Abstract
There is more to the sea than what you can see! Did you know that when you swim in the sea, you are actually swimming in a soup of carbon? The ocean has many carbon-containing things. Some are large like fish, whales, and shells, but most of them are too small to be seen unless we use microscopes. Some of these minuscule particles are living organisms, while the rest are their remains as they decompose. When land creatures die, they decompose into soil. In the ocean, creature remains are gradually dissolved in seawater. All this dead material contains the element carbon, which, at some point in time, was carbon dioxide in the air. The ocean stores a large portion of the carbon on our planet. In this article, we explain how all this carbon ends up in the ocean and how human activities affect the carbon cycle in the sea.
Why is Carbon so Important?
Carbon is the element of life. There are no other elements that have just the right properties for the job. Carbon can bind to many other elements, such as hydrogen, nitrogen, oxygen, phosphorus, and more. This means carbon can help to build a wide variety of molecules, all with very different properties. Some examples are hard, rigid structures such as carbonates, which many corals and shells are made of; flexible fibers in plants, which we can turn into cloth or rope; and sugars, such as those that give honey and soft drinks a sweet taste. Look around and you will see that carbon is everywhere, in your home and outdoors. The ocean covers of the Earth’s surface, so it may come as no surprise that it also contains a large amount of carbon. So, let us find out what shapes and forms ocean carbon is in, and why we even need to think about them.
Is All the Carbon the Same?
Carbon atoms in the ocean are always attached to other carbon atoms and atoms of other elements, forming a wide range of substances. Even though we do not know what all the carbon-containing substances are, we can still describe them based on their properties—for example, whether they sink or float and what other elements they consist of. If they are big enough to sink, we refer to them as particles. Some particles are so small that we need a microscope to see them. The substances that are too small to sink are called dissolved substances. If a substance is made of carbon atoms attached to other carbon atoms or hydrogen atoms, we refer to it as organic substance (even when atoms of other elements are present). The soft parts of all organisms (including you) such as skin, muscle, and organs, are made of organic substances. All other carbon-containing substances are called inorganic. Carbon-containing rocks such as those that make marble and chalk are examples of inorganic carbon substances, as they contain carbon attached to oxygen and calcium atoms (calcium carbonate, CaCO3). Carbon dioxide (CO2) in the atmosphere is an example of an inorganic carbon substance that is a gas (Figure 1).
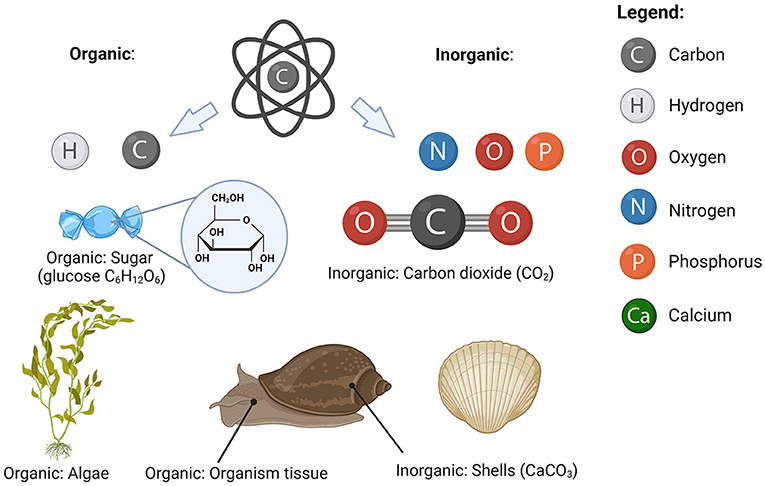
- Figure 1 - Carbon can attach to various elements to form organic and inorganic substances.
- Organic substances contain carbon attached to other carbon (C) or hydrogen (H) atoms. Inorganic substances contain carbon attached to any other element, such as oxygen (O), nitrogen (N), or phosphorus (P)(image created with BioRender.com).
Using these two properties, we can group carbon into four categories: particulate organic, particulate inorganic, dissolved organic, and dissolved inorganic (Figure 2). Most of the carbon in the ocean is dissolved inorganic carbon. In fact, the ocean has removed a large portion of the CO2 we have released into the atmosphere, by dissolving it (see this Young Minds article for some examples). Organisms in the ocean control the balance between organic and inorganic carbon, and between particulate and dissolved carbon. All organisms, from the tiniest bacteria to the largest whales, consist of organic carbon, so we classify all organisms as particulate organic carbon. Some organisms also have body parts that are made of carbonates and therefore contribute to particulate inorganic carbon. The last form of carbon to consider is dissolved organic carbon, which includes all organic particles that gradually leak into seawater (even from your body when you take a swim). When there are very high concentrations of dissolved organic carbon, the water often has a brown/yellow color (see this Young Minds article for some examples). Perhaps you have seen something similar happening when you make a cup of tea. The hot water in your teacup dissolves some of the organic carbon in the tea leaves, and the water turns brown.
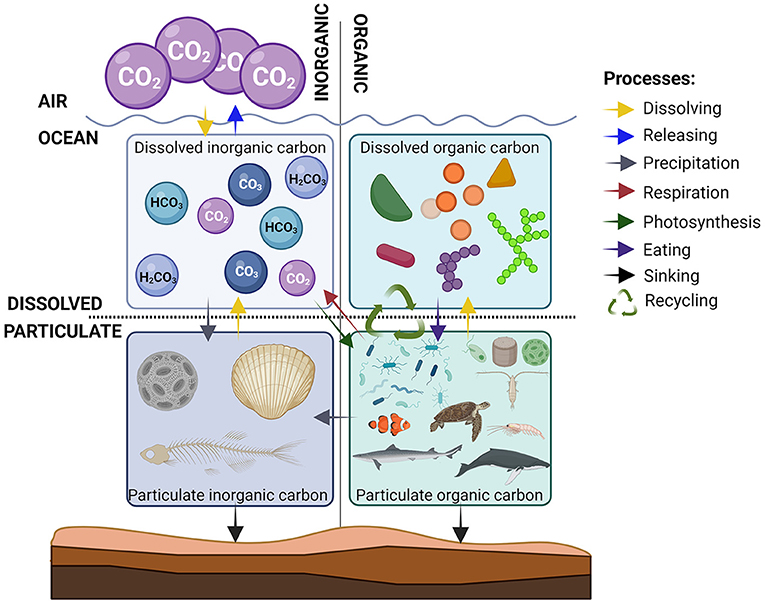
- Figure 2 - What happens to the carbon in the ocean?
- Carbon can be found in 4 forms in the ocean. The amount of carbon in each form varies, and carbon in one form can change to another form through various processes, which are shown by the colored arrows (explained in the list on the right) (image created with BioRender.com).
The Ocean as a Carbon Recycler
In the ocean, carbon is constantly being moved between these four forms and this movement makes up the ocean carbon cycle (Figure 2). A balanced carbon cycle is essential for controlling Earth’s climate and the diversity of organisms on the planet. The ocean carbon cycle consists of a collection of processes that transform carbon from one form to another. Scientists have found that all these processes are affected by changes in the concentration of CO2 in the air, which is increasing due to human activities. So, the balance between the various forms of carbon is changing. CO2 can be dissolved in or released from seawater, depending on the concentrations of CO2 in the water and in the air. If the concentration in the air is higher than in seawater, carbon dissolves into the ocean. However, CO2 can be released from the ocean to the air if the concentration in the sea is higher. As the concentration of CO2 in the air has increased over the last 100 years, the ocean has dissolved more and more CO2.
Through photosynthesis, algae and aquatic plants use the sun’s energy to produce organic carbon substances from dissolved inorganic carbon (see this Young Minds article for some examples). From here, the carbon can go four ways:
1) When algae and aquatic plants are eaten by grazers, some of the particulate organic carbon is transformed into new cells (just as we grow when we eat), so it stays as organic particles but often becomes bigger ones, starting a food web.
2) Respiration by organisms turns some of the particulate organic carbon back into dissolved inorganic carbon. The energy stored in organic substances is used by the organisms and the carbon is released as CO2, which is what happens when we breathe out.
3) Organisms die and sink to the sea floor, to be slowly buried. They form rocks and oil, and this carbon is stored underground for millions of years.
4) Particulate organic carbon releases dissolved organic carbon as the organism’s cells leak or fall apart, spilling their contents into seawater.
Some of the dissolved organic carbon substances from leaking or dead cells are food for bacteria living in seawater (Figure 3). This recycles some of the carbon, either back to dissolved inorganic carbon or into particulate organic carbon, where it contributes once more to the food web. This cycling of carbon between organic and inorganic, and between particulate and dissolved, is mainly done by microscopic organisms such as bacteria, phytoplankton, and small grazers, and is therefore called the microbial loop (see this Young Minds article for some examples) [1]. When you think of life in the ocean, your first thought might be fish, sharks, and whales. Even though they are large, these animals represent only a very small portion of the carbon in the ocean. Most of it is “spinning” around in the microbial loop.
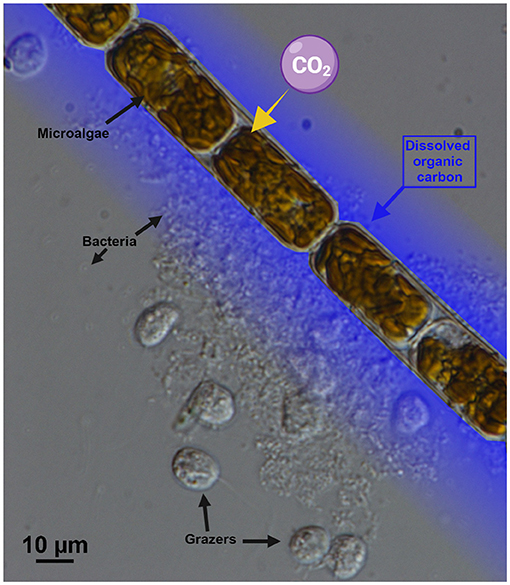
- Figure 3 - A photograph, taken using a microscope, showing microalgae surrounded by bacteria (tiny gray dots) and other organisms that feed on the bacteria (larger, roundish, gray forms).
- The microalgae release dissolved organic carbon (represented by the blue shading), which helps bacteria to grow. The scale bar at the bottom represents 10 μm. A human hair is about 70 μm thick (photo credit: L. Haraguchi).
Bacteria are carbon-recycling champions, but they cannot use all the dissolved organic carbon in the sea. Like us, they can also be picky eaters, selecting the organic substances that they like best [2]. Eventually, the remaining substances may accumulate as “leftovers” from the ocean buffet. If this is true then this could be a new way in which the ocean ecosystem can help reduce the amount of CO2 in the air and store it as dissolved organic carbon [3].
A Changing Carbon Cycle
It is important to understand how much carbon is in the ocean, what form it exists in, and what processes are transforming it. Once we know this, we can try to predict how the world may change in the future.
What happens when CO2 increases? Will the carbon shown in the four boxes in Figure 2 increase as well? The answer is… it depends! More CO2 in the ocean might affect not only the size but also the contents in each box. For the particulate organic carbon box, more CO2 will influence the number of organisms (the box size) and which types of organisms are there. This happens because some organisms like more CO2 than others do, so they thrive under these new conditions. A similar change can happen if temperatures change. Some organisms like it hot, and others do not. This is important because the presence of different kinds of organisms changes the food web and affects how carbon travels within the carbon cycle.
One challenge is that particulate organic carbon exists as many different life forms, and dissolved organic carbon is in so many kinds of substances that we cannot identify them all. Without such basic information, it becomes harder to understand the processes of the carbon cycle and how they relate to other factors, such as temperature changes.
Glossary
Dissolved: ↑ When a substance breaks down to smaller units, which do not sink, and is incorporated into a liquid, forming a solution.
Organic Substance: ↑ Is a kind of chemical containing carbon atoms attached to other carbon or hydrogen atoms.
Particulate: ↑ Refers to particles in suspension in the water, including both living and non-living substances that can sink.
Ocean Carbon Cycle: ↑ Is how carbon atoms travel from the atmosphere into organisms in the Ocean and then back into the atmosphere, again and again.
Grazers: ↑ Predators that consume plants and algae.
Respiration: ↑ The process of obtaining energy by breaking sugar molecules apart using oxygen. It is done by all living organisms and produces CO2 and water as waste products.
Phytoplankton: ↑ Minuscule algae that can make their own food using CO2, water, and sunlight.
Microbial Loop: ↑ Is how carbon and other nutrients are processed by the smallest organisms (such as bacteria and phytoplankton) in the Ocean.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
LH received funding from Academy of Finland grant agreement No. 342223. RG-A has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant Agreement No. 839311. CS has received funding from the Independent Research Fund Denmark Grant No. 9040-00266B. This work was partly funded by the European Union H2020 as part of the EU Project ARICE grant agreement no. 730965 through the project NoTAC (“Novel Tracers of Arctic Carbon and water exchange in the Fram Strait”).
References
[1] ↑ Azam, F., Frenzel, T., Field, J. G., Gray, J. S., Meyer-Rell, L. A., and Thingstand, F. 1983. The ecological role of water-column microbes in the sea. Marine Ecol. Prog. Ser. 10:257–63. doi: 10.3354/meps010257
[2] ↑ Hansell, D. A. 2013. Recalcitrant dissolved organic carbon fractions. Ann. Rev. Mari. Sci. 5:421–45. doi: 10.1146/annurev-marine-120710-100757
[3] ↑ Jiao, N., Herndl, G. J., Hansell, D. A., Benner, R., Kattner, G., Wilhelm, S. W., et al. 2010. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nat. Rev. Microbiol. 8:593–9. doi: 10.1038/nrmicro2386