- 1Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia
- 2Sir Charles Gairdner Hospital, Nedlands, WA, Australia
- 3Neuroepidemiology Unit, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia
- 4Biostatistics Unit, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia
Multiple sclerosis (MS) is a major cause of disability and poor quality of life (QOL). Previous studies have shown differences in MS health outcomes between countries. This study aimed to examine the associations between international regions and health outcomes in people with MS. Self-reported data were taken from the Health Outcomes and Lifestyle In a Sample of people with Multiple Sclerosis online survey collected in 2012. The 2,401 participants from 37 countries were categorized into three regions: Australasia, Europe, and North America. Differences were observed between regions in disability, physical and mental health QOL, fatigue, and depression, but most of these disappeared after adjusting for sociodemographic, disease, and lifestyle factors in multivariable regression models. However, adjusted odds for disability were higher in Europe [odds ratio (OR): 2.17, 95% confidence interval (CI): 1.28 to 3.67] and North America (OR: 1.79, 95% CI: 1.28 to 2.51) compared to Australasia. There may be other unmeasured factors that vary between regions, including differences in access and quality of healthcare services, determining disability in MS. When assessing differences in MS health outcomes, lifestyle factors and medication use should be taken into consideration.
Introduction
Multiple sclerosis (MS) is a chronic autoimmune neurological disorder involving inflammation and demyelination of the central nervous system (CNS). An estimated 2.5 million people are affected by MS across the world. MS can cause debilitating physical and mental health symptoms and often affects young people. Symptoms vary and depend on which part of the CNS is affected and include numbness, tingling, fatigue, and problems with balance and vision. Common comorbidities of MS are back pain, arthritis, anxiety, and depression (1). There is a genetic predisposition to MS, however, genetics play little role in progression, severity and presence of comorbidities, which have been closely linked to environmental and lifestyle factors (1–3). There is a higher prevalence of MS in developed countries compared to developing countries. Furthermore, prevalence increases as latitude from the equator increases thought to be associated with sun exposure and vitamin D (4). Other environmental risk factors for MS onset and progression include infections, and lifestyle factors such as smoking, stress, diet, and weight (5–7).
Multiple sclerosis is one of the major causes of disability in young adults in developed countries and is associated with poorer quality of life (QOL) than the general population (8, 9). Fatigue is the most common symptom affecting people with MS (PwMS) with a prevalence of up to 75%, significantly higher than the general population (10, 11). Depression is the most common mental health comorbidity of MS with a lifetime prevalence of up to 50%, which is markedly elevated compared to the general population (10–15%), and higher compared to people with other chronic illnesses (12–14). Comparisons of these health outcomes for different countries or regions are difficult to interpret because the tools used to measure health outcomes and inclusion and exclusion criteria vary between studies. To date, few studies have compared health outcomes such as disability, physical and mental health QOL, fatigue, and depression in PwMS between countries globally.
We have previously reported international differences in obesity and many comorbidities including depression, diabetes, and cardiovascular disease in PwMS, with a higher prevalence in the US and Canada using date from the Health Outcomes and Lifestyle In a Sample of people with Multiple Sclerosis (HOLISM) study (14). Lifestyle factors, including diet, smoking, and physical activity, were also associated with a number of comorbidities (14). Differences in other health outcomes are also likely to be associated with differences in health care access, latitude, lifestyle, and other sociodemographic factors that vary from country to country (15, 16).
The aim of the current study was to assess differences in health outcomes between countries and assess if these differences were associated with sociodemographic, disease, and lifestyle variables in the HOLISM study.
Materials and Methods
The methodology of the HOLISM study has previously been described in detail (17). A summary of the relevant details is provided here.
Participants and Procedures
Participants were recruited via MS society websites and newsletters, MS blogs, MS forums, Facebook pages, and Twitter accounts specific for PwMS over 15 weeks in 2012. The study ad and link to the survey were posted through these media, many of which had a health and lifestyle focus and were in English language. The web-based tool, SurveyMonkey®, was used to provide respondents with an information sheet, electronic consent indicator, and the survey itself. To be eligible for the study, participants were required to be over 18 years of age and to have a self-reported physician-confirmed diagnosis of MS. Contact details from all participants were required to facilitate follow-up. The study was approved by the Health Sciences Human Ethics Sub-Committee at the University of Melbourne (Ethics ID: 1545102), and all participants indicated consent before entering the survey.
Measures
The online survey was in English and used validated tools where possible. It took approximately 40 min to complete, and all data were self-reported.
Sociodemographic and Disease Characteristics
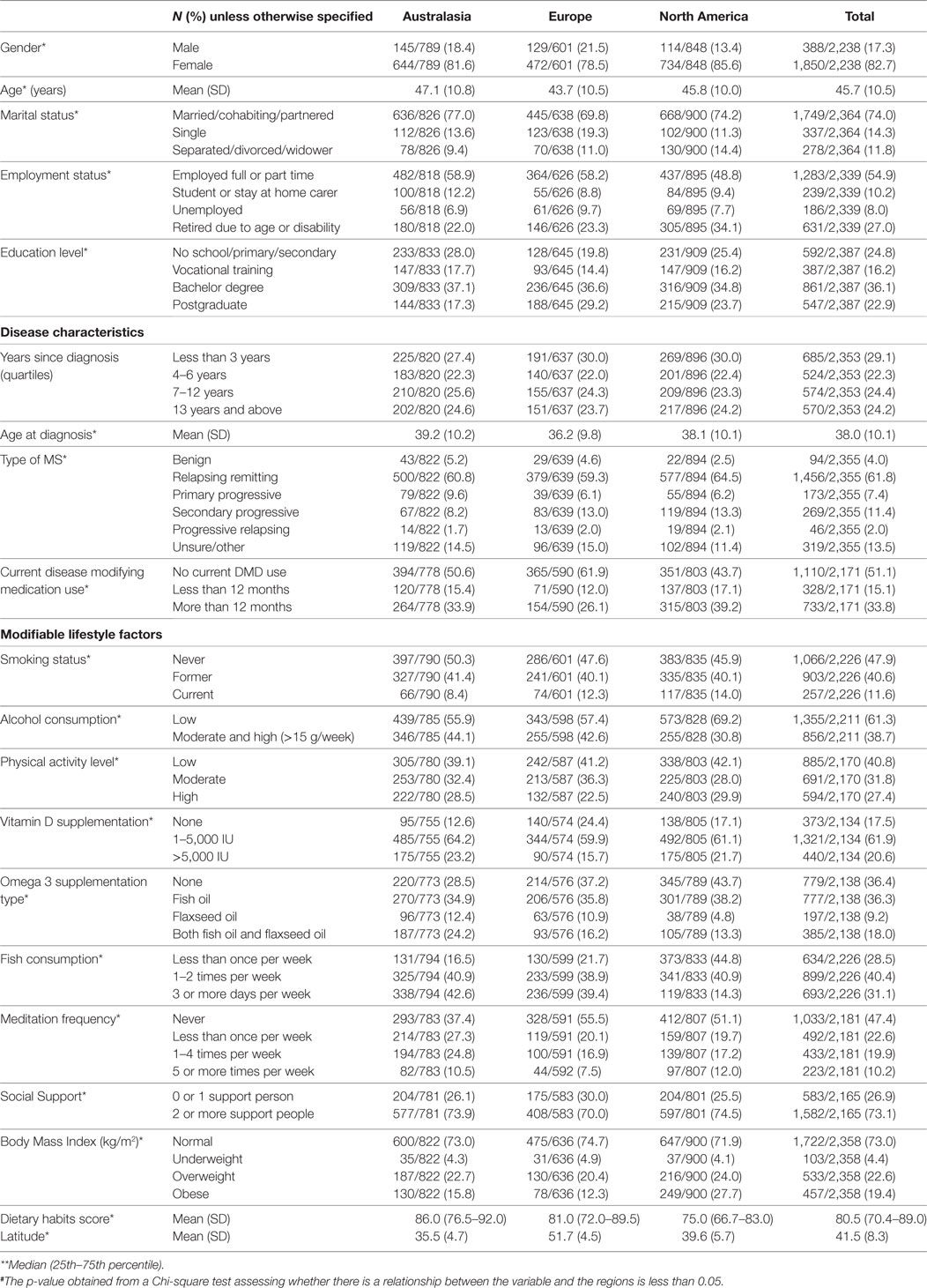
The sociodemographics and disease characteristics that were assessed included age, gender, years since diagnosis, age at diagnosis, type of MS, level of disability, disease-modifying drug (DMD) use, employment status, marital status, education level, and latitude derived from data for location (country and city). Country of residence was grouped into three categories that had sufficient sample size to enable meaningful analysis: Australasia (Australia and New Zealand), Europe, and North America (United States and Canada). Participants from Africa, Asia, and Latin America were excluded because of small sample size.
Disability
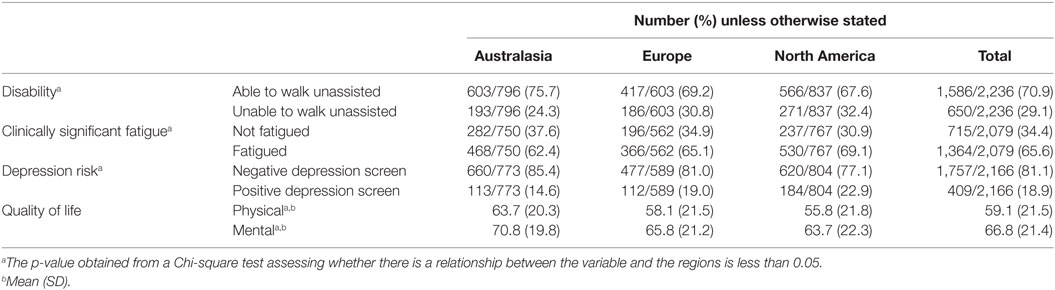
Current level of disability was measured using a self-reported Patient Determined Disease Step (PDDS) score. The PDDS has been validated against the EDDS, which is commonly used by neurologists and clinicians (18). PDDS was categorized into a binary outcome, such that scores of 0–3 were classified as able to walk unassisted or “no disability” and 4–8 as unable to walk unassisted or “disability.”
Quality of Life
The Multiple Sclerosis Quality Of Life-54 scale was used to measure health-related QOL and was specifically designed for PwMS (19). Scores range from zero to 100, indicating lowest to highest QOL, respectively. The physical composite score accounts for factors such as physical role limitation, energy/fatigue, pain, and sexual function. The mental health composite includes health distress, emotional wellbeing, emotional role limitation, and cognitive function (19).
Fatigue
The Fatigue Severity Scale (20) is a validated tool that consists of nine fatigue-related statements that are rated over the past week on a seven-point scale from “disagree” to “agree.” A mean score of ≥4 was used as a cutoff to indicate clinically significant fatigue as is commonly done.
Depression
Depression risk was assessed using the two-item Patient Health Questionnaire-2, which has been validated for use in PwMS (21). A cutoff of ≥3 is commonly used as a positive screen for depression risk.
Modifiable Lifestyle Factors
A range of lifestyle factors was assessed. Dietary habits were assessed using a modified version of the Diet Habits Questionnaire in which items assessing salt intake and alcohol use were excluded. A higher score meant a better diet as prescribed for cardiac health (22). Alcohol consumption was instead assessed in more detail separately and was classified as low (<15 g/week) and moderate/high consumption (≥15 g/week). Physical activity level was assessed using the International Physical Activity Questionnaire (23). Social support was measured using the Single Item Measure of Social Support (24). Other lifestyle factors assessed with researcher-devised questions were smoking status, vitamin D supplementation, omega-3 supplementation, type of omega-3 supplementation, meditation frequency, and body mass index (BMI, derived from weight and height). BMI was classified as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30 kg/m2).
Data Analysis
Stata 13.0 was used to analyze the data of this cross-sectional study. Sample characteristics were first described to examine sociodemographics, disease characteristics, and modifiable lifestyle factors of participants overall and within each region (Australasia, Europe, and North America). Categorical, continuous, and discrete or skewed data were presented as frequency (percentages), mean (standard deviation), and median (25th–75th percentile), respectively. Multivariable linear and logistic regression models were used to explore the associations between regions and the health outcomes, adjusting for confounders identified a priori based on our previous research (11, 13, 25, 26). We assessed for any potential interactions between region and sociodemographics, disease characteristics, and modifiable lifestyle factors using likelihood ratio tests. No significant interactions were found for any of the health outcomes and were therefore not included in the models.
Results
Descriptive data outlining regional differences in sociodemographics, disease characteristics, and lifestyle factors are presented in Table 1. Significant differences between the regions existed in nearly all variables.
Descriptive data on major health outcomes (disability, physical and mental health QOL, clinically significant fatigue, and depression risk) in our sample are presented per region in Table 2. Significant differences existed between regions for all health outcomes.
Multivariable regression models for disability, physical QOL, mental health QOL, clinically significant fatigue, and depression risk were adjusted for sociodemographic, disease, and lifestyle determinants (Tables 3 and 4). Adjusted odds for disability were higher in Europe and North America compared to Australasia (Table 4). Adjusted odds for physical and mental health QOL, clinically significant fatigue, and depression risk were not significantly associated with region (Tables 3 and 4).
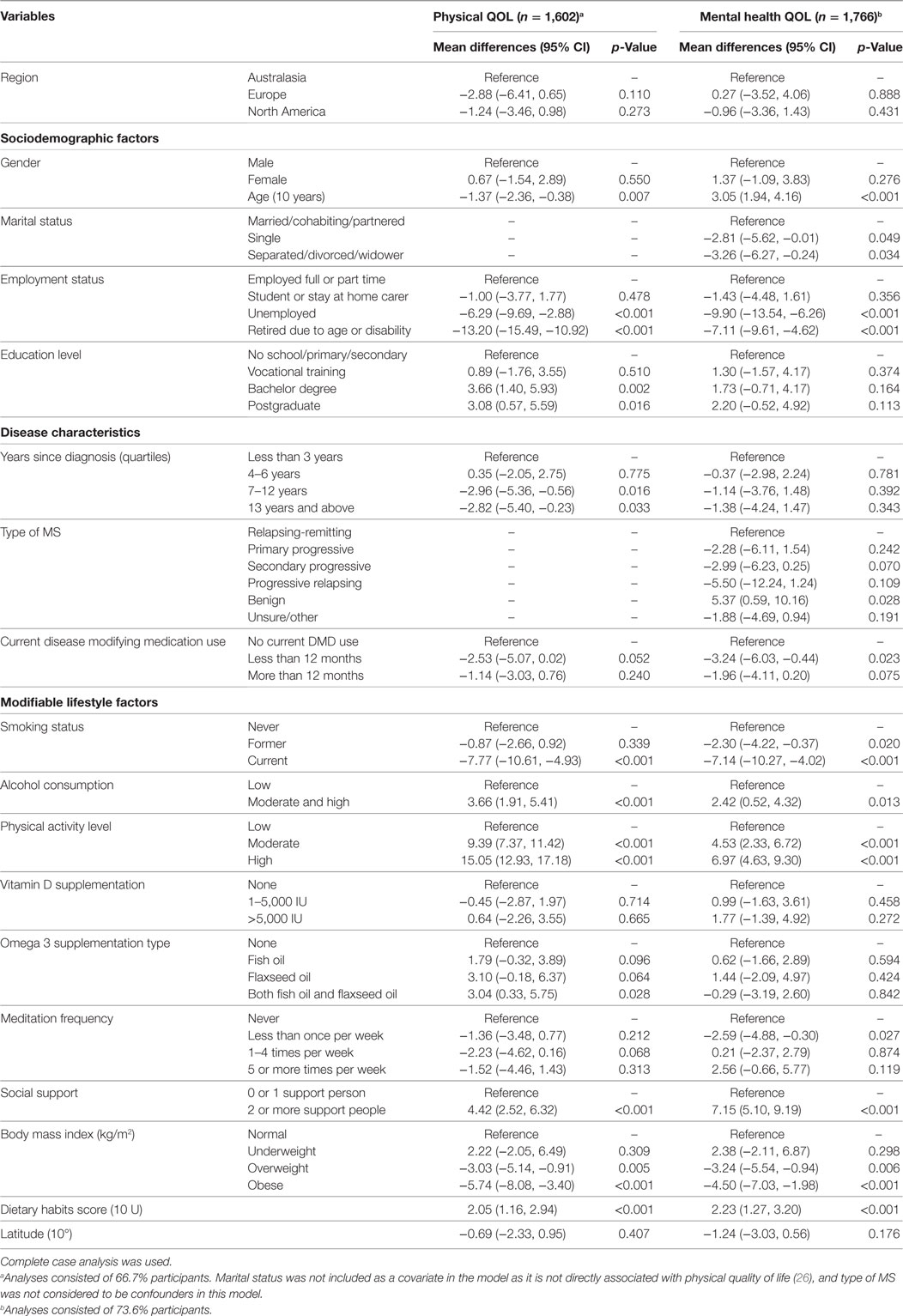
Table 3. Associations between regions and other covariates with physical and mental health quality of life (QOL) obtained using multivariable linear regression models.
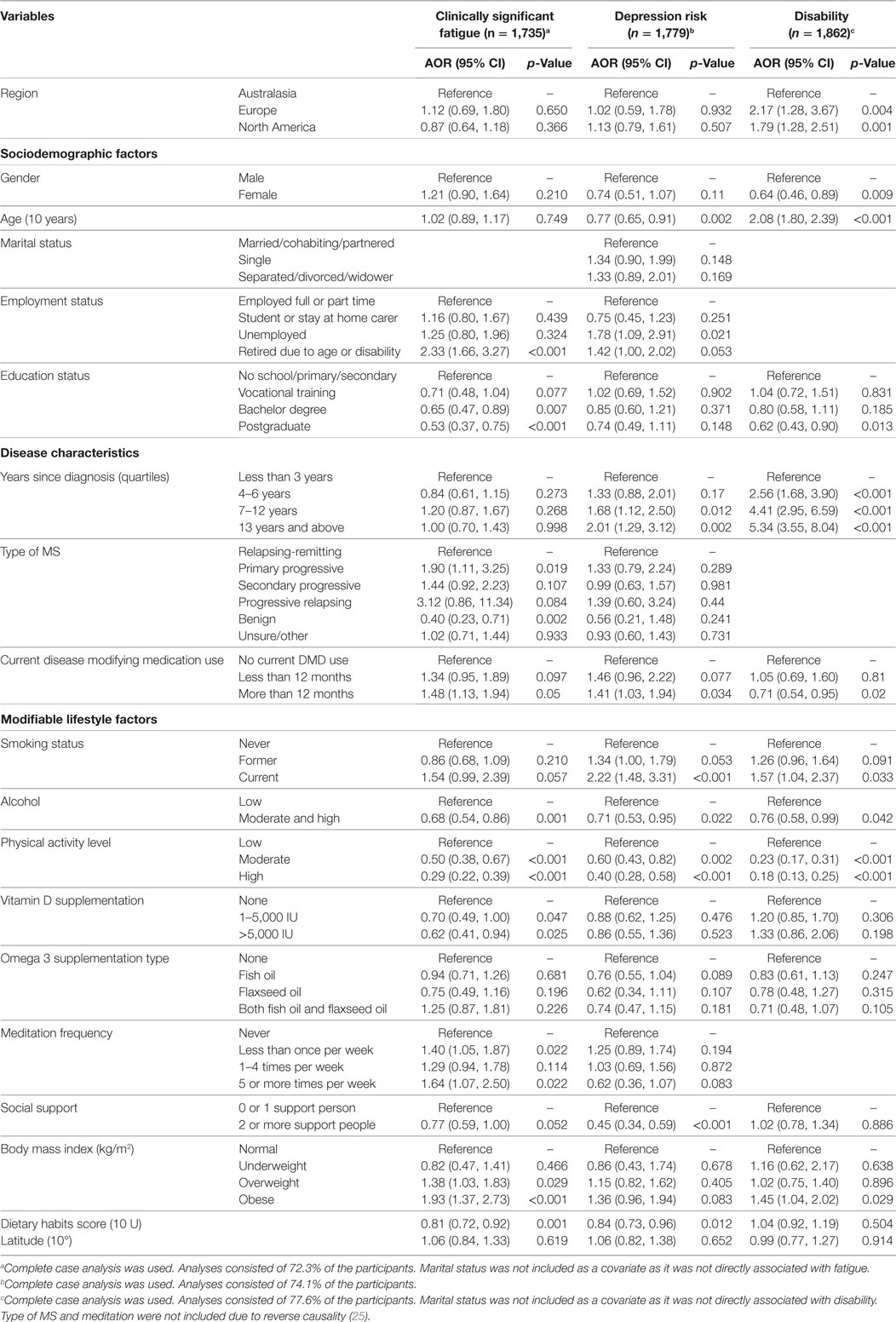
Table 4. Associations between regions and other covariates with clinically significant fatigue, depression screen, disability obtained using multivariable logistic regression.
Many of the sociodemographic and disease characteristics and modifiable lifestyle factors had independent associations with the health outcomes. The main variables associated with a clinically significant difference (5 points) in physical and mental QOL score were age, employment status, type of MS, smoking status, physical activity, social support, BMI, and diet (Table 3).
The main variables associated with a reduced or increased AOR for fatigue were employment status, education status, type of MS, DMD use, smoking status, alcohol use, physical activity, vitamin D supplementation, meditation, social support, BMI, and diet (Table 4). The main variables associated with reduced or increased AOR for depression were age, employment status, years since diagnosis, DMD use, smoking status, alcohol use, physical activity, social support, and diet (Table 4). The main variables associated with reduced or increased AOR for unassisted walking ability were region, gender, education status, years since diagnosis, DMD use, smoking status, alcohol consumption, physical activity level, and BMI (Table 4).
Modifiable Variables Associated with Health Outcomes
Current smoking was associated with a 7–8 point lower score on both QOL domains, and 2.2 times higher odds for depression risk compared to never smoking. Moderate/high alcohol use was associated with 3 points higher physical QOL score, 2 points higher mental health QOL score, 24% lower odds of disability, 38% lower odds of fatigue, and 29% lower odds of depression compared to low alcohol use. It must be noted that very few people consumed high levels of alcohol.
High levels of physical activity were associated with 15 points higher physical QOL score, 7 points higher mental health QOL score, 72% lower odds of disability, 71% lower odds of clinically significant fatigue, and 60% lower odds of depression risk compared to low levels of physical activity. Moderate levels of physical activity were also associated with higher physical and mental health QOL scores, and lower odds of disability, fatigue, and depression. Vitamin D supplementation of 1–5,000 IU was associated with 30% lower odds of fatigue and vitamin D supplementation of >5,000 IU was associated with 38% lower odds of fatigue compared to no vitamin D supplementation. Having two or more people to rely on for support was associated with 4 points higher physical QOL score, 7 points higher mental health QOL score, and 55% lower odds of depression compared to having zero or no support people.
Overweight BMI was associated with 38% higher odds of fatigue compared to normal BMI, and obese BMI associated with 93% higher odds of fatigue and 45% higher odds of disability compared to normal BMI. Overweight and obese BMI were associated with 3 and 6 points lower physical QOL scores, respectively, and 3 and 5 points lower mental health QOL scores, respectively, compared to normal BMI. Healthier dietary habits scores were associated with 19% lower odds of fatigue and 16% lower odds of depression.
Disease-modifying drug use for more than 12 months was associated with 48% higher odds of clinically significant fatigue and 41% higher odds of depression compared to no DMD use. However, DMD use for more than 12 months was associated with 29% lower odds of disability compared to no DMD use.
Discussion
A previous study from our group identifying comorbidities in PwMS using HOLISM data reported international regional differences in the comorbidities assessed and in BMI (14). The question of whether there were international regional differences in disability, QOL, fatigue, and depression in PwMS, and what their determinants might be arose from this study.
Disability (inability to walk unassisted), depression, and clinically significant fatigue were most common in North American, followed by European and Australasian participants of HOLISM. Small but statistically significant differences were seen in physical and mental QOL in a similar pattern. A wide range of prevalence is reported for depression, comorbidities, and other health outcomes in different countries (27), and QOL differences between countries have also been reported (28). These may be attributable to different study inclusion criteria, recruitment methods but may also reflect population differences in demographics, disease characteristics, lifestyles, or other factors. The international breadth of the HOLISM study enabled these questions to be studied in detail in this article.
Multivariable regression analyses showed that QOL, fatigue, and depression were associated with sociodemographics, disease characteristics, and modifiable lifestyle factors. The sociodemographic and disease variables associated with better health outcomes were younger age, female gender, current employment, higher level of education, shorter disease duration, and non-progressive type of MS. DMD use was associated with higher odds of being fatigued or depressed, which may be common side effects of DMDs (29, 30), but lower odds for inability to walk unassisted. Lifestyle factors, of special interest given their modifiable character, associated with better health outcomes were non-smoking status, moderate alcohol use (vs. low), high level of physical activity (vs. low), normal BMI, healthier diet, vitamin D supplementation, and having several support people, in line with current literature (1, 2, 7). Regular meditation was associated with increased odds for fatigue, which may be due to reverse causality as our analysis here is a cross-sectional; a trial is currently underway which may provide further insights (31).
However, even after adjusting for these factors, disability differences between those in North America, Europe, and Australasia still existed. There may be determinants other than the variables included in our models that could explain these significant geographical differences in disability in our sample. It could be speculated that health system factors and accessibility, economic status, access to MS support facilities, previous medication use, and local culture may influence disability in PwMS and may contribute to the differing levels of disability between regions. Incidence of a number of infections also differs between countries and might also be associated with regional differences in disability (32). Future studies should focus on examining these and other specific factors that may be implicated in the observed differences.
A consistent approach to measurement, conducted in large samples, ideally registries, would yield more valuable results (27). Understudied regions such as Asia and Africa still warrant further study, although it is likely that there are fewer studies in these areas because they are less developed or closer to the equator, where the prevalence of MS is lower (4).
The findings of this study have significant clinical implications. From a clinical perspective, physicians should be aware that differences in QOL, depression, and fatigue in PwMS are associated with sociodemographic and disease variables, as well as differences in lifestyle factors. Modification of these lifestyle factors, particularly smoking cessation, regular physical activity, healthy diet, and normal weight, may contribute to improved QOL and reductions in depression and fatigue. While we have previously shown important associations between lifestyle and health outcomes in our sample (11, 13, 25, 26, 33), there are likely other factors at play in explaining differences in disability between regions including variation in health services and access to health care and medications, cultural factors, infection prevalence, and chronic disease prevalence. These factors should be the focus of future research and may provide alternative strategies as part of a comprehensive approach to management of patients with MS.
Limitations
Participants who were not from the three main regions were excluded from this study. Thus, results are not generalizable to PwMS living in other countries. Most PwMS live in the three regions studied, which are generally developed countries and further from the equator. Data provided via the online survey were self-reported and therefore were unable to be verified. Where possible validated tools were used, but for some domains that have not been extensively investigated such as vitamin D supplementation and meditation, validated tools were not available. We dichotomized PDDS responses for ease of analysis, however, this has not previously been validated. Despite the varied backgrounds of participants and large sample, our findings may not be generalizable to all PwMS, as respondents were all English-speaking, mostly young women, overall highly educated, and able to complete the survey online. Selection bias is therefore a likely limitation of the study. Ethnicity was not taken into account and may be a variable of interest for future research. Causality cannot be inferred between factors assessed due to this cross-sectional study design. Temporality is likely to be a significant limitation particularly in cross-sectional analysis of disability due to the time taken to develop disability. Longitudinal data from HOLISM may clarify the associations found in this study and the issue of temporality. Our analyses here were restricted to complete case analyses, that is, the analyses were restricted to participants with complete data, however, the Table S1 in Supplementary Material shows that the sociodemographics and disease characteristics of the participants with and without any missing values are not systematically different.
Conclusion
We observed differences in physical and mental health-related QOL, disability, clinically significant fatigue, and depression risk between three regions in an international sample of PwMS. However, after adjusting for sociodemographic characteristics, disease profile, and modifiable lifestyle factors, these differences disappeared for all health outcomes except disability. Unlike sociodemographics and stable disease characteristics, lifestyle factors can be modified. These results support existing evidence that smoking, low physical activity, and normal weight are associated with better health outcomes. Differences in disability may be associated with other unmeasured factors, such as differences in health system factors and accessibility, economic variations, access to support facilities, medication, and local culture between countries. Future studies are needed to investigate these differences and potentially identify other modifiable factors related to disability.
Ethics Statement
This study was carried out in accordance with the recommendations of Health Sciences Human Ethics Sub-Committee at the University of Melbourne with digitally indicated informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Health Sciences Human Ethics Sub-Committee at the University of Melbourne (Ethics ID: 1545102).
Author Contributions
GJ conceived and obtained funding for the study. GR, AM, GJ, CM, and TW contributed to the design of the study. GR and AM were involved in data collection, analysis of data, and drafted the manuscript with PJ and CM. AL contributed to the data analysis. All the authors edited and approved the final manuscript.
Conflict of Interest Statement
GJ receives royalties for his books Overcoming Multiple Sclerosis and Recovering from Multiple Sclerosis. GJ, SN, and KT have received remuneration for conducting lifestyle educational interventions for people with MS. The other authors declare no competing interests.
Acknowledgments
We thank all participants who provided their data for the study.
Funding
The Bloom Foundation and the Horne Family Charitable Trust, both philanthropic funders, funded the study. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. CM is funded by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (1120014).
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fneur.2017.00229/full#supplementary-material.
Abbreviations
BMI, body mass index; CI, confidence interval; DHQ, diet habits questionnaire; DMDs, disease-modifying drugs; EDSS, expanded disability status scale; FSS, Fatigue Severity Scale; HOLISM, health outcomes and lifestyle in a sample of people with multiple sclerosis; IPAQ, International Physical Activity Questionnaire; MS, multiple sclerosis; OR, odds ratio; PDDS, Patient Determined Disease Step; PHQ-2, Patient Health Questionnaire-2; PwMS, people with multiple sclerosis; QOL, quality of life; SIMSS, Single Item Measure of Social Support; US, United States.
References
1. Marrie RA. Demographic, genetic, and environmental factors that modify disease course. Neurol Clin (2011) 29:323–41. doi: 10.1016/j.ncl.2010.12.004
2. D’hooghe MB, Nagels G, Bissay V, De Keyser J. Modifiable factors influencing relapses and disability in multiple sclerosis. Mult Scler (2010) 16:773–85. doi:10.1177/1352458510367721
3. International Multiple Sclerosis Genetics Consortium. Genome-wide association study of severity in multiple sclerosis. Genes Immun (2011) 12:615–25. doi:10.1038/gene.2011.34
4. Simpson S Jr, Blizzard L, Otahal P, Van Der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry (2011) 82:1132–41. doi:10.1136/jnnp.2011.240432
5. Spelman T, Gray O, Trojano M, Petersen T, Izquierdo G, Lugaresi A, et al. Seasonal variation of relapse rate in multiple sclerosis is latitude dependent. Ann Neurol (2014) 76:880–90. doi:10.1002/ana.24287
6. Tao C, Simpson S Jr, Van Der Mei I, Blizzard L, Havrdova E, Horakova D, et al. Higher latitude is significantly associated with an earlier age of disease onset in multiple sclerosis. J Neurol Neurosurg Psychiatry (2016) 87:1343–9. doi:10.1136/jnnp-2016-314013
7. Amato MP, Derfuss T, Hemmer B, Liblau R, Montalban X, Sørensen PS, et al. Environmental modifiable risk factors for multiple sclerosis: report from the 2016 ECTRIMS focused workshop. Mult Scler (2017). doi:10.1177/1352458516686847
8. Murphy N, Confavreux C, Haas J, Konig N, Roullet E, Sailer M, et al. Quality of life in multiple sclerosis in France, Germany, and the United Kingdom. Cost of Multiple Sclerosis Study Group. J Neurol Neurosurg Psychiatry (1998) 65:460–6. doi:10.1136/jnnp.65.4.460
9. McCabe MP, McKern S. Quality of life and multiple sclerosis: comparison between people with multiple sclerosis and people from the general population. J Clin Psychol Med Settings (2002) 9:287–95. doi:10.1023/A:1020734901150
10. Hadjimichael O, Vollmer T, Oleen-Burkey M; North American Research Committee on Multiple Sclerosis. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes (2008) 6:100. doi:10.1186/1477-7525-6-100
11. Weiland TJ, Jelinek GA, Marck CH, Hadgkiss EJ, Van Der Meer DM, Pereira NG, et al. Clinically significant fatigue: prevalence and associated factors in an international sample of adults with multiple sclerosis recruited via the internet. PLoS One (2015) 10:e0115541. doi:10.1371/journal.pone.0115541
12. Jones KH, Ford DV, Jones PA, John A, Middleton RM, Lockhart-Jones H, et al. A large-scale study of anxiety and depression in people with multiple sclerosis: a survey via the web portal of the UK MS Register. PLoS One (2012) 7:e41910. doi:10.1371/journal.pone.0041910
13. Taylor KL, Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, et al. Lifestyle factors, demographics and medications associated with depression risk in an international sample of people with multiple sclerosis. BMC Psychiatry (2014) 14:327. doi:10.1186/s12888-014-0327-3
14. Marck CH, Neate SL, Taylor KL, Weiland TJ, Jelinek GA. Prevalence of comorbidities, overweight and obesity in an international sample of seople with multiple sclerosis and associations with modifiable lifestyle factors. PLoS One (2016) 11:e0148573. doi:10.1371/journal.pone.0148573
15. Haug E, Rasmussen M, Samdal O, Iannotti R, Kelly C, Borraccino A, et al. Overweight in school-aged children and its relationship with demographic and lifestyle factors: results from the WHO-collaborative health behaviour in school-aged children (HBSC) study. Int J Public Health (2009) 54(Suppl 2):167–79. doi:10.1007/s00038-009-5408-6
16. Sung C, Chiu C-Y, Lee E-J, Bezyak J, Chan F, Muller V. Exercise, diet, and stress management as mediators between functional disability and health-related quality of life in multiple sclerosis. Rehabil Couns Bull (2013) 56:85–95. doi:10.1177/0034355212439899
17. Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, Van Der Meer DM. Methodology of an international study of people with multiple sclerosis recruited through web 2.0 platforms: demographics, lifestyle, and disease characteristics. Neurol Res Int (2013) 2013:580596. doi:10.1155/2013/580596
18. Learmonth Y, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol (2013) 13:37. doi:10.1186/1471-2377-13-37
19. Vickrey BG. A health-related quality of life measure for multipe sclerosis. Qual Life Res (1995) 4:43–7. doi:10.1007/BF02260859
20. Krupp LB, Larocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol (1989) 46:1121–3. doi:10.1001/archneur.1989.00520460115022
21. Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire-2: validity of a two-item depression screener. Med Care (2003) 41:1284–92. doi:10.1097/01.MLR.0000093487.78664.3C
22. McKellar S, Horsley P, Chambers R, Pullen M, Vendersee P, Clarke C, et al. Development of the diet habits questionnaire for use in cardiac rehabilitation. Aust J Prim Health (2008) 14:43–7.
23. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc (2003) 35:1381–95. doi:10.1249/01.MSS.0000078924.61453.FB
24. Blake R, McKay D. A single-item measure of social supports as a predictor of morbidity. J Fam Pract (1986) 22:82–4.
25. Jelinek GA, De Livera AM, Marck CH, Brown CR, Neate SL, Taylor KL, et al. Associations of lifestyle, medication, and socio-demographic factors with disability in people with multiple sclerosis: an international cross-sectional study. PLoS One (2016) 11:e0161701. doi:10.1371/journal.pone.0161701
26. Jelinek GA, De Livera AM, Marck CH, Brown CR, Neate SL, Taylor KL, et al. Lifestyle, medication and socio-demographic determinants of mental and physical health-related quality of life in people with multiple sclerosis. BMC Neurol (2016) 16:235. doi:10.1186/s12883-016-0763-4
27. Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler (2015) 21:305–17. doi:10.1177/1352458514564491
28. Pluta-Fuerst A, Petrovic K, Berger T, Fryze W, Fuchs S, Gold R, et al. Patient-reported quality of life in multiple sclerosis differs between cultures and countries: a cross-sectional Austrian-German-Polish study. Mult Scler (2011) 17:478–86. doi:10.1177/1352458510391341
29. Jankovic SM. Injectable interferon beta-1b for the treatment of relapsing forms of multiple sclerosis. J Inflamm Res (2010) 3:25–31. doi:10.2147/JIR.S9480
30. Yamout BI, Zeineddine MM, Tamim H, Khoury SJ. Safety and efficacy of fingolimod in clinical practice: the experience of an academic center in the Middle East. J Neuroimmunol (2015) 289:93–7. doi:10.1016/j.jneuroim.2015.10.015
31. Cavalera C, Pagnini F, Rovaris M, Mendozzi L, Pugnetti L, Garegnani M, et al. A telemedicine meditation intervention for people with multiple sclerosis and their caregivers: study protocol for a randomized controlled trial. Trials (2016) 17:4. doi:10.1186/s13063-015-1136-9
32. WHO. Disease incidence, prevalence and disability. Global Burden Disease 2004 Update [Internet]. Switzerland: WHO (2004). p. 27–37. Available from: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf
33. Jelinek GA, Marck CH, Weiland TJ, Pereira N, Van Der Meer DM, Hadgkiss EJ. Latitude, sun exposure and vitamin D supplementation: associations with quality of life and disease outcomes in a large international cohort of people with multiple sclerosis. BMC Neurol (2015) 15:132. doi:10.1186/s12883-015-0394-1
Keywords: multiple sclerosis, disability, quality of life, fatigue, depression
Citation: Reilly GD, Mahkawnghta AS, Jelinek PL, De Livera AM, Weiland TJ, Brown CR, Taylor KL, Neate SL, Jelinek GA and Marck CH (2017) International Differences in Multiple Sclerosis Health Outcomes and Associated Factors in a Cross-sectional Survey. Front. Neurol. 8:229. doi: 10.3389/fneur.2017.00229
Received: 07 April 2017; Accepted: 11 May 2017;
Published: 31 May 2017
Edited by:
Björn Tackenberg, Philipps University of Marburg, GermanyReviewed by:
Benedikt Frank, Essen University Hospital, GermanyMarkus Kraemer, Alfried Krupp von Bohlen und Halbach Foundation, Germany
Copyright: © 2017 Reilly, Mahkawnghta, Jelinek, De Livera, Weiland, Brown, Taylor, Neate, Jelinek and Marck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia H. Marck, claudia.marck@unimelb.edu.au
†These authors have contributed equally as first authors.
 Grace D. Reilly
Grace D. Reilly Awng Shar Mahkawnghta
Awng Shar Mahkawnghta Pia L. Jelinek
Pia L. Jelinek Alysha M. De Livera
Alysha M. De Livera Tracey J. Weiland
Tracey J. Weiland Chelsea R. Brown
Chelsea R. Brown Keryn L. Taylor
Keryn L. Taylor Sandra L. Neate
Sandra L. Neate George A. Jelinek
George A. Jelinek Claudia H. Marck
Claudia H. Marck