Immune responses against classical swine fever virus: between ignorance and lunacy
- Institute of Virology and Immunology – IVI, Bern, Switzerland
Classical swine fever virus infection of pigs causes disease courses from life-threatening to asymptomatic, depending on the virulence of the virus strain and the immunocompetence of the host. The virus targets immune cells, which are central in orchestrating innate and adaptive immune responses such as macrophages and conventional and plasmacytoid dendritic cells. Here, we review current knowledge and concepts aiming to explain the immunopathogenesis of the disease at both the host and the cellular level. We propose that the interferon type I system and in particular the interaction of the virus with plasmacytoid dendritic cells and macrophages is crucial to understand elements governing the induction of protective rather than pathogenic immune responses. The review also concludes that despite the knowledge available many aspects of classical swine fever immunopathogenesis are still puzzling.
Introduction
Classical swine fever (CSF) is a highly contagious disease of pigs caused by the classical swine fever virus (CSFV), which is a member of the genus pestivirus within the Flaviviridae family. CSFV is a spherical virus particle of 40–60 nm in diameter, consisting of a lipid envelope surrounded by a nucleocapsid packaging a positive-strand RNA genome of 12.3 kb. The RNA carries a single large open reading frame (ORF) which encodes a large polyprotein that is co- and post-translationally cleaved into the twelve proteins Npro, C, Erns, E1, E2, p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B by cellular and viral proteases. The four structural proteins C, Erns, E1, and E2 are components of the virion, while the others are non-structural proteins with various functions in the viral life cycle. Virus replication is restricted to the cytoplasm and does normally not result in a cytopathic effect in cell culture. Virion assembly occurs on intracellular membranes of the endoplasmic reticulum (ER), and first progeny virus is released from the cells at 5–6 h post-infection via exocytosis (1).
Classical swine fever leads to important economic losses worldwide. In Europe, the wild boar population is an important reservoir for the virus, and represents a source for reintroduction of the disease in domestic pigs.
After oronasal infection, CSFV probably passes through the epithelial cells and M-cells of the tonsilar crypts, the primary target tissue for virus replication. Thereafter, the virus is found in the tonsils and local oropharyngeal lymph nodes (2, 3). A particular affinity of the virus for the reticuloendothelial cell system has been noted with macrophages (MΦ), dendritic cells (DC), and endothelial cells (EDC) being primary targets (2–10). From these primary sites of replication, the virus spreads to other lymphoid organs. Such secondary target organs include the spleen, lymph nodes, gut-associated lymphoid tissue, bone marrow, and thymus (2, 3, 11). CSFV has also been found in the pancreas, brain, heart, gall and urinary bladders, mandibular salivary and adrenal glands, thyroid, liver, and kidney, particularly in association with EDC and MΦ (3). More recent investigations using quantitative RT-PCR confirmed these older studies, also demonstrating a wider tissue distribution with longer durations of infection (12). This is also reflected at the level of cell tropism. For example, only in late stages of the disease, viral antigen is found in peripheral lymphocytes and immature granulocytes (7, 11, 13). Furthermore, in the skin, efficient infection of keratinocytes, hair follicle epithelial cells, and mesenchymal cells in the dermis was demonstrated at later time points after infection (14).
Highly Virulent Strains Induce a Disastrous Infection for the Immune System
Strong Peripheral and Central Lymphoid Depletion Affecting Primary and Secondary Lymphoid Tissue
The numerous field isolates and laboratory strains cover an almost continuous spectrum of virulence, from highly virulent viruses to low-virulent strains. Accordingly, the clinical outcome of CSF in pigs can vary from peracute to acute, subacute, chronic, and subclinical disease outcomes. The peracute and acute disease is characterized by pyrexia, anorexia, central nervous disorders, diarrhea, and in some cases also hemorrhages of the skin, mucosa, and various other organs. In fact, virulent CSFV can induce a typical hemorrhagic fever with immunological characteristics common to all viral hemorrhagic fevers. The disease is associated with severe lymphopenia and lymphocyte apoptosis (6, 11, 15), thrombocytopenia (3), platelet aggregation (16), bone marrow depletion affecting myelopoiesis and magakaryocytopoiesis (11, 17), and thymus atrophy as well as thymocyte apoptosis (5, 13). Lymphoid depletion is generalized, not only affecting peripheral blood and lymph nodes but also the mucosal tissue (18). At later stages, disseminated intravascular coagulation (DIC), petechial bleedings, and hemoconcentration can be found (3), which can result in a circulation failure, hypotension, and death. A recent study, however, suggests that the hemorrhagic lesions observed in the late stages of the disease are not attributable to DIC. Inhibition of diffuse fibrin and thrombi formation did not influence the extent of hemorrhagic lesions. From this, it was concluded that DIC was not the cause for the thrombocytopenia and hemorrhages observed in acute–lethal CSF (19).
Massive Induction of Interferon-α
Very high levels of serum interferon-(IFN)-α are a hallmark of the acute disease phase induced by virulent CSFV. It appears that the levels of IFN-α found in the serum correlate with disease severity and the virulence of the isolate used for infection (20, 21). Nevertheless, the association between virulence and high IFN-α levels was less clear in 6-month-old pigs (22). Our experience in younger animals clearly indicated a correlation between serum IFN-α levels and the degree of lymphopenia induced by CSFV. In fact, the onset of severe lymphopenia was concomitant with the IFN-α responses, and all animals with serum IFN-α had depleted peripheral B and T lymphocytes (21). These observations indicate that high levels of IFN-α cannot control the virus but may rather mediate aberrant responses leading to immunopathology (Figure 1). Microarray analyses of PBMC isolated from infected pigs confirmed not only the dominance of IFN-stimulated genes but also of cell death receptor and apoptosis pathways such as TRAIL, FAS, and TNF (23), relating to previous studies performed with peripheral blood cells using flow cytometry (15). To our knowledge, compared with other virus infections of pigs, CSFV can induce not only the most long-lasting but also the most intense systemic IFN-α responses (24).
Figure 1. Critical immunological pathways for protective (green) versus pathogenic (red) immune responses during acute CSFV. CSFV targets both monocytic cells with their MΦ descendants and conventional and plasmacytoid DC (cDC and pDC). MΦ are mainly responsible for the typical pro-inflammatory responses, although conventional DC may contribute to this response. We propose that the large quantities of IFN-α produced by pDC play a central role in the innate immune response to CSFV. Prolonged systemic responses are associated with pathogenic host responses while time-limited production appears to promote protective adaptive Th1 effector responses.
Infection and Activation of MΦ
In vivo, MΦ infection and morphological signs of activation were found in the spleen (4, 25), the kidney (26) the lung (27), the liver (28), and the intestine (18). In addition, infection of pigs was associated with MΦ producing pro-inflammatory cytokines, such as IL-1α, IL-1β, IL-6, and TNF-α (5, 8, 25, 28). There is also evidence for macrophage activation leading to the production of vasoactive mediators including prostaglandin E2 (8) and platelet activation (16). Finally, during acute and severe CSF, an activation of the complement system has been observed (5, 25). Therefore, MΦ infection and activation have been proposed to play an important role in CSF pathogenesis, in particular, through release of pro-inflammatory and vasoactive mediators (Figure 1).
Infection and Activation of Dendritic Cells
It was also shown that CD11R1+CD172a+ cells, probably representing a subset of conventional DC (29), are activated in vivo in the blood, the tonsil, and the spleen at 24–48 h post-infection to produce TNF-α and IL-10 (10). The same study also demonstrated IFN-α and IL-12 producing CD4+CD172a+ plasmacytoid DC (pDC) in the same immunological compartments but possibly with an ever faster kinetic of response. Immunofluorescence analysis indicated that these two populations of DC do represent early target cells of CSFV (10). Only recently it became clear that the monocytic cells and DC represent two distinct lineage of cells with respect to their ontogenic development (30), and it is now also possible to clearly differentiate bona fide DC from monocytic cells in the pig (29). Although these cells do have overlapping functions, they also have a clear functional specialization. During CSF, MΦ are probably mainly responsible for the typical pro-inflammatory responses, but conventional DC appear to contribute to this response although they may also be involved in counteracting it by secretion of anti-inflammatory IL-10 (10). Finally, pDC typically secrete large quantities of IFN-α, but possibly also Th1-promoting IL-12. The impact of the virus on the antigen-presenting functions of DC is not clear but it appears that the cells are not depleted in the lymphoid tissues at least in the first 2–3 days post-infection (10).
Effects on Lymphocytes
Despite the severe lymphoid depletion, acute CSF is also associated with a pronounced anergy of T lymphocytes in the acute phase of the disease (13, 15). At later stages of severe CSF, T cell activation events (31, 32) with the detection of serum IL-2 and IFN-γ (33) have been found. Similarly, indication of B-cell activation has been described in terms of an increase in cells expressing the lambda light chain and IgM (34).
Low-Virulent Strains: From Controlled to Chronic Infections
In contrast to severe forms of the disease described above, infection with low-virulent strains of CSFV induces no obvious clinical symptoms or only weak and transient disease. In the serum of such animals, no or lower levels of IFN-α and pro-inflammatory cytokines can be detected (21, 22, 35). However, these animals often also develop transient lymphopenia (36, 37). If controlled, such infections result in life-long immunity against CSFV. Nevertheless, depending on the age and immune status, infection with low or moderately virulent CSFV may lead to forms of chronic disease, which can last up to 3 months before the animals die (38–40). Due to the inability of the immune system to clear the infection, these animals shed large quantities of virus and play an important role in epidemiology of the disease (41, 42). Initially, the immunopathogenic events in such animals can be similar to those described above albeit milder. At later stages, signs of lymphocyte activation and proliferation are found, which are not well defined (43, 44).
Virus–Host Interactions at the Cellular Level
CSFV Proteins Targeting Innate Immune Responses
Npro
The Npro is a cysteine autoprotease that cleaves itself from the viral polyprotein co-translationally and targets IRF3, an essential transcription factor for IFNB1. The C-terminal half of Npro carries a zinc-binding domain that is required for interaction with IRF3 (45, 46). Through this interaction, Npro induces efficient proteosomal degradation and depletion of IRF-3, which is the basis of the very potent antagonism of IFN type I induction by CSFV (47–51) (Figure 2). However, pDC are unique by constitutively expressing IRF7, and in contrast to other cells do not require IRF3 for induction of IFN-α/β (52), explaining why this cell type is exclusively able to respond to CSFV by IFN-α/β production. Nonetheless, Npro was found to be also partially active in pDC, presumably through its ability to interact also with IRF7 and prevent IRF7-mediated IFN type I induction (53) (Figure 2). Furthermore, also in GM-CSF-driven bone marrow hematopoietic cell-derived DC, which have been induced to express IRF7 by IFN type I pre-treatment, Npro was still inhibitory (54). In addition to its ability to suppress IFN type I responses, Npro also mediates anti-apoptotic effects induced by synthetic double stranded (ds) RNA but not by FasL or staurosporine (48, 55, 56), preventing activation of caspases 8, 9, and 3 and inhibiting the loss of mitochondrial membrane potential and cytochrome c release (48, 55, 56). Interestingly, Npro interacts with the anti-apoptotic HS-1-associated protein X-1 (HAX-1), inducing the redistribution of HAX-1 to the ER compartment. This HAX-1 redistribution to the ER during CSFV infection may increase cellular resistance to apoptosis, similar to other HAX-1 interacting proteins (56). Npro also interacts with IκBα known to prevent NFκB p65 nuclear translocation but this interaction apparently has no impact on NFκB translocation (57).
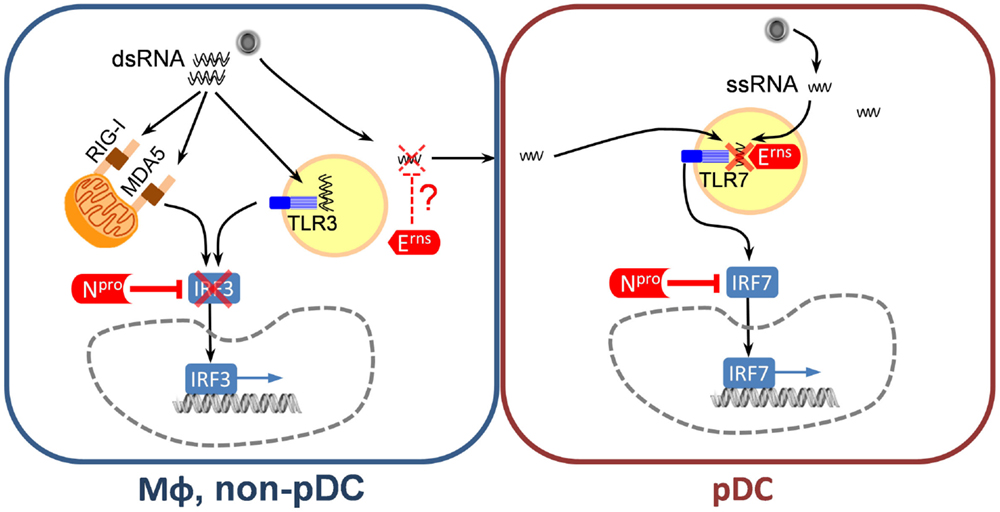
Figure 2. Classical swine fever virus-encoded inhibitors of the IFN type I system. In MΦ and non-pDC target cells, Npro represents the main IFN antagonist, which almost completely inhibits IRF3-mediated IFN type I induction induced by sensing viral dsRNA via RIG-I, MDA-5, and/or TLR3. In pDC, Npro is also active by inhibiting IRF7-mediated IFN-α induction although this inhibition is not complete. In addition, Erns represents a potent inhibitor of pDC responses through its ability to degrade viral ssRNA and thereby prevent TLR7 activation. Viral ssRNA can originate from virus replicating in pDC or in neighboring cells. The mechanism of viral RNA transfer as well as the subcellular location of RNA degradation is not clear.
Studies in pigs indicate that the Npro-mediated interference with IFN type I induction contributes to pathogenicity. Single amino acid mutations specifically eliminating the ability of Npro to interact with IRF3 partially attenuated moderately virulent but not highly virulent CSFV (20). On the other hand, reintroduction of functional Npro into moderately virulent GPE−-derived virus with unfunctional Npro enhanced virulence by preventing IFN type I induction at local replication sites (58).
Erns
This essential structural glycoprotein of pestiviruses has a remarkable RNase activity, with structural similarities to plant T2 RNases. The optimal catalytic activity is at acidic pH (59), with preferential cleavage of single-stranded (ss) RNA (60, 61). The protein also has an unusual membrane anchor composed of an amphipathic helix without a typical membrane anchor (62, 63), but a retention signal ensuring its association with the intracellular membrane compartments (64). Based on the observation that a minor fraction of the protein was found to be secreted from infected cells or cells expressing Erns (62, 64, 65), a role for secreted Erns acting in the extracellular compartment where it could degrade RNA has been postulated (66–68). In addition, Erns can be rapidly endocytosed to also degrade endosomal RNA in adjacent cells (69). However, these studies were performed with recombinant Erns. In the viral context, the antagonistic activity of Erns on IFN-α induction was only demonstrated in pDC (70). On one side, CSFV expressing Erns lacking RNase activity in contrast to wild-type virus was able to induce very strong IFN-α responses in pDC. On the other hand, cells infected with virus replicon particles lacking Erns or CSFV expressing an Erns without RNase activity were much more efficient at stimulating pDC than cells infected with the parent virus. This very potent stimulation of pDC by infected cells was demonstrated not to be mediated by virions but by a transfer of viral RNA to the TLR7 compartment of pDC. Based on this data it can be concluded that Erns degrades viral ssRNA preventing its interaction with TLR7 in pDC. Considering that the RNase activity of Erns is particularly high at acidic pH, an attractive model is that degradation would happen in the endosomal compartment (70) (Figure 2). We consider these findings as relevant since the stimulation of pDC by infected cells results in much higher levels of IFN-α as compared to the direct pDC stimulation by virions (70). The role of Erns in other cell types expressing TLR7 and TLR8 or even TLR3 such as monocyte/MΦ, B cells, and other DC subsets still needs to be investigated. The first step would be to characterize the TLR expression in pigs. In vivo removal of the RNase activity results in virus attenuation (71) and abrogation of the capacity of pestiviruses to establish immunotolerance and persistent infection after infection of fetuses (72). The relationship between this inhibitory activity of Erns on the innate immune responses mediated via TLR7 and the establishment of tolerance will be an important area of future investigations.
Quiescent In vitro Infections
With a few exceptions, CSFV is absolutely non-cytopathogenic. In all target cells analyzed so far, except pDC, the virus does not induce IFN type I responses, and no or low cytokine responses are found. This is independent of the virulence of the strains investigated. MΦ activation following in vitro infection with CSFV is surprisingly weak compared to stimulation with TLR ligands such as lipopolysaccharide, and many reports confirm that CSFV only induces a minor activation of monocytic cells including monocyte-derived MΦ, monocyte-derived DC, as well as their bone marrow-derived counterparts (8, 54, 73, 74). Zaffuto et al. (75) reported microarray data showing only 11 out of 7712 genes (0.14%) induced by the virus, including arginase-1, phosphoinositide 3-kinase, chemokine receptor 4, and interleukin-1β. Obviously, these characteristics are dependent on the potent ability of Npro to counteract virus sensing (76). Previous work also demonstrated that CSFV neither induces nor interferes with NFκB signaling (57). These reports with cell lines, monocyte-derived cells, and ex vivo isolated macrophages are remarkable, considering the replicative ability of the virus in these cells. This demonstrates that the viral innate immune system antagonist Npro is most efficient in hiding the infection. For pestiviruses, it is well known that the balance of viral dsRNA accumulating during replication is regulated by a tightly controlled expression of NS3 (77). Cytopathogenic mutants typically have higher levels of dsRNA. Accordingly, such mutants do induce IFN-β and activate monocytic cells even with functional Npro, indicating that evolution has driven a well-balanced relationship between Npro and viral dsRNA (76). In fact, using non-functional Npro mutants of CSFV, we have demonstrated that in PK-15 cells viral RNA is sensed by TLR3, RIG-I, and MDA-5 (78).
Plasmacytoid DC Responses In vitro
Classical swine fever virus activates pDC to produce IFN-α. This activation requires live virus and pDC infection (79). Nevertheless, compared to other viruses such as influenza virus the levels of IFN-α are relatively low (24). In fact, this can be explained by the action of Npro targeting IRF7 (53) and of Erns degrading viral RNA to prevent the triggering of TLR7 by viral RNA (70). A very efficient TLR7-dependent induction of IFN-α in pDC by CSFV-infected cells in the absence of virions has been demonstrated. This pathway is mediated by a transfer of RNA from an infected donor cell to pDC in a cell contact-dependent manner requiring intact lipid rafts and cytoskeleton of the donor cell. Erns blocks both direct stimulation of pDC by virions and stimulation by infected cells (70). Although on a per cell basis CSFV is a weak activator of pDC, its strong tropism for lymphoid tissue and pDC is likely to result in the overall high and prolonged responses found in vivo (24).
Proposed Mechanisms Leading to Control
Dysregulated Responses
Published data indicate that at the initial sites of virus replication – involving principally MΦ and epithelial cells – CSFV Npro inhibits virus-induced IFN-α/β allowing the virus to replicate and generate the virus load leading to viremia and spread within the organism. The speed and level by which CSFV replicates and spreads appears to be critical for the outcome of disease. The virus then infects more MΦ and pDC, resulting in massive IFN-α and pro-inflammatory cytokine release as described above (Figure 1). Based on the known effects of IFN-α/β on MΦ activation it is tempting to postulate that pDC activation may enhance these effects. Nevertheless, to our surprise even IFN-primed MΦ did not respond to CSFV by production of IFN-α, IL-1β, or IL-6 production (54). It is thus still puzzling to observe the discrepancy between in vitro and in vivo with regards to MΦ activation.
In vaccinated and immune animals, there are no or less immunopathological events such as development of leukopenia and systemic inflammatory responses after challenge infection (80–82). However, vaccinated animals still respond to CSFV infection with a serum IFN-α response, even in absence of viremia, but, in contrast to naïve animals, this response is lower and only of short duration (79–81, 83). A possible explanation for this observation is the fact that pDC from vaccinated pigs carry cytophilic antibodies, which mediate efficient capture of CSFV, resulting in early strong pDC stimulation (79). This observation underlines that in contrast to strong long-lasting systemic IFN-α responses, a short-lived IFN-α response is probably beneficial for the immunity against CSFV. In vivo administration of high levels of IFN type I is known to have comparable negative effects on the hematopoietic system (84–87). Moreover, when IFNAR knockout mice were employed in a lymphocytic choriomeningitis virus model, no induction of hematopoietic cell depletion and leukopenia was observed (88). In fact, the known antiproliferative and proapoptotic effects of IFNs (89) could be directly responsible for hematologic cytopenia (Figure 1).
Several attempts to shed light into host responses related to control of CSFV by the immune system have used transcriptomic profiling (23, 90, 91). In response to CSFV infection, increased expression of IFN-stimulated genes as well as other immune response genes, genes related to cell cycle, apoptosis, metabolism, and others were observed. The profiles described reflected what was expected in terms of IFN and cytokine responses measured by ELISA, apoptosis of lymphocytes, and general changes in immune cell composition described for CSF. Using a moderately virulent strain of CSFV, Hulst and co-workers compared groups of pigs able to control the infection with those developing chronic disease and excreting high quantities of virus over a period of 35 days (92). Interestingly, the animals that recovered later had a generally more robust early response in terms of genes associated with IFN type I responses and macrophage activation, whereas those developing chronic disease were found to express inhibitors of the NFκB pathway. This study also indicated a dysregulation of the complement cascade and the vitamin D3 metabolism in animals not controlling the infection. On the other hand, this work also showed that immunoregulatory molecules such as indoleamine 2,3-dioxygenase 1 (IDO1) were expressed early in controller pigs but late in non-controllers (92). Certainly, such analyses highlight the complexity of protective immune responses, which are composed of both stimulatory and regulatory elements required to prevent tissue damage at the right moment. It appears that this is a central theme in understanding the complex pathogenesis of CSF (Figure 1).
Protective Immune Mechanisms
It is well established that conventional live attenuated CSFV vaccines have an extraordinary protective capacity inducing protection as early as 3–5 days post vaccination (93, 94). Remarkably, transmission can even be prevented when animals are vaccinated on the day of challenge (95). Obviously, this protection is found in complete absence of neutralizing antibodies indicating alternative mechanisms of protection early post vaccination.
For this reason, several groups investigated the potential role of IFN-γ-secreting T cells (32, 35, 94, 96–98). Typically after challenge infection of pigs with virulent CSFV, only the previously vaccinated animals had circulating T cells secreting IFN-γ. In contrast, vaccination alone using C-strain based vaccine was not efficient at inducing detectable levels of activated peripheral T cells. Only in one study using three shots of a DNA vaccine, CSFV-specific IFN-γ spots were also found before infection (98). Also, in unvaccinated animals, which are challenged with a virulent strain of CSFV, no T cell activity can be detected in the peripheral blood. This is certainly caused by the severe defects in their T cell compartment which is even unable to respond to polyclonal stimulation (13, 15). Most of the IFN-γ-producing lymphocytes found in the peripheral blood belong to the CD4−CD8β+ T cell subset and co-express perforin indicating effector functions (82, 97) and are probably effector CTL’s since they express CD107a on their surface. This is in line with previous work demonstrating cytotoxic T cell activity against CSFV-infected target cells (96). From the latter study, it appears that the ability to detect CSFV-specific cytotoxic T cell activity requires a certain level of virus replication; since in this study, CTL activity was found only in the peripheral blood of animals kept unvaccinated but challenged with a moderately virulent virus. A recent report showed induction of MHC class II on NK and γδ T cells by IFN-α derived from CSFV-infected pDC in vitro. However, in vivo this was only found in tonsils and retropharygeal lymph nodes of pigs infected with virulent virus, but not following vaccination with attenuated vaccines. Furthermore, neither an increase in perforin nor IFN-γ was found both in vitro and in vivo. From this, the authors concluded that these cell types are probably not contributing to early protection induced by attenuated vaccines (99).
These studies showing an association of T cell responses with protection alone do not permit a conclusion that IFN-γ secreting T cells are a correlate of protection or even have protective value and the general contribution of T cells to protection remains unclear. A main problem is the immunopathological effects of CSFV on the T lymphocyte compartment, which if present do not permit the detection of any T cell activation. Furthermore, NS3 protein known to contain T cell epitopes (100) was not able to confer partial protection in vaccination-challenge studies (101, 102) indicating that T cell immunity alone is unlikely to control CSFV. On the other hand, neutralizing antibodies against E2 are well known to be associated with protection (83, 103–106). But E2 also contains CTL epitopes (107) whose contribution to protection is not yet clear.
Conclusion and Future Research
Despite the knowledge available on the pathogenesis of CSF, many essential aspects remain enigmatic and can only be clarified with well-defined gain- as well as loss-of-function in vivo experimental models. In our view, the most important questions are to identify the precise contribution of various cell types including pDC, conventional DC, and MΦ to disease pathogenesis and immunity both during the acute forms of CSF and during chronic disease. Similarly, correlates of early vaccine-induced protection remain unproven. The functions of various subsets of T cells in protection need to be defined to understand both chronic disease and vaccine-mediated early protection. Furthermore, the exact role of evolvement of viral inhibitors of the IFN type I system, targeting both non-pDC and pDC remains puzzling considering that in vivo IFN-α/β responses are induced. Finally, the cellular systems and the viral inhibitors described in this review need to be understood in the light of the ability of pestiviruses to induce immunotolerance, if infection occurs during certain stages of the development of the immune system.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Part of this work was supported by the Swiss National Science Foundation grants # 3100-68237, 310030-116800/1, 31003A-116608, and 310030_141045. We are grateful for the precious contributions of all present and former members of our laboratory.
References
1. Thiel H-J, Plagemann PGW, Moennig V. Pestiviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Philadelphia, PA: Lippincott-Raven Publishers (1996). p. 1059–73.
2. Ressang AA. Studies on the pathogenesis of hog cholera. II. Virus distribution in tissue and the morphology of the immune response. Zentralbl Veterinarmed B (1973) 20:272–88. doi:10.1111/j.1439-0450.1973.tb01127.x
3. Trautwein G, Leiss B. Pathology and Pathogenesis of the Disease, Classical Swine Fever and Related Infections. Boston, MA: Martinus Nijhoff Publishing (1988). p. 27–54.
4. Gomez-Villamandos JC, Ruiz-Villamor E, Bautista MJ, Sanchez CP, Sanchez-Cordon PJ, Salguero FJ, et al. Morphological and immunohistochemical changes in splenic macrophages of pigs infected with classical swine fever. J Comp Pathol (2001) 125:98–109. doi:10.1053/jcpa.2001.0487
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
5. Sanchez-Cordon PJ, Romanini S, Salguero FJ, Nunez A, Bautista MJ, Jover A, et al. Apoptosis of thymocytes related to cytokine expression in experimental classical swine fever. J Comp Pathol (2002) 127:239–48. doi:10.1053/jcpa.2002.0587
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
6. Susa M, Konig M, Saalmuller A, Reddehase MJ, Thiel HJ. Pathogenesis of classical swine fever: B-lymphocyte deficiency caused by hog cholera virus. J Virol (1992) 66:1171–5.
7. Summerfield A, Hofmann MA, McCullough KC. Low density blood granulocytic cells induced during classical swine fever are targets for virus infection. Vet Immunol Immunopathol (1998) 63:289–301. doi:10.1016/S0165-2427(98)00108-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
8. Knoetig SM, Summerfield A, Spagnuolo-Weaver M, McCullough KC. Immunopathogenesis of classical swine fever: role of monocytic cells. Immunology (1999) 97:359–66. doi:10.1046/j.1365-2567.1999.00775.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
9. Carrasco CP, Rigden RC, Schaffner R, Gerber H, Neuhaus V, Inumaru S, et al. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology (2001) 104:175–84. doi:10.1046/j.1365-2567.2001.01299.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
10. Jamin A, Gorin S, Cariolet R, Le Potier MF, Kuntz-Simon G. Classical swine fever virus induces activation of plasmacytoid and conventional dendritic cells in tonsil, blood, and spleen of infected pigs. Vet Res (2008) 39:7. doi:10.1051/vetres:2007045
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
11. Summerfield A, Knoetig SM, Tschudin R, McCullough KC. Pathogenesis of granulocytopenia and bone marrow atrophy during classical swine fever involves apoptosis and necrosis of uninfected cells. Virology (2000) 272:50–60. doi:10.1006/viro.2000.0361
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
12. Liu J, Fan XZ, Wang Q, Xu L, Zhao QZ, Huang W, et al. Dynamic distribution and tissue tropism of classical swine fever virus in experimentally infected pigs. Virol J (2011) 8:201. doi:10.1186/1743-422X-8-201
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
13. Pauly T, Konig M, Thiel HJ, Saalmuller A. Infection with classical swine fever virus: effects on phenotype and immune responsiveness of porcine T lymphocytes. J Gen Virol (1998) 79(Pt 1):31–40.
14. Kaden V, Lange E, Faust A, Teifke JP. Value of skin punch biopsies for the diagnosis of acute classical swine fever. J Vet Diagn Invest (2007) 19:697–701. doi:10.1177/104063870701900614
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
15. Summerfield A, Knotig SM, McCullough KC. Lymphocyte apoptosis during classical swine fever: implication of activation-induced cell death. J Virol (1998) 72:1853–61.
16. Bautista MJ, RuizVillamor E, Salguero FJ, SanchezCordon PJ, Carrasco L, GomezVillamandos JC. Early platelet aggregation as a cause of thrombocytopenia in classical swine fever 1842. Vet Pathol (2002) 39:84–91. doi:10.1354/vp.39-1-84
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
17. Gomez-Villamandos JC, Salguero FJ, Ruiz-Villamor E, Sanchez-Cordon PJ, Bautista MJ, Sierra MA. Classical swine fever: pathology of bone marrow. Vet Pathol (2003) 40:157–63. doi:10.1354/vp.40-2-157
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
18. Sanchez-Cordon PJ, Romanini S, Salguero FJ, Ruiz-Villamor E, Carrasco L, Gomez-Villamandos JC. A histopathologic, immunohistochemical, and ultrastructural study of the intestine in pigs inoculated with classical swine fever virus. Vet Pathol (2003) 40:254–62. doi:10.1354/vp.40-3-254
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
19. Blome S, Meindl-Bohmer A, Nowak G, Moennig V. Disseminated intravascular coagulation does not play a major role in the pathogenesis of classical swine fever. Vet Microbiol (2013) 162:360–8. doi:10.1016/j.vetmic.2012.10.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
20. Ruggli N, Summerfield A, Fiebach AR, Guzylack-Piriou L, Bauhofer O, Lamm CG, et al. Classical swine fever virus can remain virulent after specific elimination of the interferon regulatory factor 3-degrading function of Npro. J Virol (2009) 83:817–29. doi:10.1128/JVI.01509-08
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
21. Summerfield A, Alves M, Ruggli N, de Bruin MG, McCullough KC. High IFN-alpha responses associated with depletion of lymphocytes and natural IFN-producing cells during classical swine fever. J Interferon Cytokine Res (2006) 26:248–55. doi:10.1089/jir.2006.26.248
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
22. von Rosen T, Lohse L, Nielsen J, Uttenthal A. Classical swine fever virus infection modulates serum levels of INF-alpha, IL-8 and TNF-alpha in 6-month-old pigs. Res Vet Sci (2013) 95:1262–7. doi:10.1016/j.rvsc.2013.09.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
23. Renson P, Blanchard Y, Le Dimna M, Felix H, Cariolet R, Jestin A, et al. Acute induction of cell death-related IFN stimulated genes (ISG) differentiates highly from moderately virulent CSFV strains. Vet Res (2010) 41:7. doi:10.1051/vetres/2009055
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
24. Summerfield A. Viewpoint: factors involved in type I interferon responses during porcine virus infections. Vet Immunol Immunopathol (2012) 148:168–71. doi:10.1016/j.vetimm.2011.03.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
25. Sanchez-Cordon PJ, Nunez A, Salguero FJ, Pedrera M, Fernandez de MM, Gomez-Villamandos JC. Lymphocyte apoptosis and thrombocytopenia in spleen during classical swine fever: role of macrophages and cytokines. Vet Pathol (2005) 42:477–88. doi:10.1354/vp.42-4-477
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
26. Gomez-Villamandos JC, Ruiz-Villamor E, Bautista MJ, Quezada M, Sanchez CP, Salguero FJ, et al. Pathogenesis of classical swine fever: renal haemorrhages and erythrodiapedesis. J Comp Pathol (2000) 123:47–54. doi:10.1053/jcpa.2000.0385
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
27. Carrasco L, Ruiz-Villamor E, Gomez-Villamandos JC, Salguero FJ, Bautista MJ, Macia M, et al. Classical swine fever: morphological and morphometrical study of pulmonary intravascular macrophages. J Comp Pathol (2001) 125:1–7. doi:10.1053/jcpa.2001.0470
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
28. Nunez A, Gomez-Villamandos JC, Sanchez-Cordon PJ, Fernandez de Marco M, Pedrera M, Salguero FJ, et al. Expression of proinflammatory cytokines by hepatic macrophages in acute classical swine fever. J Comp Pathol (2005) 133:23–32. doi:10.1016/j.jcpa.2005.01.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
29. Summerfield A, Auray G, Ricklin M. Comparative dendritic cell biology of veterinary mammals. Annu Rev Anim Biosci (2014) 3:533–57. doi:10.1146/annurev-animal-022114-111009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
30. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol (2013) 31:563–604. doi:10.1146/annurev-immunol-020711-074950
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
31. Suradhat S, Sada W, Buranapraditkun S, Damrongwatanapokin S. The kinetics of cytokine production and CD25 expression by porcine lymphocyte subpopulations following exposure to classical swine fever virus (CSFV). Vet Immunol Immunopathol (2005) 106:197–208. doi:10.1016/j.vetimm.2005.02.017
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
32. Suradhat S, Intrakamhaeng M, Damrongwatanapokin S. The correlation of virus-specific interferon-gamma production and protection against classical swine fever virus infection. Vet Immunol Immunopathol (2001) 83:177–89. doi:10.1016/S0165-2427(01)00389-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
33. Sanchez-Cordon PJ, Nunez A, Salguero FJ, Carrasco L, Gomez-Villamandos JC. Evolution of T lymphocytes and cytokine expression in classical swine fever (CSF) virus infection. J Comp Pathol (2005) 132:249–60. doi:10.1016/j.jcpa.2004.10.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
34. Sanchez-Cordon PJ, Romero-Trevejo JL, Pedrera M, Raya AI, Gomez-Villamandos JC. The role of B cells in the immune response to pestivirus (classical swine fever virus). J Comp Pathol (2006) 135:32–41. doi:10.1016/j.jcpa.2006.04.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
35. Tarradas J, de la Torre ME, Rosell R, Perez LJ, Pujols J, Munoz M, et al. The impact of CSFV on the immune response to control infection. Virus Res (2014) 185:82–91. doi:10.1016/j.virusres.2014.03.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
36. Nielsen J, Lohse L, Rasmussen TB, Uttenthal A. Classical swine fever in 6- and 11-week-old pigs: haematological and immunological parameters are modulated in pigs with mild clinical disease. Vet Immunol Immunopathol (2010) 138:159–73. doi:10.1016/j.vetimm.2010.07.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
37. Summerfield A, McNeilly F, Walker I, Allan G, Knoetig SM, McCullough KC. Depletion of CD4(+) and CD8(high+) T-cells before the onset of viraemia during classical swine fever. Vet Immunol Immunopathol (2001) 78:3–19. doi:10.1016/S0165-2427(00)00248-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
38. Depner KR, Hinrichs U, Bickhardt K, Greiser-Wilke I, Pohlenz J, Moennig V, et al. Influence of breed-related factors on the course of classical swine fever virus infection. Vet Rec (1997) 140:506–7. doi:10.1136/vr.140.19.506
39. Moennig V, Floegel-Niesmann G, Greiser-Wilke I. Clinical signs and epidemiology of classical swine fever: a review of new knowledge. Vet J (2003) 165:11–20. doi:10.1016/S1090-0233(02)00112-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
40. Van Oirschot JT, Liess B. Description of the Virus Infection, Classical Swine Fever and Related Viral Infections. Boston, MA: Martinus Nijhoff Publishing (1988). p. 1–18.
41. Weesendorp E, Backer J, Stegeman A, Loeffen W. Transmission of classical swine fever virus depends on the clinical course of infection which is associated with high and low levels of virus excretion. Vet Microbiol (2011) 147:262–73. doi:10.1016/j.vetmic.2010.06.032
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
42. Weesendorp E, Stegeman A, Loeffen W. Dynamics of virus excretion via different routes in pigs experimentally infected with classical swine fever virus strains of high, moderate or low virulence. Vet Microbiol (2009) 133:9–22. doi:10.1016/j.vetmic.2008.06.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
43. Korn G, Lorenz RJ. [Blood-pictures in different courses of swine fever infection concerning lymphocytic reactions, cellnecrobiosis, moving forms of lymphocytes (“lysocytes”) and reactions of the bone-marrow (author’s transl)]. Zentralbl Bakteriol Orig A (1974) 229:299–322.
44. Narita M, Kawashima K, Shimizu M. Viral antigen and B and T lymphocytes in lymphoid tissues of gnotobiotic piglets infected with hog cholera virus. J Comp Pathol (1996) 114:257–63. doi:10.1016/S0021-9975(96)80047-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
45. Szymanski MR, Fiebach AR, Tratschin JD, Gut M, Ramanujam VM, Gottipati K, et al. Zinc binding in pestivirus N(pro) is required for interferon regulatory factor 3 interaction and degradation. J Mol Biol (2009) 391:438–49. doi:10.1016/j.jmb.2009.06.040
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
46. Gottipati K, Ruggli N, Gerber M, Tratschin JD, Benning M, Bellamy H, et al. The structure of classical swine fever virus N(pro): a novel cysteine autoprotease and zinc-binding protein involved in subversion of type I interferon induction. PLoS Pathog (2013) 9:e1003704. doi:10.1371/journal.ppat.1003704
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
47. Bauhofer O, Summerfield A, Sakoda Y, Tratschin JD, Hofmann MA, Ruggli N. Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation. J Virol (2007) 81:3087–96. doi:10.1128/JVI.02032-06
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
48. Ruggli N, Bird BH, Liu L, Bauhofer O, Tratschin JD, Hofmann MA. N(pro) of classical swine fever virus is an antagonist of double-stranded RNA-mediated apoptosis and IFN-alpha/beta induction. Virology (2005) 340(2):265–76. doi:10.1016/j.virol.2005.06.033
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
49. Seago J, Hilton L, Reid E, Doceul V, Jeyatheesan J, Moganeradj K, et al. The Npro product of classical swine fever virus and bovine viral diarrhea virus uses a conserved mechanism to target interferon regulatory factor-3. J Gen Virol (2007) 88:3002–6. doi:10.1099/vir.0.82934-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
50. Chen Z, Rijnbrand R, Jangra RK, Devaraj SG, Qu L, Ma Y, et al. Ubiquitination and proteasomal degradation of interferon regulatory factor-3 induced by Npro from a cytopathic bovine viral diarrhea virus. Virology (2007) 366:277–92. doi:10.1016/j.virol.2007.04.023
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
51. Hilton L, Moganeradj K, Zhang G, Chen YH, Randall RE, McCauley JW, et al. The NPro product of bovine viral diarrhea virus inhibits DNA binding by interferon regulatory factor 3 and targets it for proteasomal degradation. J Virol (2006) 80:11723–32. doi:10.1128/JVI.01145-06
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
52. Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature (2005) 434:772–7. doi:10.1038/nature03464
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
53. Fiebach AR, Guzylack-Piriou L, Python S, Summerfield A, Ruggli N. Classical swine fever virus N(pro) limits type I interferon induction in plasmacytoid dendritic cells by interacting with interferon regulatory factor 7. J Virol (2011) 85:8002–11. doi:10.1128/JVI.00330-11
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
54. Husser L, Ruggli N, Summerfield A. N(pro) of classical swine fever virus prevents type I interferon-mediated priming of conventional dendritic cells for enhanced interferon-alpha response. J Interferon Cytokine Res (2012) 32:221–9. doi:10.1089/jir.2011.0068
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
55. Ruggli N, Tratschin JD, Schweizer M, McCullough KC, Hofmann MA, Summerfield A. Classical swine fever virus interferes with cellular antiviral defense: evidence for a novel function of N(pro). J Virol (2003) 77:7645–54. doi:10.1128/JVI.77.13.7645-7654.2003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
56. Johns HL, Bensaude E, La Rocca SA, Seago J, Charleston B, Steinbach F, et al. Classical swine fever virus infection protects aortic endothelial cells from pIpC-mediated apoptosis. J Gen Virol (2010) 91:1038–46. doi:10.1099/vir.0.016576-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
57. Doceul V, Charleston B, Crooke H, Reid E, Powell PP, Seago J. The Npro product of classical swine fever virus interacts with IkappaBalpha, the NF-kappaB inhibitor. J Gen Virol (2008) 89:1881–9. doi:10.1099/vir.0.83643-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
58. Tamura T, Nagashima N, Ruggli N, Summerfield A, Kida H, Sakoda Y. Npro of classical swine fever virus contributes to pathogenicity in pigs by preventing type I interferon induction at local replication sites. Vet Res (2014) 45:47. doi:10.1186/1297-9716-45-47
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
59. Krey T, Bontems F, Vonrhein C, Vaney MC, Bricogne G, Rumenapf T, et al. Crystal structure of the pestivirus envelope glycoprotein E(rns) and mechanistic analysis of its ribonuclease activity. Structure (2012) 20:862–73. doi:10.1016/j.str.2012.03.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
60. Hausmann Y, Roman-Sosa G, Thiel HJ, Rumenapf T. Classical swine fever virus glycoprotein E rns is an endoribonuclease with an unusual base specificity. J Virol (2004) 78:5507–12. doi:10.1128/JVI.78.10.5507-5512.2004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
61. Windisch JM, Schneider R, Stark R, Weiland E, Meyers G, Thiel HJ. RNase of classical swine fever virus: biochemical characterization and inhibition by virus-neutralizing monoclonal antibodies. J Virol (1996) 70:352–8.
62. Fetzer C, Tews BA, Meyers G. The carboxy-terminal sequence of the pestivirus glycoprotein E(rns) represents an unusual type of membrane anchor. J Virol (2005) 79:11901–13. doi:10.1128/JVI.79.18.11901-11913.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
63. Tews BA, Schurmann EM, Meyers G. Mutation of cysteine 171 of pestivirus E rns RNase prevents homodimer formation and leads to attenuation of classical swine fever virus. J Virol (2009) 83:4823–34. doi:10.1128/JVI.01710-08
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
64. Burrack S, Aberle D, Burck J, Ulrich AS, Meyers G. A new type of intracellular retention signal identified in a pestivirus structural glycoprotein. FASEB J (2012) 26:3292–305. doi:10.1096/fj.12-207191
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
65. Rumenapf T, Unger G, Strauss JH, Thiel HJ. Processing of the envelope glycoproteins of pestiviruses. J Virol (1993) 67:3288–94.
66. Iqbal M, Poole E, Goodbourn S, McCauley JW. Role for bovine viral diarrhea virus Erns glycoprotein in the control of activation of beta interferon by double-stranded RNA. J Virol (2004) 78:136–45. doi:10.1128/JVI.78.1.136-145.2004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
67. Magkouras I, Matzener P, Rumenapf T, Peterhans E, Schweizer M. RNase-dependent inhibition of extracellular, but not intracellular, dsRNA-induced interferon synthesis by Erns of pestiviruses. J Gen Virol (2008) 89:2501–6. doi:10.1099/vir.0.2008/003749-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
68. Matzener P, Magkouras I, Rumenapf T, Peterhans E, Schweizer M. The viral RNase E(rns) prevents IFN type-I triggering by pestiviral single- and double-stranded RNAs. Virus Res (2009) 140:15–23. doi:10.1016/j.virusres.2008.10.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
69. Zurcher C, Sauter KS, Mathys V, Wyss F, Schweizer M. Prolonged activity of the pestiviral RNase Erns as an interferon antagonist after uptake by clathrin-mediated endocytosis. J Virol (2014) 88:7235–43. doi:10.1128/JVI.00672-14
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
70. Python S, Gerber M, Suter R, Ruggli N, Summerfield A. Efficient sensing of infected cells in absence of virus particles by plasmacytoid dendritic cells is blocked by the viral ribonuclease E(rns.). PLoS Pathog (2013) 9:e1003412. doi:10.1371/journal.ppat.1003412
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
71. Meyers G, Saalmuller A, Buttner M. Mutations abrogating the RNase activity in glycoprotein E(rns) of the pestivirus classical swine fever virus lead to virus attenuation. J Virol (1999) 73:10224–35.
72. Meyers G, Ege A, Fetzer C, von Freyburg M, Elbers K, Carr V, et al. Bovine viral diarrhea virus: prevention of persistent fetal infection by a combination of two mutations affecting Erns RNase and Npro protease. J Virol (2007) 81:3327–38. doi:10.1128/JVI.02372-06
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
73. Alves MP, Carrasco CP, Balmelli C, Ruggli N, McCullough KC, Summerfield A. Mycoplasma contamination and viral immunomodulatory activity: dendritic cells open Pandora’s box. Immunol Lett (2007) 110:101–9. doi:10.1016/j.imlet.2007.04.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
74. Carrasco CP, Rigden RC, Vincent IE, Balmelli C, Ceppi M, Bauhofer O, et al. Interaction of classical swine fever virus with dendritic cells. J Gen Virol (2004) 85:1633–41. doi:10.1099/vir.0.19716-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
75. Zaffuto KM, Piccone ME, Burrage TG, Balinsky CA, Risatti GR, Borca MV, et al. Classical swine fever virus inhibits nitric oxide production in infected macrophages. J Gen Virol (2007) 88:3007–12. doi:10.1099/vir.0.83042-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
76. Bauhofer O, Summerfield A, McCullough KC, Ruggli N. Role of double-stranded RNA and N(pro) of classical swine fever virus in the activation of monocyte-derived dendritic cells. Virology (2005) 343:93–105. doi:10.1016/j.virol.2005.08.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
77. Schweizer M, Peterhans E. Pestiviruses. Annu Rev Anim Biosci (2014) 2:141–63. doi:10.1146/annurev-animal-022513-114209
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
78. Husser L, Alves MP, Ruggli N, Summerfield A. Identification of the role of RIG-I, MDA-5 and TLR3 in sensing RNA viruses in porcine epithelial cells using lentivirus-driven RNA interference. Virus Res (2011) 159:9–16. doi:10.1016/j.virusres.2011.04.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
79. Balmelli C, Vincent IE, Rau H, Guzylack-Piriou L, McCullough K, Summerfield A. FcgammaRII-dependent sensitisation of natural interferon-producing cells for viral infection and interferon-alpha responses. Eur J Immunol (2005) 35:2406–15. doi:10.1002/eji.200525998
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
80. Renson P, Le Dimna M, Keranflech A, Cariolet R, Koenen F, Le Potier MF. CP7_E2alf oral vaccination confers partial protection against early classical swine fever virus challenge and interferes with pathogeny-related cytokine responses. Vet Res (2013) 44:9. doi:10.1186/1297-9716-44-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
81. Renson P, Le Dimna M, Gabriel C, Levai R, Blome S, Kulcsar G, et al. Cytokine and immunoglobulin isotype profiles during CP7_E2alf vaccination against a challenge with the highly virulent Koslov strain of classical swine fever virus. Res Vet Sci (2014) 96:389–95. doi:10.1016/j.rvsc.2014.01.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
82. Franzoni G, Kurkure NV, Edgar DS, Everett HE, Gerner W, Bodman-Smith KB, et al. Assessment of the phenotype and functionality of porcine CD8 T cell responses following vaccination with live attenuated classical swine fever virus (CSFV) and virulent CSFV challenge. Clin Vaccine Immunol (2013) 20:1604–16. doi:10.1128/CVI.00415-13
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
83. Rau H, Revets H, Cornelis P, Titzmann A, Ruggli N, McCullough KC, et al. Efficacy and functionality of lipoprotein OprI from Pseudomonas aeruginosa as adjuvant for a subunit vaccine against classical swine fever. Vaccine (2006) 24:4757–68. doi:10.1016/j.vaccine.2006.03.028
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
84. Quesada JR, Talpaz M, Rios A, Kurzrock R, Gutterman JU. Clinical toxicity of interferons in cancer patients: a review. J Clin Oncol (1986) 4:234–43.
85. Gillespie JH, Scott FW, Geissinger CM, Czarniecki CW, Scialli VT. Levels of interferon in blood serum and toxicity studies of bacteria-derived bovine alpha I1 interferon in dairy calves. J Clin Microbiol (1986) 24:240–4.
86. Mann EA, Markovic SN, Murasko DM. Inhibition of lymphocyte recirculation by murine interferon: effects of various interferon preparations and timing of administration. J Interferon Res (1989) 9:35–51. doi:10.1089/jir.1989.9.35
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
87. Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med (2004) 10:187–92. doi:10.1038/nm987
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
88. Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med (1997) 185:517–30. doi:10.1084/jem.185.3.517
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
89. Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis (2003) 8:237–49. doi:10.1023/A:1023668705040
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
90. Durand SV, Hulst MM, de Wit AA, Mastebroek L, Loeffen WL. Activation and modulation of antiviral and apoptotic genes in pigs infected with classical swine fever viruses of high, moderate or low virulence. Arch Virol (2009) 154:1417–31. doi:10.1007/s00705-009-0460-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
91. Li J, Yu YJ, Feng L, Cai XB, Tang HB, Sun SK, et al. Global transcriptional profiles in peripheral blood mononuclear cell during classical swine fever virus infection. Virus Res (2010) 148:60–70. doi:10.1016/j.virusres.2009.12.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
92. Hulst M, Loeffen W, Weesendorp E. Pathway analysis in blood cells of pigs infected with classical swine fever virus: comparison of pigs that develop a chronic form of infection or recover. Arch Virol (2013) 158:325–39. doi:10.1007/s00705-012-1491-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
93. Kaden V, Lange B. Oral immunisation against classical swine fever (CSF): onset and duration of immunity. Vet Microbiol (2001) 82:301–10. doi:10.1016/S0378-1135(01)00400-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
94. Graham SP, Everett HE, Haines FJ, Johns HL, Sosan OA, Salguero FJ, et al. Challenge of pigs with classical swine fever viruses after C-strain vaccination reveals remarkably rapid protection and insights into early immunity. PLoS One (2012) 7:e29310. doi:10.1371/journal.pone.0029310
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
95. Dewulf J, Laevens H, Koenen F, Mintiens K, de Kruif A. Efficacy of E2-sub-unit marker and C-strain vaccines in reducing horizontal transmission of classical swine fever virus in weaner pigs. Prev Vet Med (2004) 65:121–33. doi:10.1016/j.prevetmed.2004.05.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
96. Piriou L, Chevallier S, Hutet E, Charley B, Le Potiera MF, Albina E. Humoral and cell-mediated immune responses of d/d histocompatible pigs against classical swine fever (CSF) virus. Vet Res (2003) 34:389–404. doi:10.1051/vetres:2003013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
97. Graham SP, Haines FJ, Johns HL, Sosan O, La Rocca SA, Lamp B, et al. Characterisation of vaccine-induced, broadly cross-reactive IFN-gamma secreting T cell responses that correlate with rapid protection against classical swine fever virus. Vaccine (2012) 30:2742–8. doi:10.1016/j.vaccine.2012.02.029
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
98. Tarradas J, Argilaguet JM, Rosell R, Nofrarias M, Crisci E, Cordoba L, et al. Interferon-gamma induction correlates with protection by DNA vaccine expressing E2 glycoprotein against classical swine fever virus infection in domestic pigs. Vet Microbiol (2010) 142:51–8. doi:10.1016/j.vetmic.2009.09.043
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
99. Franzoni G, Edwards JC, Kurkure NV, Edgar DS, Sanchez-Cordon PJ, Haines FJ, et al. Partial activation of natural killer and gammadelta T cells by classical swine fever viruses is associated with type I interferon elicited from plasmacytoid dendritic cells. Clin Vaccine Immunol (2014) 21:1410–20. doi:10.1128/CVI.00382-14
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
100. Armengol E, Wiesmuller KH, Wienhold D, Buttner M, Pfaff E, Jung G, et al. Identification of T-cell epitopes in the structural and non-structural proteins of classical swine fever virus. J Gen Virol (2002) 83:551–60.
101. Rau H, Revets H, Balmelli C, McCullough KC, Summerfield A. Immunological properties of recombinant classical swine fever virus NS3 protein in vitro and in vivo. Vet Res (2006) 37:155–68. doi:10.1051/vetres:2005049
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
102. Voigt H, Wienhold D, Marquardt C, Muschko K, Pfaff E, Buettner M. Immunity against NS3 protein of classical swine fever virus does not protect against lethal challenge infection. Viral Immunol (2007) 20:487–94. doi:10.1089/vim.2006.0111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
103. Bouma A, de Smit AJ, de Kluijver EP, Terpstra C, Moormann RJ. Efficacy and stability of a subunit vaccine based on glycoprotein E2 of classical swine fever virus 373. Vet Microbiol (1999) 66:101–14. doi:10.1016/S0378-1135(99)00003-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
104. Konig M, Lengsfeld T, Pauly T, Stark R, Thiel HJ. Classical swine fever virus: independent induction of protective immunity by two structural glycoproteins. J Virol (1995) 69:6479–86.
105. van Rijn PA, Bossers A, Wensvoort G, Moormann RJ. Classical swine fever virus (CSFV) envelope glycoprotein E2 containing one structural antigenic unit protects pigs from lethal CSFV challenge. J Gen Virol (1996) 77(Pt 11):2737–45. doi:10.1099/0022-1317-77-11-2737
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
106. Terpstra C, Wensvoort G. The protective value of vaccine-induced neutralising antibody titres in swine fever. Vet Microbiol (1988) 16:123–8. doi:10.1016/0378-1135(88)90036-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
107. Ceppi M, de Bruin MG, Seuberlich T, Balmelli C, Pascolo S, Ruggli N, et al. Identification of classical swine fever virus protein E2 as a target for cytotoxic T cells by using mRNA-transfected antigen-presenting cells. J Gen Virol (2005) 86:2525–34. doi:10.1099/vir.0.80907-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: classical swine fever, macrophages, dendritic cells, virulence, interferon
Citation: Summerfield A and Ruggli N (2015) Immune responses against classical swine fever virus: between ignorance and lunacy. Front. Vet. Sci. 2:10. doi: 10.3389/fvets.2015.00010
Received: 06 January 2015; Paper pending published: 14 February 2015;
Accepted: 20 April 2015; Published: 07 May 2015
Edited by:
Dirk Werling, Royal Veterinary College, UKReviewed by:
Carol Geralyn Chitko-McKown, U.S. Meat Animal Research Center, USALinda Kathleen Dixon, The Pirbright Institute, UK
Copyright: © 2015 Summerfield and Ruggli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Artur Summerfield, Institute of Virology and Immunology – IVI, Sensemattstrasse 293, Mittelhäusern, Bern 3147, Switzerland, artur.summerfield@ivi.admin.ch
 Artur Summerfield
Artur Summerfield Nicolas Ruggli
Nicolas Ruggli