Visual target selection and motor planning defi ne attentional enhancement at perceptual processing stages
1
Laboratoire Psychologie de la Perception, CNRS, Université Paris Descartes, Paris, France
2
Biological Psychology and Neuropsychology, Hamburg University, Hamburg, Germany
Extracting information from the visual field can be achieved by covertly orienting attention to different regions, or by making saccades to bring areas of interest onto the fovea. While much research has shown a link between covert attention and saccade preparation, the nature of that link remains a matter of dispute. Covert presaccadic orienting could result from target selection or from planning a motor act toward an object. We examined the contribution of visual target selection and motor preparation to attentional orienting in humans by dissociating these two habitually aligned processes with saccadic adaptation. Adaptation introduces a discrepancy between the visual target evoking a saccade and the motor metrics of that saccade, which, unbeknownst to the participant, brings the eyes to a different spatial location. We examined attentional orienting by recording event-related potentials (ERPs) to task-irrelevant visual probes flashed during saccade preparation at four equidistant locations including the visual target location and the upcoming motor endpoint. ERPs as early as 130–170 ms post-probe were modulated by attention at both the visual target and motor endpoint locations. These results indicate that both target selection and motor preparation determine the focus of spatial attention, resulting in enhanced processing of stimuli at early visual-perceptual stages.
Extracting and processing relevant information from the visual environment is a fundamental aspect of vision. This can be achieved by bringing objects of interest onto the fovea with saccadic eye movements or by covertly directing attention to these objects (Posner 1980
, 1994
). Covert orienting is traditionally defined as the transient focusing of sensory resources on a limited region of the visual world. Behaviorally, this corresponds to improved detection or discrimination rates of events located in the selected region, often accompanied by worsening perception of nearby, non-attended events (Schneider, 1993
; Reynolds and Chelazzi, 2004
). The relationship between overt and covert orienting has long been studied and debated. Perceptual enhancement at the upcoming movement endpoint reveals a link between overt movement planning and covert attention (e.g., Deubel and Schneider, 1996
; Moore and Fallah, 2004
). The goal of the present study was to investigate the nature of that link. The preparation of a saccade to a specific spatial location is assumed to involve at least two distinct steps. The target location must be selected and then transformed into a motor plan appropriate to guiding gaze. Perceptual enhancement at the upcoming saccade endpoint could result from either of these two cognitive processes: intentional processes tied to target selection, or motor processes involved in programming a saccade.
Different theories about the relationship between covert attention and saccade planning emphasize one or the other process. The premotor theory of attention proposes that covert attention is a consequence of saccade planning. Orienting is labeled overt or covert depending on the execution or inhibition of the final motor command, but all modulations of visual attention are subserved by activity in the oculomotor system (Rizzolatti et al., 1994
). Support for the premotor theory comes from functional imaging studies revealing that shifts of covert attention and saccade programming involve overlapping cortical structures (Corbetta, 1998
; Corbetta et al., 1998
) and patient studies demonstrating that peripheral oculomotor deficits impair covert attention (Craighero et al., 2001
; Smith et al., 2004
). Furthermore, microstimulation of brain areas involved in saccade programming enhances visual processing in various tasks (Cavanaugh and Wurtz, 2004
; Moore and Fallah, 2004
; Muller et al., 2005
) and leads to increased sensitivity in perceptual brain areas such as V4 (Moore and Armstrong, 2003
; Armstrong and Moore, 2007
).
Selection-for-action views of attention propose, on the contrary, that attention is the mechanism which selects spatial areas of interest for subsequent action planning (Allport, 1987
; Neumann, 1990
; Schneider, 1995
; Goodale et al., 2005
). Thus, attention and target selection are the same mechanism, which provides spatial information about visual targets to the motor system. Motor planning and attention are coupled because they share this common target. The partial overlap of structures involved in covert attention and saccade planning would result from their temporary functional coupling. According to this framework, the independent processes of covert attention and overt motor preparation are coordinated by target selection mechanisms.
The present study sought to disentangle visual target selection from motor preparation and examine their respective contribution to the orienting of attention. Dissociating target selection from motor preparation is difficult because most of the time, our actions – and especially our eye movements – are appropriate to their targets. There is, however, one situation in which the correspondence between target selection and motor preparation breaks down: saccadic adaptation (see Hopp and Fuchs, 2004
for a review). Saccadic adaptation refers to the remarkable ability of the brain to adjust the motor parameters of eye movements relative to the coordinates of the saccade target. Saccadic adaptation introduces a dissociation between target selection and motor processes, because the final motor output does not correspond to the sensory representation of the selected target. Adaptation therefore provides a unique experimental technique for studying the contribution of target selection and motor preparation to visual processing (Hopp and Fuchs, 2004
). Adaptation can be observed after impairment of the extraocular muscles or nerve lesions: initially ill-directed saccades gradually adjust and after a few days are once again appropriate to the target (Kommerell et al., 1976
; Abel et al., 1978
; Optican and Robinson, 1980
). In these cases, saccadic adaptation realigns target selection and motor processes. In the laboratory, displacing the saccade target during movement execution introduces an artificial targeting error, because visual perception is reduced during eye movements and the target step goes unnoticed (Bridgeman et al., 1975
). However there is a progressive modification of saccadic amplitude such that the amplitude eventually becomes appropriate to the post-saccadic target position (McLaughlin, 1967
). The use of adaptation to dissociate target selection from motor planning is supported by recent studies of the brain site of saccadic adaptation. Modified neuronal activity after adaptation has been consistently reported in the cerebellum (Optican and Robinson, 1980
; Vilis and Hore, 1981
) but also in cerebellar inputs (Robinson et al., 2002
; nucleus reticularis tegmenti pontis: Takeichi et al., 2005
; superior colliculus (SC): Takeichi et al., 2007
). The involvement of the SC in adaptation is also supported by behavioral data investigating the characteristics of the transfer of adaptation from a given saccade to other saccades (Hopp and Fuchs, 2002
; see Hopp and Fuchs, 2004
for a review). These findings support the idea that the SC might be the site of adaptation, or that adaptation is present in collicular inputs. While the relevant research has yet to be carried out, observations that lesions of the cerebellar thalamus impair adaptation in humans (Gaymard et al., 2001
), and of specific adaptation deficits in Parkinson’s patients (MacAskill et al., 2002
) suggests that this may be the case. Current research therefore places the site of saccadic adaptation at the level of the SC, where signals relating to target selection and motor planning can be dissociated (Port and Wurtz, 2009
), thus it is reasonable to propose that adaptation spatially dissociates a motor command from a selected target at a level that could be relevant for attentional orienting (Cavanaugh and Wurtz, 2004
; Sommer and Wurtz, 2008
).
We measured attentional orienting with event-related potentials (ERPs) to task-irrelevant visual probes. Studies using ERPs have revealed a selective enhancement of early visual ERPs to stimuli within the attentional focus (reviewed in Luck et al., 2000
). Furthermore, this enhancement diminishes with increasing distance from the current attentional focus. This gradual effect of spatial location on ERPs is referred to as a spatial attentional gradient. Combining saccadic adaptation with the measurement of ERPs therefore allowed us to test if the location of attentional enhancement – operationalized as ERP amplitudes at different time intervals after stimulation – is determined by the sensory coordinates of the selected target or by the motor coordinates of the saccade. If sensory coordinates are relevant for the deployment of attention, then the amplitude of ERPs to task-irrelevant visual probes should be unaffected by saccadic adaptation. ERP amplitude should be highest for probes at the visual target location, and the farther the probe from this location, the smaller the ERP amplitude, reflecting the spatial attentional gradient. If, in contrast, motor coordinates define the location of attentional enhancement, the attentional focus is expected to shift to the motor endpoint of the adapted saccade. Indeed, the premotor theory predicts that any change in the motor plan should be accompanied by a change in the orientation of attention. This translates to the prediction that the amplitude of early visual peaks (i.e., in the N1 and N2 time range) to the probe at the target location will be more negative before adaptation relative to after adaptation. At the adapted motor location, peak amplitude will be more negative after adaptation relative to before adaptation. Moreover, if both target selection and motor planning are relevant to attentional deployment (see Collins et al., 2008
), the after adaptation, ERPs to the probe at the target location will be indistinguishable from ERPs to the probe at the adapted motor location.
In this context, the point in time at which a modulation of ERPs due to adaptation may be observed is informative about the possible underlying mechanisms of motor-to-sensory influences. If a modulation were found at early time intervals (<200 ms), this would indicate that the adaptation of motor programs influences predominantly sensory brain areas.
Participants
Data were recorded from 21 volunteers from the University of Hamburg community in exchange for payment or course credit, and gave their informed consent prior to starting the experiment. All had normal vision and did not wear glasses or contact lenses. Before running the experiment, participants completed a pre-test during which they practiced the task without EEG recording for 20 min. Saccades were not adapted during the practice session. Seven participants were excluded from EEG analysis because they either reported in an exit interview that they were aware of the target jumps during the adaptation and post-adaptation phases (see Section “Procedure”), or their adaptation was sudden rather than gradual, suggesting that they detected the jump and corrected for it. The remaining 14 participants (aged 22–43 years, 7 women, 13 right-handed) were included in the following analyses. The experiments were carried out according to the ethical standards laid down in the Declaration of Helsinki.
Stimuli
Stimuli consisted of black, blue and red 1° × 1° crosses (+) and 1° × 1° black dots (·) on a white background, presented on an Ilyama MS103DT 21′ screen (vertical refresh rate 170 Hz). Two black crosses were located immediately above and below a blue or red central fixation cross. A probe (dot) could appear at one of four locations 8° away, always in the upper hemisphere, directly above the fixation cross (0°) or to the left at an angle of 22.5°, 45° or 67.5° (Figure 1
A). All probes were equidistant from the center of gaze.
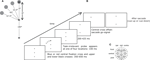
Figure 1. (A) Spatial lay-out of the stimuli. (B) Procedure. The color of the central cross cued the saccade to be made: to the upper or lower target. After 350–650 ms, a probe appeared at one of four equidistant locations in the upper hemisphere for 100 ms. After another 200–450 ms, the central cross disappeared, which constituted the go-signal to make the required saccade. When this saccade was detected, the target could step to a different location, disappear, or remain present (see Section “Materials and Methods” for details). (C) Locations of electrodes and clusters.
Eye Movement Paradigm, Recording and Analysis
In the present experiment, vertical saccades were adapted such that their direction was shifted from 0° (straight up) to approximately 45° to the left. Saccadic adaptation influences the saccade at a level where it is coded in vector coordinates (amplitude and direction; Hopp and Fuchs, 2006
), and both parameters can be adaptively modified. Amplitude adaptation has been more extensively studied, however direction adaptation shares some basic properties (Deubel, 1987
). Eye movements were recorded using an Eyelink 1000 Remote (SR Research, Osgoode, ON, Canada), at 500 Hz sampling rate. Saccades were detected online based on velocity (>30°/s) and acceleration (>3000°/s) thresholds. At the beginning of each phase (pre-adaptation, adaptation, post-adaptation), the Eyelink was calibrated. At the beginning of each trial, central fixation was checked and compared to the calibration. If the distance between the fixation check and the calibration was greater than 1°, fixation was refused and a full calibration was initiated.
Eye movement traces were subsequently analyzed offline. Instantaneous velocity and acceleration were computed for each data sample and compared to a threshold (30° and 8000°/s). Saccade onset was defined as two consecutive above-threshold samples. Saccade offset was defined as the beginning of the next 20-ms period of below-threshold samples.
Saccades were grouped according to experimental condition (probe location, cueing condition, pre-adaptation and post-adaptation). Specific ANOVAs and t-tests are detailed in Section “Results”. When Mauchly’s sphericity test was significant and sphericity could not be assumed, Huynh–Feldt adjustments to the degrees of freedom were used (although uncorrected dfs are reported in Section “Results”).
Procedure
Participants performed a delayed saccade to one of two targets as instructed by the color of the central fixation cross. They first fixated a central dot and pressed on a button to initiate the trial. The button press also executed a fixation check. The central dot was replaced by a red or blue cross with one black cross above and another below. Participants were instructed to prepare a saccade but to withhold execution until the central cross disappeared, 650–1175 ms later. Two hundred to 425 ms before central cross offset, a task-irrelevant probe was presented for 100 ms at one of four locations (Figure 1
B). Participants were instructed to ignore the probe. If a saccade was detected before central cross offset, a warning instructed participants to wait until the go-signal, and the trial was placed at the end of the trial queue.
In the pre-adaptation phase (960 trials), the proportion of upward and downward saccades was equal, and the visual stimulus location was not changed during participants’ saccades. In the adaptation phase (140 trials), 80% of saccades were cued up, and during these trials the target cross was stepped 45° to the left when participants made a saccade and remained at the new location until participants responded. The lower cross was extinguished to discourage comparison of the stepped target with vertical. Saccade detection did not alter visual stimuli when the saccade was cued down. In the post-adaptation phase (960 trials), the proportion of upward and downward saccades was equal. During 67% of upward saccade trials, saccade detection led to a 45° target step to maintain saccadic adaptation. To make sure that adaptation was not the result of visual feedback about the target step, the target disappeared in the remaining 33% of trials.
EEG Recording and Analysis
The EEG and EOG were recorded continuously from 73 Ag/AgCl scalp electrodes (EasyCap GmbH, Herrsching, Germany) and one electrode under the left eye, referenced to the left earlobe and re-referenced offline to a linked earlobe reference. Electrode impedance was kept under 5 kΩ. Electrodes were mounted according to the 10-10 system (Oostenveld and Praamstra, 2001
), an extension of the 10-20 system, using an elastic cap (EasyCap GmbH, Herrsching, Germany). Electrode positions were FP1/2, AFz, AF3/4, AF7/8, Fz, F1/2, F3/4, F5/6, F7/8, F9/10, FCz, FC1/2, FC3/4, FC5/6, FT7/8, FT9/10, Cz, C1/2, C3/4, C5/6, T7/8, CPz, CP1/2, CP3/4, CP5/6, TP7/8, TP9/10, Pz, P1/2, P3/4, P5/6, P7/8, P9/10, POz, PO3/4, PO7/8, PO9/10, Oz, O1/2, Iz, I1, and I2.
The electrode signals were amplified using three BrainAmp DC amplifiers with 32 channels each (Brain Products GmbH, Gilching, Germany) and digitally stored using the BrainVision Recorder software (Brain Products GmbH, Gilching, Germany). The analog EEG signal was sampled at 5000 Hz, filtered online with a bandpass of 0.1–250 Hz and then downsampled online to 500 Hz to be stored on disk. EEG data processing was done with VisionAnalyzer 1.05 (Brain Products GmbH, Gilching, Germany). To obtain ERPs, the continuous signal was segmented around the visual probes (100 ms pre-stimulus and 400 ms post-stimulus); this interval was chosen to exclude any saccade motor activity (see Section “Procedure”). Trials in which central fixation was broken before the instruction to move were cancelled during testing by monitoring eye movements with the EyeLink (see above) and did therefore not enter in the ERP analyses. ERP segments were rejected if they contained absolute voltage differences >120 μV. The maximum number of trials eliminated in any participant was 32% (average 20%). The number of remaining trials after artefact rejection did not differ statistically between the different conditions of the experiment. Segments were averaged by condition and were baseline corrected (100 ms pre-stimulus baseline).
For the purpose of visualization, average data of the different conditions were imported into MATLAB, and voltage maps and voltage difference maps, were created using EEGLAB (Delorme and Makeig, 2004
) using all 73 scalp electrodes. Voltage difference maps show the difference between two conditions.
ERP Analysis
All probes were equidistant from the center of gaze and differed only in their position relative to the focus of attention. ERPs are enhanced to visual stimuli at attended compared to unattended locations (Mangun and Hillyard, 1991
; Eimer, 1994
). These effects occur relatively early and influences have been reported for positive and negative peaks, termed P1 and N1, starting at ∼100 and 130 ms after stimulus presentation, respectively. Amplitude modulations have also been observed at a second negative peak, termed N2, starting at ∼200 ms post-stimulus. These visual ERPs originate partially from posterior cortex, presumably in the visual pathway (see Luck et al., 2000
for a review), suggesting that these attentional processes are mediated by sensory cortical areas. Similar perceptual enhancement, reflected in modulations in both the N1 and the N2 (but not the P1) time ranges, has been reported during the preparation of a saccade as well (i.e., preparation of overt orienting; Eimer et al., 2006
, 2007
). Based on these previous results, we chose two time intervals for statistical analyses of the ERPs. The first interval was centered on the prominent negative peak mainly over posterior electrodes at 130–170 ms post-stimulus. The second interval spanned from 200 to 400 ms post-stimulus.
Beyond general differences between the processing of stimuli at attended versus unattended locations in opposite hemifields, attentional modulation declines as a function of the distance of a visual event from the attentional focus within one hemifield (Downing and Pinker, 1985
; Shulman et al., 1986
). This “spatial attentional gradient” has been shown to give rise to graded ERPs (Mangun and Hillyard, 1988
; Röder et al., 1999
; Eimer, 2000
).
Electrodes were clustered into nine groups of five electrodes for statistical analyses (see Figure 1
C) to increase the signal-to-noise ratio of the ERPs. Each cluster contained at least one electrode position of the 10-20 system and were chosen to cover both hemispheres and the midline, as well as the frontal, central, and posterior scalp (frontal ipsilateral: AF7, AF3, F3, F5, F7; central ipsilateral cluster: FC5, T7, C5, C3, Cp5; posterior ipsilateral cluster: P3, P5, P7, PO3, PO7; frontal central cluster: AFz, F1, Fz, F2, FCz; central midline cluster: Cz, CP1, CPz, CP2, Pz; posterior central cluster: POz, O1, Oz, O2, Iz; frontal contralateral cluster: AF4, AF8, F4, F6, F8; central contralateral cluster: FC6, C4, C6, T8; posterior contralateral cluster: P4, P6, P8, PO4, PO8).
The ERP signal was averaged over all sampling points for each cluster, interval (130–170 and 200–400 ms post-stimulus), condition, and participant. We analyzed each of the two time intervals separately with an ANOVA comprising four repeated measurement factors, Electrode Cluster (nine clusters, see above), Cueing Condition [probe location cued (i.e., saccade instructed toward the upper hemisphere) versus uncued (i.e., saccade instructed in the opposite direction)], Adaptation phase (pre-adaptation versus post-adaptation), and Probe Location (four locations, as detailed in Section “Procedure”). Significant interactions in these ANOVAs were followed up by ANOVAs including only those factors which interacted significantly. Comparisons of only two levels of a single factor were done using paired-sample t-tests.
Saccade Characteristics and Adaptation
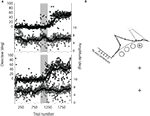
Saccades were directed to one of two visual targets, 8° above or below central fixation, based on the color of the fixation point. During the pre-adaptation phase, saccade endpoints were concentrated near the upper and lower targets with very few direction errors (<1% in each participant), and presented an undershoot of the target typical for saccades (Becker, 1972
) (Figure 2
A). During the adaptation phase, the upper target stepped to a location 45° to the left of central fixation while the saccade was in mid-flight. Saccade endpoints shifted accordingly; this shift was induced during the adaptation phase (gray area in Figure 2
A) and maintained during the post-adaptation phase. To determine the number of trials necessary for adaptation to emerge, the time course (saccade direction as a function of trial) was analyzed with the following method. Linear regressions on the relationship between saccade direction and trial number T were performed, by varying the limits Tmin and Tmax. First, Tmin was fixed as the first trial of the pre-adaptation phase and Tmax varied, each time taking one more trial into account. Each trial is thus associated with a particular slope value. During the pre-adaptation phase, the slope is close to 0, but when saccade direction changes during the adaptation phase, the slope exhibits a breaking point which corresponds to the onset of adaptation. Second, Tmax was fixed as the last trial of the post-adaptation phase and Tmin varied. Again, the slope of the linear function exhibits a breaking point, which corresponds to the offset of adaptation. On average, saccade direction needed 132 ± 71 trials (range 45–251) to become adapted, which is well within the normally reported range for humans (Hopp and Fuchs, 2004
). Figure 2
B presents the pre- and post-adaptation endpoint distributions over all participants.
Figure 2. (A) Time course of saccadic adaptation (saccade direction (filled symbols) and amplitude (open symbols) as a function of trial number) in two typical participants. The shaded area corresponds to the adaptation phase. Each point corresponds to one saccade and only upward saccades are shown. The curve corresponds to a running average with a 50-trial sliding window. (B) Distribution of saccade directions in the pre-adaptation (gray) and post-adaptation (black) phases relative to the four probe locations, over all 14 participants. Dashed lines represent ±SEM.
Average saccade latency (the time from the go-signal to saccade onset) was 254 ± 30 ms. We ran a three-way ANOVA on latency with Cued Saccade (saccade up; saccade down), Probe Location (0°; 22.5°; 45°; 67.5°) and Adaptation (pre-adaptation phase; post-adaptation phase) as factors. None of the factors had an effect on latency and no interaction reached significance (all F values <3.1, all p values >0.1). This suggests that participants were successful at ignoring the probes and that adaptation itself did not increase latency.
Saccade direction and amplitude were analyzed with two ANOVAs with the same factors as latency. Probe Location did not influence saccade direction (F < 1). Adaptation and Cued Saccade interacted [F(1,13) = 310.0, p < 0.001], revealing that there was an effect of Adaptation on direction when the saccade was cued up [F(1,13) = 402.1, p < 0.001], but not when it was cued down (F < 1). This reflects that only upward saccades were modified by adaptation, with an average pre-adaptation direction of −0.2 ± 4° (i.e., straight up) and an average post-adaptation direction of 43 ± 7°. Direction of downward saccades was on average 181 ± 4°. Probe Location and Adaptation did not influence saccade amplitude (F values <1), but there was an effect of direction [F(1,13) = 8.1, p < 0.02], revealing a slight bias for larger downward than upward saccades (7.0 ± 0.7° versus 6.4 ± 0.6° respectively). None of the interactions reached significance (all F values <1.6, all p values >0.20).
Event-Related Potentials to Visual Probes
Effect of cueing condition
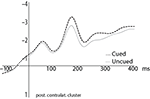
We first examined the effect of attention on the ERPs to the probes, in order to replicate earlier findings showing an enhanced response to probes located within the focus of attention relative to outside the focus of attention (defined by an upcoming movement endpoint). To do so, we compared ERPs to the visual probe stimuli in the cued versus uncued conditions. There was a marginally significant main effect of Cueing Condition [F(1,13) = 4.1, p = 0.064] with a marginal interaction with Cluster [F(8,104) = 2.7, p = 0.076] in the time interval 130–170 ms after probe presentation. Each cluster was therefore analyzed with an ANOVA including factors Adaptation, Cueing Condition, and Probe. The main effect of Cueing Condition was significant at the central midline cluster [F(1,13) = 6.5, p < 0.025] and central and posterior contralateral clusters [F(1,13) = 8.9, p < 0.015 and F(1,13) = 8.1, p < 0.015 respectively]. In these three clusters, ERPs to the cued (i.e., attended) condition were more negative than the uncued (i.e., unattended) condition (Figure 3
). Electrode Cluster and Cueing Condition interacted in the 200–400 ms post-probe time frame [F(8,104) = 3.2, p < 0.02]; we thus again ran ANOVAs including factors Adaptation, Cueing Condition, and Probe for each cluster. The effect of Cueing Condition was marginally significant in the posterior midline cluster [F(1,13) = 4.3, p = 0.058] and significant in the posterior contralateral cluster [F(1,13) = 4.7, p < 0.049]. In both clusters, the ERP amplitude was more negative in the cued than in the uncued condition. Figure 3
presents grand averaged ERPs for the cued and uncued conditions for the posterior contralateral electrode cluster that revealed statistically significant differences in both analyzed time intervals (130–170 and 200–400 ms).
Figure 3. Grand averaged ERPs to probes in the cued (dashed) and uncued (gray) conditions, averaged over the four probe locations.
Effect of saccadic adaptation
The main goal of the present study was to determine the effect of motor planning, manipulated by means of saccadic adaptation, on the orientation of attention. We therefore examined ERPs elicited by probes in the cued condition (in which the probe was presented near the planned movement goal) in the pre-adaptation versus post-adaptation phases.
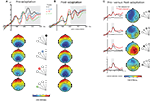
We first examined whether the differential response to the probes was modified by adaptation. Figure 4
illustrates the gradual decrease of negativity in the ERPs over the four probes and the corresponding voltage maps for to the 200–400 time interval. In the pre-adaptation phase (Figure 4
A), the amplitude of the ERPs (200–400 ms) was ordered from most to least negative as a function of the probe’s distance from the saccade target location, thus demonstrating an attentional gradient: the most negative ERP was evoked by the probe at the saccade target, the least negative by the probe at 67.5°, with 45° and 22.5° lined up between these two. We ran an ANOVA including factors Electrode Cluster, Adaptation and Probe Location, restricting Probe Location to include probes at 0° and 45° for which we had specific hypotheses about how adaptation should influence the response. The ANOVA revealed a significant interaction of Adaptation and Probe Location in the 200–400 ms time interval [F(1,13) = 4.6, p < 0.05]. The difference between the two probes was significant in the pre-adaptation phase [F(1,13) = 7.7, p < 0.016] but not in the post-adaptation phase (F < 1)
1
.
Figure 4. (A) Grand averaged ERPs to each of the four probes in the pre-adaptation cued condition, from an example cluster (central posterior, depicted in each map) (probe located at 0°, black; 22. 5°, blue dashed; 45°, red; 67.5°, green dashed). Topological maps below show the distribution of the effect for each probe in the second time interval (200–400 ms), corresponding to the gray shaded area in the ERP traces. (B) ERPs from an example cluster (central posterior) in the post-adaptation cued condition, and the corresponding maps for each probe. (C) Grand averaged ERPs to each probe location in the cued condition for pre-adaptation (black dashed lines) versus post-adaptation (red lines), from an example cluster (central contralateral, depicted in the maps). The gray shaded area in the ERPs corresponds to the first time interval (130–170 ms) which is also illustrated in the corresponding difference maps (pre-adaptation–post-adaptation).
In a second analysis, we examined the effect of adaptation for each separate probe to see whether adaptation modified the attentional enhancement at the four tested spatial locations. The overall ANOVA including factors Electrode Cluster, Probe Location, here including the four locations, and Adaptation revealed a significant Probe Location by Adaptation interaction in the 130–170 ms time interval [F(3,39) = 3.5, p < 0.036]. We subsequently carried out t-tests comparing pre- and post-adaptation in the cued condition for each probe location. The effect of Adaptation was significant at probe location 45° [t(1,13) = 2.3, p < 0.036] and non-significant at probe location 67.5° (t < 1). Probe locations 22.5° and 0° were also non-significant although they were marginal [t(1,13) = 2.1, p = 0.051 and t(1,13) = 1.8, p = 0.10, respectively]. At probe location 45° (i.e., the location of the saccade endpoint in post-adaptation), the ERP was more negative in post-adaptation than in pre-adaptation. At other probe locations, the ERP did not change between the two phases. Figure 4
C compares the grand averaged ERPs for pre- and post-adaptation for each probe at the central contralateral electrode cluster, as well as the difference maps (pre-adaptation minus post-adaptation) for the 130–170 ms time interval. These different effects of phase for each probe carried over into the later time interval (200–400 ms post-probe), with a non-significant effect of Adaptation for probe locations 0° and 22.5° [t(1,13) = 1.4, p > 0.19 and F < 1] and a significant effect for probe locations 45° and 67.5° [t(1,13) = 2.8, p < 0.015 and t(1,13) = 2.4, p < 0.03].
We dissociated target selection from motor preparation using saccadic adaptation. In this paradigm, saccades are adaptively modified such that their endpoint is spatially distinct from the target that evoked them. The aim of our study was to examine the contribution of target selection and motor preparation to attentional orienting, in order to contrast different theories of attention that emphasize one or the other process.
In order to probe visual processing, we examined ERPs evoked by stimuli presented briefly during the delay period in a delayed-saccade paradigm. The use of task-irrelevant probes has the advantage of revealing automatic processing of visual stimuli because response requirements for attended and unattended stimuli are equated (and null). The probe could not be used as a timing signal to know when to prepare the saccade because the delay between probe onset and go-signal varied randomly on each trial. Furthermore, there was no effect of probe location on saccade latency, suggesting participants were successful at ignoring the probes. While the probe may have been a kind of general alerting cue, this did not differ between the experimental conditions and so cannot explain the differences observed on the evoked response. The delay between probe presentation and saccade onset varied randomly, but the longest delay was around 600 ms. It is crucial to note that we used a delayed saccade paradigm and thus that the effect on probe processing can be tied to saccade preparation even in these cases. Indeed, attentional orienting toward the saccade target occurs when it is presented and remains there until the saccade is executed, even if the paradigm introduces a delay of up to 1200 ms between the two (Deubel and Schneider, 2003
).
In agreement with previous studies (e.g., Mangun and Hillyard, 1987
, 1991
; Eimer, 1994
; Eimer et al., 2006
, 2007
), we found an effect of attention on ERPs revealed by a difference in amplitude between cued (i.e., attended) and uncued (i.e., unattended) conditions. Indeed, ERPs in our study were more negative when the visual stimulus was presented at an attended (cued) as compared to an unattended (uncued) location. This attentional enhancement was observed over posterior central and contralateral areas 130–170 ms post-probe presentation and over posterior midline and contralateral areas 200–400 ms post-probe. Both the timing and distribution of this attentional effect are in line with earlier reports (e.g., Eimer, 1994
; Hopf et al., 2000
; Luck et al., 2000
).
However, beyond a coarse difference in ERP amplitude between diametrically opposed attended and unattended locations, we observed an effect of the distance between the visual probe and the focus of attention in the form of a spatial attentional gradient. Here the relevant experimental manipulation was not between cued and uncued conditions but between the different probe locations as a function of phase (pre-adaptation versus post-adaptation) in the cued condition. Before saccades were adapted, ERPs between 200 and 400 ms were more negative the closer probes were to the saccade target. As all probes had the same eccentricity (8°), differences in the evoked potentials cannot result from physical differences between the probes but must be due to their location relative to the focus of attention. The graded evoked potentials and their time range are in line with previous ERP studies investigating attentional gradients in vision (Mangun and Hillyard, 1988
; Eimer, 2000
). The gradually modulated ERP response in our study therefore most likely reflects a gradient of visuo-spatial attention, which before adaptation is centered on the saccade target (which also corresponds in this phase to the endpoint) and declines in strength with increasing distance from this location. After adaptation, the focus of the gradient seems to expand to include several probe locations. Probes located at the upcoming (adapted) endpoint were on the periphery of the gradient before adaptation but benefited from greater enhancement after adaptation. Enhanced processing of this probe was observed as early as 130 ms post-probe presentation, as indicated by the effect of adaptation for this probe location. This result pattern is consistent with a shift of the focus of attention to the adapted motor location, suggesting that the orientation of attention during saccade preparation depends on the upcoming motor endpoint: when the endpoint changes, so does the orientation of attention. However, at the target location (0°), processing of the visual probe was not modified by adaptation, suggesting that the focus of attention remained at this location whatever the motor plan. Therefore, it appears that after adaptation there were two foci of attention: one centered on the selected target location, and the other centered on the motor endpoint. Another interpretation is that there was one attentional focus but that after adaptation its size increased to encompass both target and motor locations. Whatever the case may be, both interpretations are compatible with the novel finding reported here: that both motor planning and target selection are relevant to attentional orienting.
Our results are due to the adaptive change of saccade direction brought about by the plasticity of the saccadic system. We checked that participants were not aware of the target step by explicitly enquiring in an exit interview and eliminating from our analyses the participants who reported seeing the step. We also examined the time course of adaptation, as abrupt changes in amplitude may signal awareness of the step even in the absence of explicit report. Due to this conservative criterion, one additional participant was eliminated. In the remaining participants, progressive adaptation (as has been described previously) was observed. Finally, there was no latency change after adaptation, again suggesting no strategic change of saccade direction.
Our results differ in some regard from those of previous studies on saccadic adaptation and visual perception. When participants had to discriminate a probe presented at the target location or at the motor endpoint, best perceptual performance was found at the motor endpoint. When the endpoint differed from the target, performance decreased at the target location. These results suggested that motor preparation processes were primary in determining the orientation of attention (Doré-Mazars and Collins, 2005
; Collins and Doré-Mazars, 2006
). However, in these studies participants repeatedly made saccades to the same location; target selection is minimal in this situation, possibly rendering less relevant the target to perception. In our study participants made saccades to two different locations (up and down); this situation makes target selection task-relevant. This, in turn, may have lead to an attentional deployment to the target in addition to the motor location of the planned movement.
Results from other paradigms have also suggested that attention can be concurrently directed to several locations in space in conjunction with movement planning. For example, when participants prepare a sequence of saccades or manual reaches to several locations, perception is enhanced at all target locations before the execution of the first movement (Baldauf et al., 2006
; Baldauf and Deubel, 2008
). Furthermore, attentional modulations of ERPs were evoked by task-irrelevant probes at both the first and second target of a pointing sequence (Baldauf and Deubel, 2009
), and each target in a two-target pointing sequence activated single cells in the cortical parietal reach region in monkeys (Baldauf et al., 2008
). Finally, we recently demonstrated two foci of attention in a pointing task by dissociating the movement goal from the motor endpoint by asking participants to use a tool for pointing (Collins et al., 2008
). In summary, in addition to investigations of sequential saccades, pointing movements, and tool use, our results suggest that the focus of attention can be distributed to several spatial locations (Eimer, 2000
; VanRullen et al., 2007
). Crucially, two different processes seem to be involved in the selection of such distributed attentional foci: target selection enhances perception of objects at the selected location, as does the motor planning of a saccade to a spatial location.
Thus, in line with the predictions of the premotor theory of attention, motor preparation processes can drive attentional orienting. We show that modifying the motor characteristics of a saccade leads to proportional changes in the distribution of spatial attention throughout the visual field. Our results are therefore compatible with the framework proposed by what has been termed an “embodied” approach to cognition (e.g., Cisek, 2007
). Indeed, our results provide direct evidence for the prediction of such approaches that changes in the motor exploration of the world or the motor affordances of objects are accompanied by perceptual changes. However, contrary to the predictions of the premotor theory of attention, our results suggest that motor preparation processes alone cannot explain the pattern of attentional effects observed here, because despite a change in the motor plan, attentional enhancement was still observed at the location of the visual target. This result is compatible with theories proposing that target selection determines the orientation of attention and enables the programming of an action toward the target (e.g., Goodale et al., 2005
). These theories follow the serial perception–cognition-action architecture of traditional cognitive science; however these approaches alone also cannot explain our results, because attentional enhancement was found at the motor endpoint when it differed from the visual target location. This suggests top-down influences of motor preparation on perceptual processing, or, alternatively, parallel processing of perceptual and motor functions.
The debate about whether enhanced activity corresponds to target selection or motor preparation has its counterpart in neurophysiological research in animals. Cortical parietal activity has been interpreted as a representation of attended targets, a kind of salience map modulated by behavioral context (Gottlieb et al., 1998
; Kusunoki et al., 2000
; Gottlieb, 2002
), which would function as perceptual input to the motor system. Other interpretations propose on the contrary that parietal activity represents the preparation of actions or motor intentions (Andersen, 1995
; Snyder et al., 2000a
,b
), classifying parietal processing rather as motor output. Rather than supporting either the premotor or the selection-for-action theory, therefore, our results suggest that motor planning is one of several processes that contribute to the distribution of attention throughout the visual field. In most situations, the selected visual target and the corresponding motor plan are aligned, and there is then only one enhanced location. However, by dissociating target selection from motor planning, we show that both processes contribute to perceptual processing. These results raise the possibility that both task-relevant targets and motor planning might contribute to parietal activity, in line with proposals that the parietal cortex is involved in both sensory and motor processing, and in particular in sensory-motor transformation (e.g., Buneo and Andersen, 2006
). A task-relevant stimulus such as a saccade target should activate a salience map representing visual objects in terms of their behavioral significance (Gottlieb et al., 1998
), however the action afforded by this target should also render that part of space to which the action is oriented salient (Andersen et al., 1997
). Such unified processing of perception and action through a direct influence of motor on perceptual processing is well suited to serve the efficient planning and execution of behavior.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Thérèse Collins was supported by an Alexander von Humboldt Foundation Research Fellowship. The authors thank S. Röper, D. Tödter and J. Schubert for help with data acquisition.
- ^ Because an ANOVA involving all four probe locations was not sensitive enough to discover the descriptively identified changes in the attentional gradient from pre- to post-adaptation (non-significant interaction of Adaptation × Location), we concentrated our analysis on those two probe locations for which we had specific hypotheses: the target (0°) and the adapted (45°) locations.