task test (Figures 7
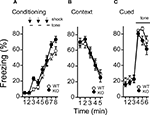
A,B) and in context testing or in cued testing with an altered context between kf-1+/+ and kf-1−/−, although immediate freezing was significantly enhanced in the kf-1−/− mice (Figures 8
A–C).
Figure 3. No significant differences between kf-1+/+ and kf-1−/− mice in open field test. The kf-1+/+ and kf-1−/− mice were subjected to the open-field test. (A) Total locomotion distance (p = 0.2879, F1,38 = 1.162). (B) Count of vertical activity (p = 0.7164, F1,38 = 0.134). (C) Time spent in the center of the compartment (p = 0.3930, F1,38 = 0.747). (D) Count of stereotypic behavior (p = 0.4435, F1,38 = 0.600) (see Section ‘Open field test’). WT and KO stand for kf-1+/+ and kf-1−/− mice, respectively.
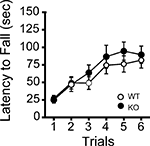
Figure 4. Normal motor coordination/learning of kf-1+/+ and kf-1−/− mice in the rotarod test. The kf-1+/+ and kf-1−/− mice were tested on an accelerating rotarod and no significant differences were found between the two genotypes (p = 0.5108, F1,38 = 0.441) (see Section ‘Rotarod test’). WT and KO stand for kf-1+/+ and kf-1−/− mice, respectively.
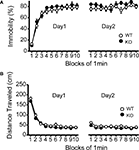
Figure 5. No significant differences in despair-like behavior between kf-1+/+ and kf-1−/− mice in Porsolt forced swim test. (A) Immobility time (%) in each block for day 1 (p = 0.3525, F1,38 = 0.886) and day 2 (p = 0.1145, F1,38 = 2.610). (B) Total distance (centimeters) traveled in each block for day 1 (p = 0.1304, F1,38 = 2.390) and day 2 (p = 0.0435, F1,38 = 4.362). No significant differences were found between kf-1+/+ and kf-1−/− mice, although significant reduction of distance traveled was barely observed in kf-1−/− mice compared to kf-1+/+ littermates in day 2 (see Section ‘Porsolt forced swim test’). WT and KO stand for kf-1+/+ and kf-1−/− mice, respectively.
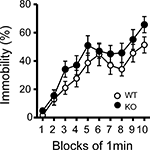
Figure 6. Immobility in the tail suspension assay. Immobility time is presented as % time in each block. There was no significant differences between kf-1+/+ and kf-1−/− in immobility in the tail suspension test (p = 0.0855, F1,38 = 3.118) (see Section ‘Tail Suspension Test’). WT and KO stand for kf-1+/+ and kf-1−/− mice, respectively.
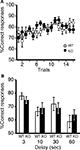
Figure 7. Spatial working memory in T-maze forced alternation task test. (A) No significant differences were observed in spatial working memory between kf-1+/+ and kf-1−/− mice as presented by % correct responses (p = 0.6146, F1,36 = 0.258). (B) On the 17th to 20th trials, the delay was applied after sample trial for 3 s (p = 0.3829, F1,36 = 0.779), 10 s (p = 0.6371, F1,36 = 0.226), 30 s (p = 0.6267, F1,36 = 0.241), or 60 s (p = 0.4110, F1,36 = 0.691). Data are given as mean (±SEM; see Section ‘T-maze forced alternation task test’). WT and KO stand for kf-1+/+ and kf-1−/− mice, respectively.
Figure 8. Levels of freezing in kf-1−/− mice during conditioning, cued testing with altered context, and context testing. (A) Immediate freezing during conditioning phase (p = 0.0211, F1,38 = 5.788). (B) Contextual testing conducted 1 day after conditioning (p = 0.9891, F1,38 = 0.0002). (C) Cued test with altered context (p = 0.2352, F1,38 = 1.455) and pre-tone period (p = 0.0774, F1,38 = 3.294) are shown. There were no significant differences observed between kf-1+/+ and kf-1−/− mice in contextual and cued fear conditioning test, although significant difference was observed in the immediate freezing without conditioning (see Section ‘Contextual and cued fear conditioning’). WT and KO stand for kf-1+/+ and kf-1−/− mice, respectively.
Light/Dark Transition and Elevated Plus Maze Tests: Increased Anxiety in kf-1−/− Mice
In the light/dark transition test, kf-1−/− mice traveled significantly shorter distances than kf-1+/+ mice in the light but not in the dark (Figure 9
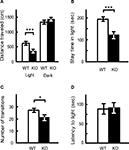
A; p = 0.0005, F1,38 = 14.482 and p = 0.1471, F1,38 = 2.190, respectively), and their stay time in the light was reduced significantly as well (Figure 9
B; p = 0.0004, F1,38 = 15.280). The number of transitions between light and dark was also decreased significantly in kf-1−/− mice (Figure 9
C; p = 0.0392, F1,38 = 4.561). As can be seen in Figures 9
A,B, the distance traveled and stay time of kf-1−/− mice were reduced in parallel against those of kf-1+/+ mice in the light, implying that mice had equivalent moving capability per second between the two genotypes. These results suggest an elevated level of anxiety, but not a reduced level of physical capability, specific to light conditions in kf-1−/− mice, although they did not show an increased photophobic response as observed in the latency time for first entering the light compartment from the dark (Figure 9
D; p = 0.8986, F1,38 = 0.016).
Figure 9. Increased anxiety-like behavior in kf-1−/− mice in the light/dark transition test compared to kf-1+/+ littermates. Mice were placed individually in the dark compartment at the start of the test. Data are given as mean (±SEM). (A) Distance traveled (centimeters) in the light and dark sides. (B) Time (seconds) the mice stayed in the light side. (C) Number of transitions between the light and dark sides. (D) Latency time (seconds) before the first entry into the light side. *p < 0.05; ***p < 0.0005 (see Section ‘Light/dark transition test’). WT and KO stand for kf-1+/+ and kf-1−/− mice, respectively.
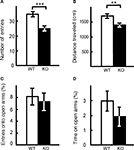
The results of elevated plus maze test were similar. The number of entries into the crossing center from open and closed arms, and the total distance traveled, were decreased significantly in kf-1−/− mice compared to their kf-1+/+ littermates (Figures 10
A,B; p = 0.0005, F1,38 = 14.758 and p = 0.0016, F1,38 = 11.555, respectively), suggesting increased anxiety in kf-1−/− mice under stressful situation on heights. However, no significant differences were observed in % number of entries into and % stay time on the open arms between the two genotypes as observed in Figures 10
C,D (p = 0.6548, F1,38 = 0.203 and p = 0.2490, F1,38 = 1.371, respectively). No significant differences in these parameters may be due to a floor effect. Some mice did not visit in fact the open arms at all in both the genotypes, 3 and 5 mice in kf-1+/+ and kf-1−/−, respectively. Alternatively, the discrepancy between the two tests (Figures 9
A–C vs. Figures 10
C,D) may reflect the different aspect of anxiety-like behaviors (see also Discussion).
Figure 10. Performance of kf-1−/− mice in the elevated plus maze test. At the start of the test, mice were placed individually in the crossing center from open and closed arms. Data are given as mean (±SEM). (A) Number of entries into the center crossing between open and closed arms. (B) Total distance (centimeters) traveled. (C) % Number of entries into open arms. (D) % Time spent on open arms. Note that the closed arms were enclosed at both sides by transparent walls, and therefore mice could recognize visibly their position on heights even on the closed arms. **p < 0.005; ***p < 0.0005 (see Section ‘Elevated plus maze test’). WT and KO stand for kf-1+/+ and kf-1−/− mice, respectively.
Prepulse Inhibition Test: Increased Sensorimotor Gating
The PPI test is used widely to measure deficits in information-processing abilities or sensorimotor gating in schizophrenic patients (Geyer and Ellenbroek, 2003
), and can be employed in both human and animal experiments (Arguello and Gogos, 2006
). PPI is defined as the degree (%) to which the acoustic startle response is reduced when the startle-eliciting stimulus is preceded by a brief, low-intensity, non-eliciting stimulus, and can be used in both human and animal experiments. The kf-1−/− mice had lower startle amplitudes than their kf-1+/+ littermates at either 110 or 120 dB in ‘startle-stimulus-only’ trials for all stimulus amplitudes (Figure 11
A; p = 0.0043, F1,38 = 9.226). Interestingly, the percent PPI, an index of sensorimotor gating, was significantly greater in kf-1−/− than in kf-1+/+ (Figure 11
B; overall ANOVA including all four conditions, p = 0.0023, F1,38 = 10.676). Although a significant difference between genotypes was not observed when the startle stimulus intensity was 120 dB (p = 0.0935, F1,38 = 2.960), the difference between genotypes was significant at the startle stimulus intensity of 110 dB (p = 0.0013, F1,38 = 11.973). The reduced PPI values at 120 dB than those at 110 dB may be a ceiling effect caused by the strong intensity of the startle stimulus.
Figure 11. Reduced startle amplitude and increased prepulse inhibition in kf-1−/− mice. (A) Startle amplitude was significantly reduced in ‘startle-stimulus-only’ trials (p = 0.0043). (B) % Prepulse inhibition was significantly increased in kf-1−/− compared to that in kf-1+/+ mice (overall ANOVA including all four conditions, p = 0.0023). Data are given as mean (±SEM; see Section ‘Startle response/prepulse inhibition test’). WT and KO stand for kf-1+/+ and kf-1−/− mice, respectively.
The mouse kf-1 gene is expressed throughout the body, except in the liver; the highest expression is in the brain (Yasojima et al., 1997
). However, the lack of kf-1 gene did not lead to any obvious abnormalities in appearance or in behaviors such as exploratory locomotion, nociception, social behavior, motor coordination, behavioral despair, spatial working memory, and context memory (Table 1
; Figures 3–8
). The exception to this is that kf-1−/− mice displayed a pronounced increase in anxiety-like behavior in the light/dark transition test (Figures 9
A–C). In addition, ‘timidity-like’ responses were observed, under stressful situations, as prolonged wire-hanging time (Table 1
), enhanced immediate freezing with an aversive foot shock (Figure 8
A), and decreased locomotor activity on heights (Figures 10
A,B) in kf-1−/− mice. Since no significant differences were observed between kf-1+/+ and kf-1−/− mice in body weight and grip strength (Table 1
), and in exploratory locomotion using the open field test (Figure 3
A), these behaviors should be rooted in psychological, but not physical, alteration in kf-1−/− mice. These responses are consistent with the increased anxiety-like behavior observed in the light/dark transition test. However, there was no significant difference regarding the stay time index on open arms between kf-1+/+ and kf-1−/− mice in the elevated plus maze test (Figures 10
C,D). How can we explain this apparent discrepancy (Figures 9
A–C and Figures 10
A,B vs. Figures 10
C,D)? In the laboratory of T.M., a comprehensive behavioral test battery has been conducted with identical protocols on >70 different KO and transgenic mice in the past 5 years (Takao et al., 2007
). Although there were several strains of mutant mice that showed increased anxiety-like behavior, kf-1−/− mice were the second greatest mice among them in terms of the size of genotype effect on ‘stay time in light’ index of the light/dark transition test {δ = −1.058, where δ = [(average of mutant stay time in light) − (average of wild type stay time in light)]/(total SD)} (Takao and Miyakawa, data not shown). Considering the large genotype effect observed in the light/dark transition test (Figures 9
A–C), we believe that this apparent discrepancy is due to a floor effect in the elevated plus maze test or to the difference of the kind of anxiety-like behaviors that were assessed by these two tests. By a factor analysis using our large set of data using >5,000 mice, we are aware that these two tests assesses different aspects of anxiety-like behavior, although there are significant correlations between indices in these tests (Yamasaki et al., 2006
). There are also published data indicating that different anxiety tests measure different aspects of anxiety-like behaviors (Holmes et al., 2003a
; Ramos and Mormède, 1998
; Turri et al., 2001
). One but not another assay could provide evidence of altered anxiety-like behavior. To distinguish different anxiety-related behavioral dimensions, kf-1−/− mice would have to be tested further by another anxiety-related behavioral tests including elevated plus maze with opaque walls, square maze, mirror chamber, and so on.
Considering the fact that the level of kf-1 gene expression is augmented in the frontal cortex and hippocampus, but not in the hypothalamus, after chronic antidepressant treatments (Kudo et al., 2005
; Nishioka et al., 2003
; Yamada et al., 2000
), these results suggest that kf-1 gene may play a role(s) in the emotional regulation such as the suppression of anxiety or fear under stressful conditions. In this context, it is worth to note that roles of prefrontal cortex and hippocampus in modulating amygdala activity have been reported to be responsible for adaptive emotional regulation of context-specific retrieval of anxiety or fear extinction (Corcoran et al., 2005
; Sotres-Bayon et al., 2006
). Therefore, our observations suggesting a possible ‘antianxiety’-like effect of KF-1 seem to be consistent with all these reports. Since KF-1 is an E3 ubiquitin ligase to modulate cellular protein level (Lorick et al., 1999
), it appears to be crucial to identify what is (are) the target protein(s) degraded depending on KF-1 ubiquitination in the future studies. Cellular concentration of this putative target protein(s) may have played an important role during the vertebral evolution to determine the anxiety level to defy or evade potential dangers in various environmental circumstances.
The kf-1 gene expression is up-regulated in the frontal cortex of rats treated by chronic SSRI administration (Yamada et al., 2000
). It may be possible that the effect of KF-1 depletion on anxiety-like behavior could be associated with the dysregulation of serotonergic system in these mutant mice. Increased anxiety-like behavior has been reported in mice lacking serotonin-related genes. For example, mice (C57BL/6J) lacking the serotonin receptor gene (5-HT1A) displayed increased ‘anxiety’ levels in the open-field, elevated zero maze, and novel-object tests (Heisler et al., 1998
). Similarly, mice lacking the gene (5-HTT) for serotonin transporter were shown to have increased ‘anxiety’-like behavior in the light/dark transition, elevated plus maze, and open-field tests on the C57BL/6J background (Holmes et al., 2003b
). It has been pointed out that the absence of the 5-HT1A gene during the postnatal developmental period, but not in adult mice, affects anxiety-like behavior (Gross et al., 2002
). It would be necessary, therefore, to determine in the future studies whether the behavioral abnormality of kf-1−/− mice is caused by a developmental impairment by KF-1 depletion or its absence at adulthood.
Concerning the possible association of the kf-1 gene with AD, no significant effects of genotype were observed on memory or cognitive function in the present studies (Figures 7 and 8
). Interestingly, kf-1−/− mice exhibited significantly increased PPI values (Figure 11
), which are usually reduced in human schizophrenic patients (Geyer and Ellenbroek, 2003
). PPI deficit has been reported also in mouse models of schizophrenia (Chen et al., 2008
; Clapcote et al., 2007
; Miyakawa et al., 2003
). Taking these reports into account, agents that can inhibit the expression or function of the kf-1 gene, if any, could possibly ameliorate sensorimotor-gating deficits that are seen in patients of schizophrenia and other psychiatric disorders. Finally, it will be interesting to see whether or not the G/A transition in front of the initiation codon of the human kf-1 gene influences the mRNA translation efficiency. In conclusion, the kf-1−/− mice and cells derived thereof may be useful for elucidating the molecular mechanism of anxiety/depression, and furthermore, the kf-1−/− mice may provide a useful tool for finding novel anxiolytic/antidepressant compounds, which may compensate KF-1 by antagonizing its target protein(s).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank M. Nakagawa for encouragement during this work; J. Miyazaki for providing CAG-Cre mice; T. Matsuo and K. Itoh for help with immunohistochemistry. T. Hashimoto-Gotoh thanks T. Mizuno, K. Hayashi, and C. Gross for valuable discussions and comments. This work was supported by a Grant-in-Aid for Geriatric Research Joint-Projects from Kyoto-fu, and in part by a Grant-in-Aid for Scientific Research on Priority Areas, Integrative Brain Research (Shien) from MEXT in Japan, and by a Grant-in-Aid from the Institute for Bioinformatics Research and Development (BIRD) at the Japan Science and Technology Agency.
Chen, Y. J., Johnson, M. A., Lieberman, M. D., Goodchild, R. E., Schobel, S., Lewandowski, N., Rosoklija, G., Liu, R. C., Gingrich, J. A., Small, S., Moore, H., Dwork, A. J., Talmage, D. A., and Role, L. W. (2008). Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J. Neurosci. 28, 6872–6883.
Clapcote, J. S., Lipina, V. T., Millar, J. K., Mackie, S., Christie, S., Ogawa, F., Lerch, J. P., Trimble, K., Uchiyama, M., Sakuraba, Y., Kaneda, H., Shiroishi, T., Houslay, M. D., Henkelman, R. M., Sled, J. G., Gondo, Y., Porteous, D. J., and Roder, J.C. (2007). Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 54, 387–402.
Hashimoto-Gotoh, T., Tsujimura, A., and Watanabe, Y. (2006). Presenilins: clarification of contradictory observations using molecular and developmental animal models. J. Kyoto Prefect. Univ. Med. 115, 809–825. (Available at: http://www.eonet.ne.jp/~kpum/Presenilin_files/Hashimoto_GotohModel.pdf
).
Holmes, A., Kinney, J. W., Wrenn, C. C., Li, Q., Yang, R. J., Ma, L., Vishwanath, J., Saavedra, M. C., Innerfield, C. E., Jacoby, A. S., Shine, J., Iismaa, T. P., and Crawley, J. N. (2003a). Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology 28, 1031–1044.
Matsuki, M., Yamashita, F., Ishida-Yamamoto, A., Yamada, K., Kinoshita, C., Fushiki, S., Ueda, E., Morishima, Y., Tabata, K., Yasuno, H., Hashida, M., Iizuka, H., Ikawa, M., Okabe, M., Kondoh, G., Kinoshita, T., Takeda, J., and Yamanishi, K. (1998). Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase I (keratinocyte transglutaminase). Proc. Natl. Acad. Sci. USA 95, 1044–1049.
Ringman, J. M., Diaz-Olavarrieta, C., Rodriguez, Y., Chavez, M., Paz, F., Murrell, J., Macias, M. A., Hill, M., and Kawas, C. (2004). Female preclinical presenilin-1 mutation carriers unaware of their genetic status have higher levels of depression than their non-mutation carrying kin. J. Neurol. Neurosurg. Psychiatry 75, 500–502.
Takao, K., and Miyakawa, T. (2006). Light/dark transition test for mice. J. Vis. Exp. (Available at: http://www.jove.com/index/details.stp?ID=104
).
Yamada, M., Yamada, M., Yamazaki, S., Takahashi, K., Nishioka, G., Kudo, K., Ozawa, H., Yamada, S., Kiuchi, Y., Kamijima, K., Higuchi, T., and Momose, K. (2000). Identification of a novel gene with RING-H2 finger motif induced after chronic antidepressant treatment in rat brain. Biochem. Biophys. Res. Commun. 278, 150–157.
Yamasaki, N., Takao, K., Tanda, K., Toyama, K., Takahashi, Y., Yamagata, S., and Miyakawa, T. (2006). Factor analyses of large-scale data justify the behavioral test battery strategy to reveal the functional significances of the genes expressed in the brain. In The 36th Annual Meeting of the Society for Neuroscience, Atlanta, Georgia, USA, October 14–18, 2006. Program number 100.15.
Yasojima, K., Tsujimura, A., Mizuno, T., Shigeyoshi, Y., Inazawa, J., Kikuno, R., Kuma, K., Ohkubo, K., Hosokawa, Y., Ibata, Y., Abe, T., Miyata, T., Matsubara, K., Nakajima, K., and Hashimoto-Gotoh, T. (1997). Cloning of human and mouse cDNAs encoding novel zinc finger proteins expressed in cerebellum and hippocampus. Biochem. Biophys. Res. Commun. 231, 481–487.