Effects of an High-Fat Diet Enriched in Lard or in Fish Oil on the Hypothalamic Amp-Activated Protein Kinase and Inflammatory Mediators
- 1Department of Experimental Medicine-Section of Human Physiology, Second University of Naples, Naples, Italy
- 2Department of Medicine, University of Padova, Padua, Italy
- 3Department of Biology, University of Naples Federico II, Naples, Italy
The high fat diet (HFD) rich in lard induces obesity, inflammation and oxidative stress, and the deregulation of hypothalamic nuclei plays an important role in this mechanism. One important factor involved in the food intake and inflammation is adenosine monophosphate-dependent kinase (AMPK), a serine/threonine kinase activated by phosphorylation. Omega (ω)3-polyunsaturated fatty acids (PUFA) are dietary compounds known to attenuate the obesity-related diseases, although the molecular mechanisms underlying their actions in the hypothalamus are not completely understood. We hypothesized that the beneficial effects of PUFA may be mediated by AMPK in the hypothalamus. To this aim, rats were fed a control diet (CD), or isocaloric HFD containing either fish oil (FD; rich in ω3-PUFA) or lard for 6 weeks, and the activation of AMPK, inflammatory state (IKKβ, TNF-α) and oxidative stress were analyzed in the hypothalamus. In addition, we also studied serum lipid profile, homeostatic model assessment (HOMA) index, and pro-inflammatory parameters. Our results showed, at the hypothalamic level of LD-fed rats, an increase of AMPK activation, inflammation and oxidative stress, while no modifications were detected in FD-fed animals compared to CD. In addition body weight gain, serum lipid profile, pro-inflammatory parameters and insulin resistance were reduced in FD animals compared to LD. In conclusion, our data indicate that the substitution of saturated by unsaturated fatty acids in the diet has beneficial effects on modulation of hypothalamic inflammation and function in obesity, underlying, at hypothalamic level, the interaction among insulin and/or leptin resistance, AMPK activation and hyperphagia.
Introduction
Obesity and diabetes are major causes of morbidity and mortality in the western world, and may lead to inflammatory responses and oxidative stress in the peripheral tissues (Hotamisligil, 2006). Several data indicate that overnutrition-associated diseases are also linked to inflammation and increased levels of reactive oxygen species (ROS) in the brain (Cai, 2009). Hypothalamus is the brain region responsible of a variety of metabolic regulations and many overnutrition-related diseases appear to be etiologically related to the deregulations of hypothalamic neurons that are very vulnerable to the nutritional oxidative stress and inflammation (Cai, 2009). It has been demonstrated that overnutrition can activate IKKβ/NF-κB in the mediobasal region of the hypothalamus, which is the area sensing nutrition status and regulating metabolism (Zhang et al., 2008). IKKβ/NF-κB is a master-switch and central regulator of innate immunity and related functions. Some studies have suggested that IKKβ/NF-κB, as mediator of a metabolic inflammation, may represent the link between overnutrition and the dysfunctions of hypothalamic signaling that cause obesity and associated problems (Zhang et al., 2008).
One of the potent counter-regulator of inflammatory signaling pathways is adenosine monophosphate-dependent kinase (AMPK; Salt and Palmer, 2012; Hernández-Aguilera et al., 2013). AMPK, an evolutionary conserved serine/threonine kinase, is a heterotrimeric complex formed by a catalytic α subunit and regulatory β and γ subunits. The phosphorylation of the α subunit, at Thr172, turns AMPK into the activated form pAMPK. AMPK is a sensor of the cellular energy status that, when activated by metabolic stress, is able to maintain cellular energy homeostasis by turning on catabolic pathways (Hardie, 2014).
Several studies have demonstrated that AMPK in the hyphotalamus regulates food intake and that its activity is modulated by several hormones such as leptin, adiponectin and insulin. Since these hormones are involved in the control of body weight and glucose and lipid metabolism, these findings implied that AMPK might serve as a downstream effector of leptin and insulin and thereby play a role as a signaling molecule during hormon-induced metabolic responses. Whereas leptin inhibits AMPK activity in the arcuate and in the paraventricular nuclei of hypotalamus, insulin is known to inhibit AMPK activity in the lateral, ventromedial, and dorsomedial hypothalamic regions (Minokoshi et al., 2004). All these brain regions are involved in the control of food intake (Monda et al., 1993; Viggiano et al., 2006) and in energy expenditure (Monda et al., 1996; Messina et al., 2013).
The ω-3 polyunsaturated fatty acids (PUFA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are dietary compounds that are intensively studied as potent anti-inflammatory products, able to reduce the risk of insulin resistance and ameliorate obesity-associated disorders affecting hormonal control and modulating AMPK activity (Xue et al., 2012; Martínez-Fernández et al., 2015). Recently, we have demonstrated that the replacement of lard, rich in saturated fatty acids (SFA), with fish oil (rich in ω-3 PUFA) in high-fat diet (HFD) is able to limit the development of systemic and tissue inflammation, hepatic steatosis and to attenuate insulin resistance (Lionetti et al., 2014a,b; Cavaliere et al., 2016).
Here, we have tested, at hypothalamic level, the effect of substitution of saturated by unsaturated fatty acid on AMPK, activated AMPK (pAMPK at Thr 172), IKKβ, inflammation and oxidative stress. Moreover, we have also analyzed, in the same animals, the involvement of insulin, leptin and inflammatory parameters in the modulation of AMPK.
Materials and Methods
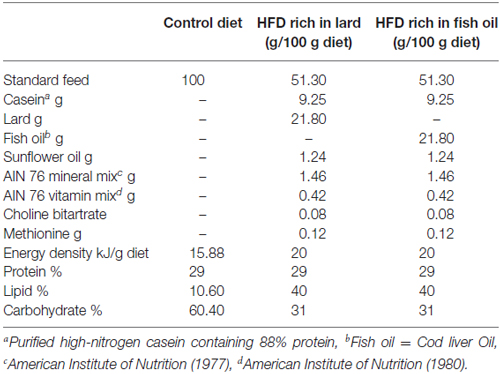
All chemicals were purchased by Sigma–Aldrich (St. Louis, MO, USA), unless otherwise specified. Young male Wistar rats (60 days old; 345 ± 7 g; Charles River, Calco, Lecco, Italy) were individually caged in a temperature-controlled room and exposed to a daily 12/12 h light/dark cycle with free access to chow diet and drinking water. Rats were divided into three experimental groups (n = 8) according to a different 6 weeks dietary regimen: the first group (control diet, CD) received a standard diet (10.6%fat J/J); the second group (LD) received the HFD rich in lard (40% fat J/J); and the third group (FD) received the HFD rich in fish oil (40% fat J/J). The composition of all dietary regimens is reported in Table 1.
Throughout the experimental period, body weights and food intakes were monitored daily to calculate the body-weight gain and the gross energy intake. Spilled food was collected and compensated in readjusting the calculation of food intake. Gross energy density for standard or HFD (15.8, or 20 kJ/g, respectively) was determined by a bomb calorimeter (Parr adiabatic calorimeter, Parr Istrumentes Co, Moline, IL, USA).
Another set of CD, LD and FD animals (n = 5 per group) at 6 weeks of treatment, were injected i.p. with insulin (homolog rapid-acting, 10 units/kg body wt; Novartis, Basel, Switzerland).
At the end of the experimental treatments, the rats were anesthesized by i.p. injection of chloral hydrate (40 mg/100 g body weight), decapitated with a guillotine, and the blood was taken from the inferior cava vein. The hypothalamus was quickly dissected from the brain and transferred in the appropriate buffer. All the samples that were not immediately used were stored at −80°C.
Serum Parameters
The serum levels of cholesterol, triglycerides, NEFA and glucose were measured with standard procedures. The serum levels of insulin (Mercodia AB, Uppsala, Sweden), TNF-α (Biovendor R&D, Brno, Czech Republic), adiponectin and leptin (B-Bridge International Mountain View, CA, USA) were measured using commercially available ELISA kits.
Lipid Peroxidation Assay
To determine the lipid peroxidation in hypothalamic homogenate, the level of malondialdehyde (MDA) was measured using the thiobarbituric acid reaction (TBAR) method. MDA reacts with thiobarbituric acid (TBA) to form a pink chromogen that is detected at the wavelength of 532. MDA values were expressed as nanomoles per milligram of brain protein (Lu et al., 2009).
Redox Status and Nuclear Factor Erythroid 2-Related Factor (Nrf2) Activated Enzymes Activities
Reduced glutathione (γ-L-Glutamyl-L-Cysteinyl-Glycine, GSH) and oxidized glutathione (γ-glutamyl-L-cyteinylglycine disulfide, GSSG) concentrations in the hypothalamus were measured using the dithionitrobenzoic acid (DTNB)-GSSG reductase recycling assay (Bergamo et al., 2007); the GSH/GSSG ratio was used as an oxidative stress marker. The enzymatic activities of glutathione S-transferase (GST) and NAD(P)H-quinone oxidoreductase (NQO1) were evaluated spectrophotometrically in brain cytoplasmic extracts with standard protocols (Benson et al., 1980; Levine et al., 1990).
Western Blot
The hypothalamus was homogenized in the lysis buffer (10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 12% glycerol, 0.5 mM DTT, 0.1 mM EGTA) with a cocktail of protease inhibitors (Sigma Aldrich). Proteins (20 or 40 μg/lane) were separated on 12% SDS-PAGE and transferred to nitrocellulose membranes. The blots were incubated with AMPKα rabbit monoclonal antibody (Cell Signaling Technology; dil 1:1000), pAMPKα (Thr172) rabbit monoclonal antibody (Cell Signaling Technology; dil 1:1000), IKK-β rabbit monoclonal antibody (Abcam; dil 1:500) or α-tubulin mouse antibody (Sigma Aldrich; dil 1:1000) overnight at 4°C and then with secondary antibody against rabbit or mouse IgG (Promega; dil 1:2500) for 1 h at RT. The signals were visualized with the ECL system (Pierce). The expression level of α-tubulin was used to normalize the data.
Statistical Analysis
Statistical analyses were carried out using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). ANCOVA or one way ANOVA followed by Tukey’s post hoc test was used to evaluate differences between the groups. P values smaller than 0.05 were considered statistically significant.
Ethics Statement
Procedures involving animals and their care were conducted in conformity with international and national law and policies (EU Directive 2010/63/EU for animal experiments, ARRIVE guidelines and the Basel declaration including the 3R concept). The procedures reported here were approved by the Institutional Committee on the Ethics of Animal Experiments (CSV) of the University of Naples Federico II and by the Ministero della Salute.
Results
Effect of High Fat Diet Enriched in Lard or Fish Oil on Body Weight Gain and Energy Intake
Figure 1 reports the changes in body weight, daily food consumption (g/day) and energy intake in LD or FD fed rats compared to CD at 1–6 weeks of treatment. At each time point, the body weight was higher in the LD compared to CD, but the difference was statistically significant only after 5 and 6 weeks of treatment (Figure 1A). On the other hand, the three groups of rats did not show any difference in the daily food consumption (g/diet, Figure 1B). whereas the energy intake (kJ/diet) was significantly higher in the LD and FD groups compared to the CD group at each time point (Figure 1C).
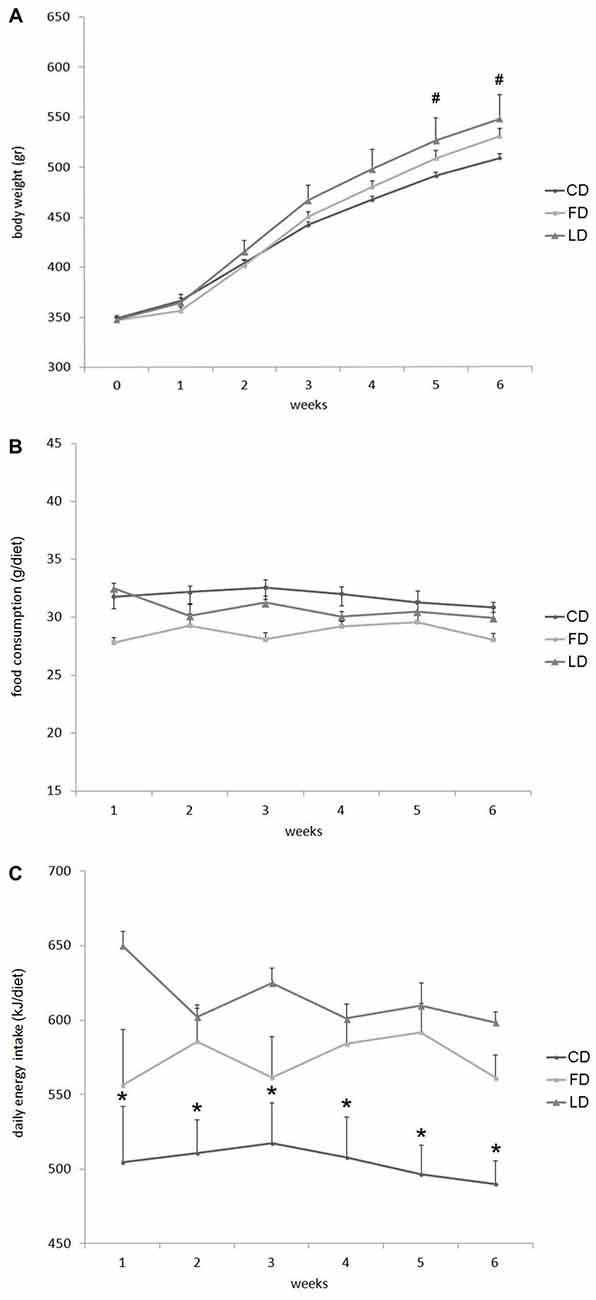
Figure 1. Effect of high fat diet enriched in lard or in fish oil on body weight, food consumption and energy intake. Body weight (A), food consumption (B) and daily energy intake (C) during 6 weeks of treatment with control diet (CD), fish oil enriched diet (FD) or lard enriched diet (LD). Values are expressed as Mean ± SEM. #P < 0.05 compared to the CD group; *P < 0.05 compared to the other groups.
Effect of High Fat Diet Enriched in Lard or Fish Oil on Plasma Lipids, Hormones and TNF-α
As shown in Figure 2, the HFD enriched with fish oil for 6 weeks increased significantly basal glycemia compared to the CD (P < 0.05), while the other parameters (non-esterified fatty acid (NEFA), cholesterol, TG, insulin, leptin, TNF-α and adiponectin) did not change compared to CD, although they were significantly different from LD group.
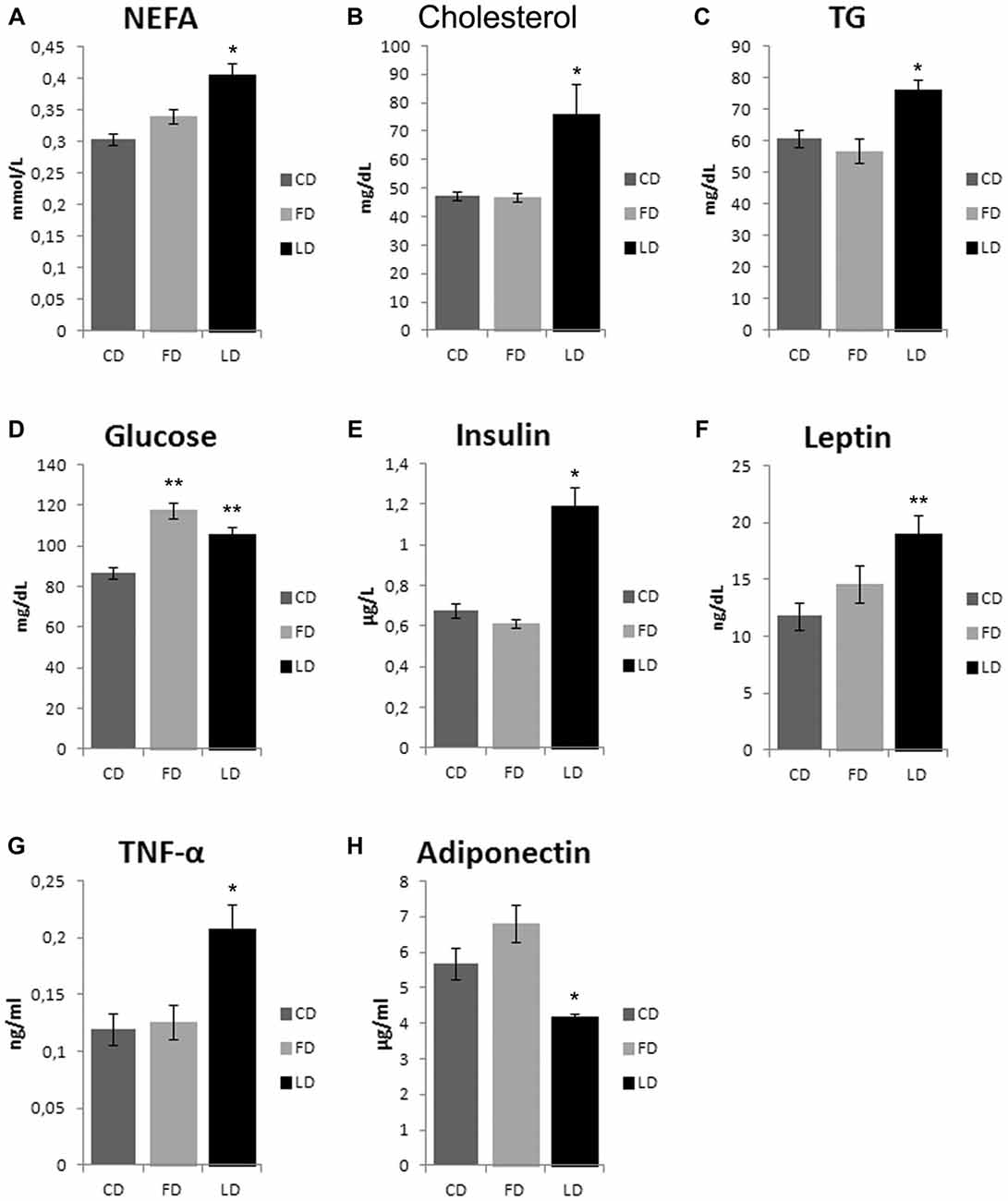
Figure 2. Effect of high fat diet enriched in lard or in fish oil on plasma lipids, glycemia, hormones and inflammatory index. Serum levels of non-esterified fatty acid (NEFA, A), cholesterol (B), triglycerides (TG, C), glucose (D) insulin (E), leptin (F), TNF-α (G) and adiponectin (H), in animals treated for 6 weeks with control diet (CD), fish oil enriched diet (FD) or lard enriched diet (LD). Values are expressed as Mean ± SEM. *P < 0.05 compared to CD and FD; **P < 0.05 compared to CD.
Effect of the High Fat Diet Enriched in Lard or Fish Oil on the Phosphorylation of the Hypothalamic AMPK
The HFD (enriched in lard or fish oil) did not modify the expression of AMPK in the hypothalamus as judged by the results of Western blot analyses (Figure 3). To investigate the effects of the HFD on the activity of this protein, we also analyzed the expression level of pAMPK (the phosphorylated active form of AMPK) and we did not observe any difference between FD and CD groups (Figure 3), indicating that the fish oil-enriched diet did not change the hypothalamic expression level of AMPK and its activation level. Interestingly however, the pAMPKα expression level significantly increased in the LD group compared to FD group (Figure 3).
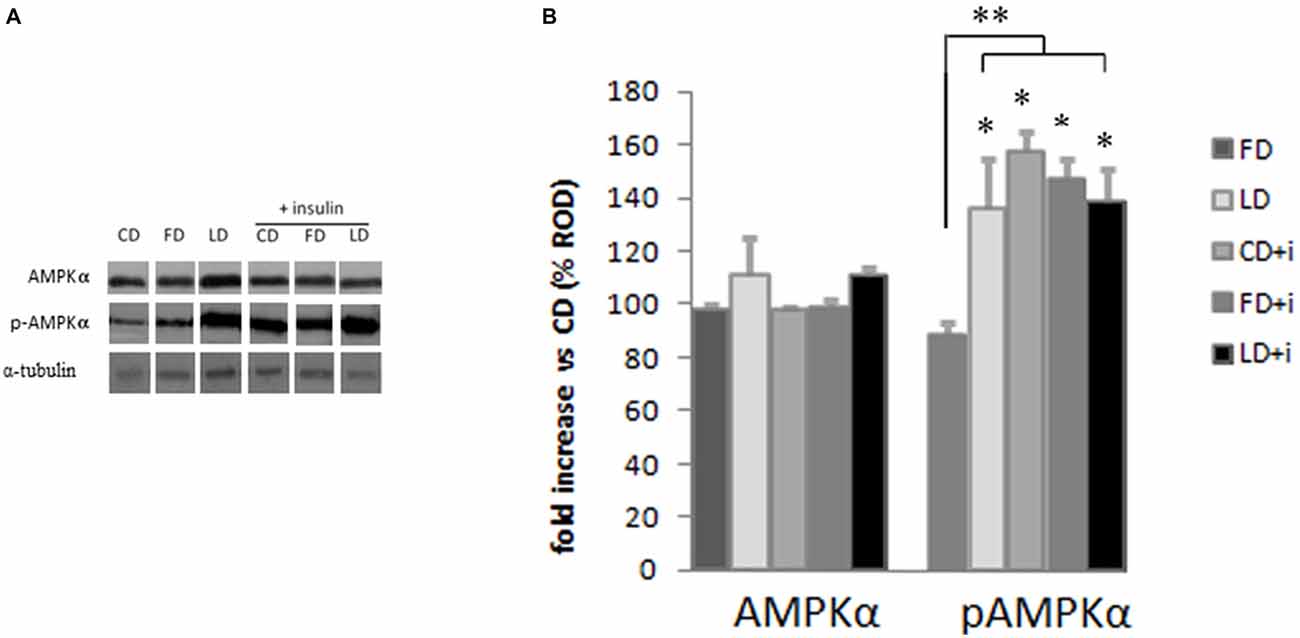
Figure 3. Effect of high fat diet enriched in lard or in fish oil on hypothalamic AMPK or pAMPK in rats with or without insuline injection. (A) Representative blots probed with antibodies against p-AMPK (phosphorylated at Thr172), AMPK and α-tubulin. Samples were obtained by hypothalamus of animals treated for 6 weeks with control diet (CD), fish oil enriched diet (FD) or lard enriched diet (LD). Some of the animals were injected with insulin (+ insulin). (B) Quantitation of protein levels of AMPK and pAMPK normalized to the α-tubulin by densitometry. The bars show the values (Mean ± SD) expressed as percentage of CD group as Relative Optical Density (ROD). *P < 0.05 compared to CD and FD. **P < 0.05 compared to FD.
To evaluate the involvement of insulin sensitivity in the LD-dependent increase of pAMPK, the three groups of animals were intraperitoneally injected with insulin. The insulin did not modify the AMPK expression level in the hypothalamus. Interestingly, the insulin in CD and FD groups increased significantly the pAMPK level in the hypothalamus compared with the corresponding groups without insulin treatment. On the other hand the pAMPK expression level did not change in LD group treated with insulin (Figure 3).
Effect of High Fat Diet Enriched in Lard or Fish Oil on Hypothalamic IKKβ
To investigate the molecular mechanisms underlying the HFD-dependent activation of AMPK we analyzed the expression level of IKKβ (activator of NFKB pathway) in the hypothalamus of treated animals. We did not observe significant changes of this mediator in LD or FD groups compared to CD, suggesting that the mechanism induced by LD-enriched diet and leading to the activation of hypothalamic AMPK is independent from IKKβ (data not shown).
Effect of High Fat Diet Enriched in Lard or Fish Oil on Inflammation and Oxidative Stress in the Hypothalamus
The HFD enriched in LD causes an increase of oxidative stress in the hypothalamus as demonstrated by the significant increase of MDA (P < 0.01) and TNFα (P < 0.05) and the significant decrease of GSH and GSH/GSSG (P < 0.01) in LD compared to FD and CD groups. On the other hand, the HFD enriched in fish oil did not affect these parameters (that are similar to control) demonstrating beneficial effects of fish oil on oxidative stress in the hypothalamus (Figure 4). In addition, the negligible differences in GST and NQO1 activities in LD and FD groups compared to CD indicated that the Nrf2 pathway is not involved in the HFD-mediated modulation of redox status (data not shown).
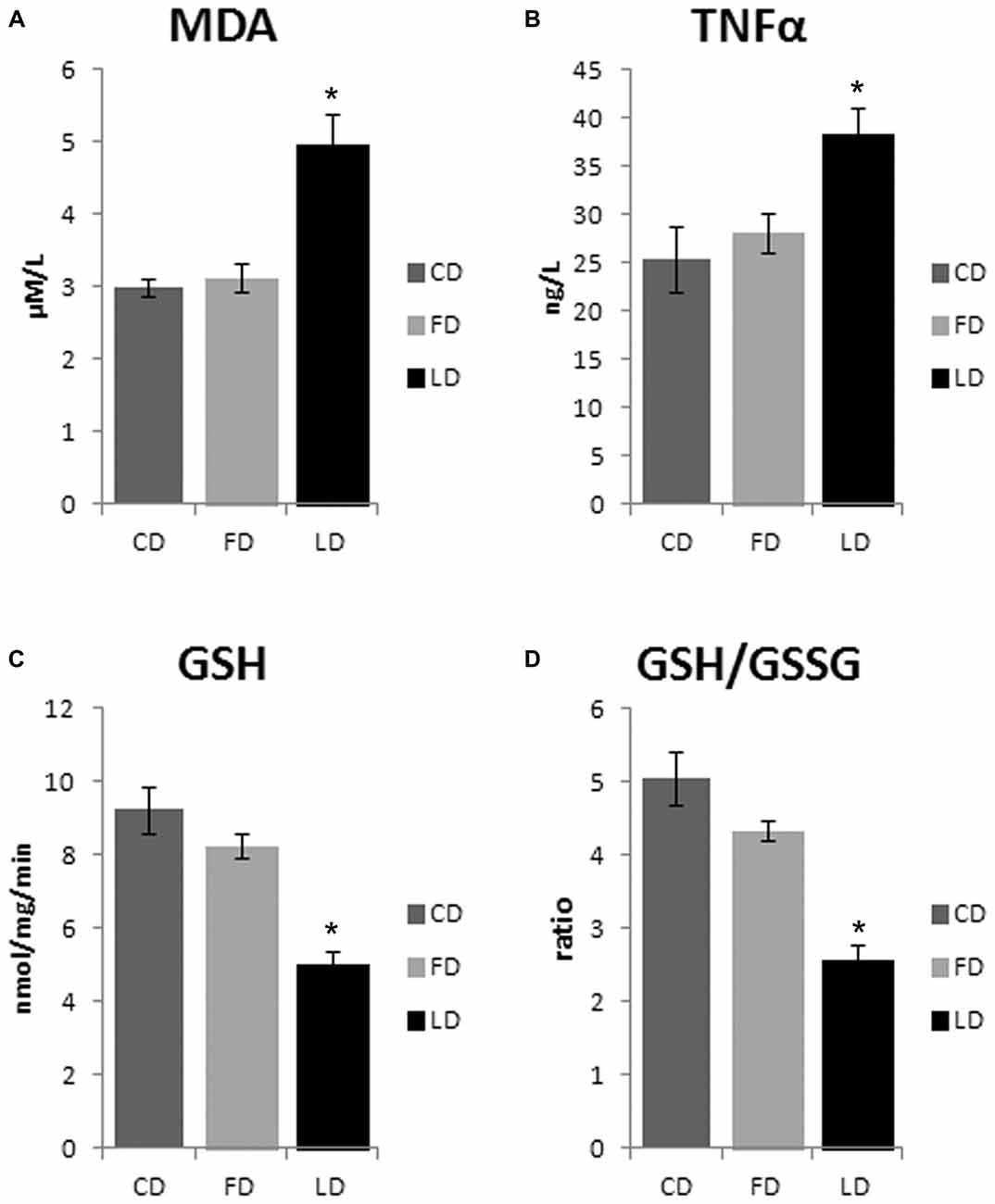
Figure 4. Effect of high fat diet enriched in lard or in fish oil on hypothalamic oxidative stress. Hypothalamic levels of malondialdehyde (MDA, A), TNF-α (B), reduced glutathione (GSH, C) and ratio reduced/oxidized glutathione (GSH/GSSG, D) in animals treated for 6 weeks with control diet (CD), fish oil enriched diet (FD) or lard enriched diet (LD). Values are expressed as Mean ± SEM. *P < 0.05 compared to CD and FD.
Discussion
The present study aims to evaluate the effect of the nutritional substitution of saturated by unsaturated fatty acids on the modulation of hypothalamic inflammation in obesity. Our data confirmed that the extent of HFD harmful effects depends on the type of fat included in the diet and in particular, the HFDs rich in poly-unsaturated fatty acids (fish oil) are less deleterious than those rich in saturated fat (lard).
Rats fed for 6 weeks with HFD enriched in lard or fish oil showed an increase in body weight when compared to a control group. On the other hand, in previous studies, using the same experimental paradigm, we found in FD group a significant decreased in body fat mass compared to LD group (Cavaliere et al., 2016). In addition, we also showed that the increase in body weight was not associated with an increase in food intake, but with an increase in energy intake, in agreement with several previous studies (Cavaliere et al., 2016). In this context, it is worth to notice that rodents are considered hyperphagic if the energy intake increases even in conditions of normal food intake (Ghibaudi et al., 2002; Woods et al., 2003; Huang et al., 2004).
To verify the hypothesis that the increase in energy intake may depend on activation of the AMPK pathway in the hypothalamus, that is strongly involved in the control of food intake (Minokoshi et al., 2004), we studied the effect of a HFD on the hypothalamic AMPK expression. Although the hypothalamic expression of AMPK was not modified by diet, the level of the active phosphorylated form of the enzyme (pAMPK, phosphorylated at Thr 172) was significantly increased in LD group, suggesting that the administration of this diet leads to the activation, at hypothalamic level, of a key enzyme regulating the food intake. This effect was observed exclusively with LD diet suggesting that the type of fats affects pAMPK expression. The activation of AMPK in LD group is independent from IKKβ, an inhibitor of NF-κB, since the IKKβ expression level did not change with the administration of HFD.
We also observed that HFD rich in lard, unlike HFD rich in fish oil, affects the insulin, leptin and adiponectin levels. Therefore, a possible mechanism underlying the AMPK activation in the hypothalamus of LD animals may involve orexinergic or anorexinergic molecules. Indeed, several orexinergic and anorexinergic molecules are known to modify the AMPK activity in the hypothalamus. In particular, glucose, leptin and insulin inhibit AMPK (Minokoshi et al., 2004; Kim and Lee, 2005), and the HFD induces resistance to leptin (Martin et al., 2006) and insulin (Lichtenstein and Schwab, 2000; Woods et al., 2003; Riccardi et al., 2004). Leptin and insulin are present in the hypothalamic neurons that are involved in body weight regulation, and the loss of leptin and insulin in the hypothalamus can promote obesity and type 2 diabetes (Morton and Schwartz, 2011). In the present study, we have shown that the insulin injection increased hypothalamic expression of pAMPKα in the CD group and in the FD group, but it had no effect in the LD group. A possible explanation could be that the increased hypothalamic expression of pAMPK, following insulin injection in the CD group and in the FD group, was mediated by hypoglycemia in agreement with previous findings that hypoglycemia activates AMPK (Han et al., 2005). The LD group instead, might have developed insulin resistance, as previously reported (Xu et al., 2003; Hancock et al., 2008; Cavaliere et al., 2016), resulting therefore in the lack of hypoglycemic effect of exogenous insulin, and in turn, no further increase in hypothalamic pAMPK expression. Central and peripheral insulin resistance could depend on inflammatory molecules. In fact, overnutrition can induce inflammatory responses in peripheral tissues and in the hypothalamus, resulting in a defective control of food intake and energy expenditure (Minokoshi et al., 2004; De Souza et al., 2005). In particular, hypothalamic inflammation affects whole body energy homeostasis mostly by controlling neural inputs to specific organs (Thaler et al., 2010, 2013). Here, we found in FD group a reduction in the hypothalamic inflammation (indicated by decreased TNFα level) and oxidative stress (indicated by decreased MDA level). Nonetheless, the modulation of inflammatory state and redox state does not depend on Nrf2 pathway since GST and NQO1 activities do not change in the three groups of rats. Further studies are necessary to investigate on other molecules that may mediate the anti-inflammatory and anti-oxidant effects of polyunsaturated fatty acid. The reduction of hypothalamic inflammation in obesity corrects simultaneously feeding, thermogenesis and metabolic disarrangements, placing this phenomenon in a central position in the pathogenesis of obesity (De Souza et al., 2005; Milanski et al., 2009).
Our data confirm that chronic overnutrition leads to hypothalamic dysregulation and to the development of overnutrition-related diseases. Interestingly, the substitution of saturated by unsaturated fatty acids in the diet has beneficial effects on modulation of hypothalamic inflammation and function in obesity, underlying, at hypotalamic level, the interaction among insulin and/or leptin resistance, AMPK activation and hyperphagia.
Author Contributions
EV and MPM conceived the original idea, designed and supervised the whole study; MPM, EV, GC, GT, CDeF and MG performed the experiments; MPM and EV analyzed and interpreted data; EV, MPM, LL, SC, AB, BDeL MC and MM critically revised the manuscript for intellectual content; EV, MPM, MM and MC wrote the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thanks Dr. Roberta Scognamiglio for the invaluable technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fncel.2016.00150/abstract
References
Benson, A. M., Hunkeler, M. J., and Talalay, P. (1980). Increase of NAD(P)H: quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc. Natl. Acad. Sci. U S A 77, 5216–5220. doi: 10.1073/pnas.77.9.5216
Bergamo, P., Maurano, F., and Rossi, M. (2007). Phase 2 enzymes induction by Conjugated Linoleic Acid improves lupus-associated oxidative stress. Free Radic. Biol. Med. 43, 71–79. doi: 10.1016/j.freeradbiomed.2007.03.023
Cai, D. (2009). NFκB-mediated metabolic inflammation in peripheral tissues versus central nervous system. Cell Cycle 8, 2542–2548. doi: 10.4161/cc.8.16.9386
Cavaliere, G., Trinchese, G., Bergamo, P., De Filippo, C., Mattace Raso, G., Gifuni, G., et al. (2016). Polyunsaturated fatty acids attenuate diet induced obesity and insulin resistance, modulating mitochondrial respiratory uncoupling in rat skeletal muscle. PLoS One 11:e0149033. doi: 10.1371/journal.pone.0149033
De Souza, C. T., Araujo, E. P., Bordin, S., Ashimine, R., Zollner, R. L., Boschero, A. C., et al. (2005). Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146, 4192–4199. doi: 10.1210/en.2004-1520
Ghibaudi, L., Cook, J., Farley, C., van Heek, M., and Hwa, J. J. (2002). Fat intake affects adiposity, comorbidity factors and energy metabolism of sprague-dawley rats. Obes. Res. 10, 956–963. doi: 10.1038/oby.2002.130
Han, S. M., Namkoong, C., Jang, P. G., Park, I. S., Hong, S. W., Katakami, H., et al. (2005). Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia 48, 2170–2178. doi: 10.1007/s00125-005-1913-1
Hancock, C. R., Han, D. H., Chen, M., Terada, S., Yasuda, T., Wright, D. C., et al. (2008). High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl. Acad. Sci. U S A 105, 7815–7820. doi: 10.1073/pnas.0802057105
Hardie, D. G. (2014). AMPK: positive and negative regulation and its role in whole-body energy homeostasis. Curr. Opin. Cell. Biol. 33, 1–7. doi: 10.1016/j.ceb.2014.09.004
Hernández-Aguilera, A., Rull, A., Rodríguez-Gallego, E., Riera-Borrull, M., Luciano-Mateo, F., Camps, J., et al. (2013). Mitochondrial dysfunction: a basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediators Inflamm. 2013:135698. doi: 10.1155/2013/135698
Hotamisligil, G. S. (2006). Inflammation and metabolic disorders. Nature 444, 860–867. doi: 10.1038/nature05485
Huang, X. F., Xin, X., McLennan, P., and Storlien, L. (2004). Role of fat amount and type in ameliorating diet-induced obesity: insights at the level of hypothalamic arcuate nucleus leptin receptor, neuropeptide Y and pro-opiomelanocortin mRNA expression. Diabetes Obes. Metab. 6, 35–44. doi: 10.1111/j.1463-1326.2004.00312.x
Kim, M. S., and Lee, K. U. (2005). Role of hypothalamic 5’-AMP-activated protein kinase in the regulation of food intake and energy homeostasis. J. Mol. Med. 83, 514–520. doi: 10.1007/s00109-005-0659-z
Levine, R. L., Garland, D., Oliver, C. N., Amici, A., Climent, I., Lenz, A. G., et al. (1990). Determination of carbonyl content in oxidatively modified proteins. Meth. Enzymol. 186, 464–478. doi: 10.1016/0076-6879(90)86141-h
Lichtenstein, A. H., and Schwab, U. S. (2000). Relationship of dietary fat to glucose metabolism. Atherosclerosis 150, 227–243. doi: 10.1016/s0021-9150(99)00504-3
Lionetti, L., Mollica, M. P., Donizzetti, I., Gifuni, G., Sica, R., Pignalosa, A., et al. (2014a). High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS One 9:e92753. doi: 10.1371/journal.pone.0092753
Lionetti, L., Mollica, M. P., Sica, R., Donizzetti, I., Gifuni, G., Pignalosa, A., et al. (2014b). Differential effects of high-fish oil and high-lard diets on cells and cytokines involved in the inflammatory process in rat insulin-sensitive tissues. Int. J. Mol. Sci. 15, 3040–3063. doi: 10.3390/ijms15023040
Lu, H., Zhang, D. M., Chen, H. L., Lin, Y. X., Hang, C. H., and Yin, H. X. (2009). N-acetylcysteine suppresses oxidative stress in experimental rats with subarachnoid hemorrhage. J. Clin. Neurosci. 16, 684–688. doi: 10.1016/j.jocn.2008.04.021
Martin, T. L., Alquier, T., Asakura, K., Furukawa, N., Preitner, F., and Kahn, B. B. (2006). Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J. Biol. Chem. 281, 18933–18941. doi: 10.1074/jbc.m512831200
Martínez-Fernández, L., Laiglesia, L. M., Huerta, A. E., Martínez, J. A., and Moreno-Aliaga, M. J. (2015). Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid. Mediat. 121, 24–41. doi: 10.1016/j.prostaglandins.2015.07.003
Messina, G., De Luca, V., Viggiano, A., Ascione, A., Iannaccone, T., Chieffi, S., et al. (2013). Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurol. Res. Int. 2013:639280. doi: 10.1155/2013/639280
Milanski, M., Degasperi, G., Coope, A., Morari, J., Denis, R., Cintra, D. E., et al. (2009). Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J. Neurosci. 29, 359–370. doi: 10.1523/jneurosci.2760-08.2009
Minokoshi, Y., Alquier, T., Furukawa, N., Kim, Y. B., Lee, A., Xue, B., et al. (2004). AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569–574. doi: 10.1038/nature02440
Monda, M., Amaro, S., and De Luca, B. (1993). The influence of exercise on energy balance changes induced by ventromedial hypothalamic lesion in the rat. Physiol. Behav. 54, 1057–1061. doi: 10.1016/0031-9384(93)90324-9
Monda, M., Sullo, A., De Luca, E., and Pellicano, M. P. (1996). Lysine acetylsalicylate modifies aphagia and thermogenic changes induced by lateral hypothalamic lesion. Am. J. Physiol. 271, R1638–R1642.
Morton, G. J., and Schwartz, M. W. (2011). Leptin and the central nervous system control of glucose metabolism. Physiol. Rev. 91, 389–411. doi: 10.1152/physrev.00007.2010
Riccardi, G., Giacco, R., and Rivellese, A. A. (2004). Dietary fat, insulin sensitivity and the metabolic syndrome. Clin. Nutr. 23, 447–456. doi: 10.1016/j.clnu.2004.02.006
Salt, I. P., and Palmer, T. M. (2012). Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert. Opin. Investig. Drugs 21, 1155–1167. doi: 10.1517/13543784.2012.696609
Thaler, J. P., Choi, S. J., Schwartz, M. W., and Wisse, B. E. (2010). Hypotalamic inflammation and energy homeostasis: resolving the paradox. Front. Neuroendocrinol. 31, 79–84. doi: 10.1016/j.yfrne.2009.10.002
Thaler, J. P., Guyenet, S. J., Dorfman, M. D., Wisse, B. E., and Schwartz, M. W. (2013). Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes 62, 2629–2634. doi: 10.2337/db12-1605
Viggiano, A., Viggiano, A., Monda, M., Turco, I., Incarnato, L., Vinno, V., et al. (2006). Annurca apple-rich diet restores long-term potentiation and induces behavioral modifications in aged rats. Exp. Neurol. 199, 354–361. doi: 10.1016/j.expneurol.2006.01.001
Woods, S. C., Seeley, R. J., Rushing, P. A., D’Alessio, D., and Tso, P. (2003). A controlled high-fat diet induces an obese syndrome in rats. J. Nutr. 133, 1081–1087.
Xu, H., Barnes, G. T., Yang, Q., Tan, G., Yang, D., Chou, C. J., et al. (2003). Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830. doi: 10.1172/jci19451
Xue, B., Yang, Z., Wang, X., and Shi, H. (2012). Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PloS One 7:e45990. doi: 10.1371/journal.pone.0045990
Keywords: high fat diet, ω3-PUFA, AMPK, inflammation, oxidative stress
Citation: Viggiano E, Mollica MP, Lionetti L, Cavaliere G, Trinchese G, De Filippo C, Chieffi S, Gaita M, Barletta A, De Luca B, Crispino M and Monda M (2016) Effects of an High-Fat Diet Enriched in Lard or in Fish Oil on the Hypothalamic Amp-Activated Protein Kinase and Inflammatory Mediators. Front. Cell. Neurosci. 10:150. doi: 10.3389/fncel.2016.00150
Received: 08 March 2016; Accepted: 27 May 2016;
Published: 09 June 2016.
Edited by:
Francesco Moccia, University of Pavia, ItalyCopyright © 2016 Viggiano, Mollica, Lionetti, Cavaliere, Trinchese, De Filippo, Chieffi, Gaita, Barletta, De Luca, Crispino and Monda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marianna Crispino, crispino@unina.it
† These authors have contributed equally to this work, and share the first authorship.
 Emanuela Viggiano
Emanuela Viggiano Maria Pina Mollica
Maria Pina Mollica Lillà Lionetti
Lillà Lionetti Gina Cavaliere
Gina Cavaliere Giovanna Trinchese
Giovanna Trinchese Chiara De Filippo
Chiara De Filippo Sergio Chieffi
Sergio Chieffi Marcello Gaita
Marcello Gaita Antonio Barletta3
Antonio Barletta3  Marianna Crispino
Marianna Crispino Marcellino Monda
Marcellino Monda