Clinical Spectrum of SCID: The Key is in the Thymus?
- 1 Division of Immunology and The Manton Center for Orphan Disease Research, Children’s Hospital Boston, Boston, MA, USA
- 2 Department of Pathology, University of Brescia, Brescia, Italy
- 3 “Angelo Nocivelli” Institute for Molecular Medicine and Department of Pediatrics, University of Brescia, Brescia, Italy
- 4 Howard Hughes Medical Institute, Children’s Hospital, Immune Disease Institute and Harvard Medical School, Boston, MA, USA
Lymphostromal cross-talk in the thymus is essential to allow generation of a diversified repertoire of T lymphocytes and to prevent autoimmunity by self-reactive T cells. Hypomorphic mutations in genes that control T cell development have been associated with immunodeficiency and immune dysregulation both in humans and in mice. We have studied T cell development and thymic stroma architecture and maturation in two mouse models of leaky severe combined immune deficiency, carrying hypomorphic mutations in rag1 and lig4 genes. Defective T cell development was associated with abnormalities of thymic architecture that predominantly affect the thymic medulla, with reduction of the pool of mature medullary thymic epithelial cells (mTECs). While the ability of mTECs to express autoimmune regulator (Aire) is preserved in mutant mice, the frequency of mature mTECs expressing Aire and tissue-specific antigens is severely reduced. Similarly, the ability of CD4+ T cells to differentiate into Foxp3+ natural regulatory T cells is preserved in rag1 and lig4 mutant mice, but their number is greatly reduced. These data indicate that hypomorphic defects in T cell development may cause defective lymphostromal cross-talk and impinge on thymic stromal cells maturation, and thus favor immune dysregulation.
Introduction
The thymus is a highly specialized lymphoid organ whose peculiar microenvironment supports homing, proliferation, survival, maturation, and migration of immature thymocytes (Takahama, 2006).
Upon entrance in the thymus, bone marrow-derived committed lymphoid progenitors undergo multiple rounds of proliferation and a distinct process of cell differentiation that culminates with the emergence of a diversified pool of mature T cells whose randomly generated T cell-receptor (TCR) repertoire has been selected for self-major-histocompatibility-complex (MHC) restriction and purged of self-reactive specificities.
Thymocyte development and selection are supported by the thymic stroma, that includes a highly organized network of specialized thymic epithelial cells (TECs) and distinct populations of thymic dendritic cells (DCs). The mature thymus is organized in two major compartments, the cortex and the medulla, that contain distinct populations of TECs that exert different functions. In particular, cortical TECs (cTECs) sustain the positive selection of CD4+ CD8+ double positive (DP) thymocytes that have successfully rearranged their TCR; in contrast, medullary TECs (mTECs) support selection, maturation, and export of single positive (SP) thymocytes (Anderson and Jenkinson, 2001; Hogquist et al., 2005; Irla et al., 2010; Takahama et al., 2010).
Moreover, mTECs display the unique ability of expressing the transcription factor autoimmune regulator (Aire), that allows mTECs to express a large number of genes that encode for peripheral tissue-specific antigens (TSAs). These self TSAs are presented to developing SP T cells either directly by mTECs or indirectly by DCs upon uptake from mTECs (Derbinski et al., 2005; Kyewski and Klein, 2006). Interaction between self-antigen-loaded thymic stromal cells and newly generated T cells expressing self-reactive TCR specificities leads to the induction of central T cell tolerance via clonal deletion of self-reactive T cells. Alternatively, it has been proposed that mTECs and medullary thymic DCs may contribute to the establishment of tolerance by facilitating diversion of self-reactive thymocytes into natural regulatory T (nTreg) cells, that exhibit suppressive properties when exposed to self-antigens in the periphery (Watanabe et al., 2005; Miyara and Sakaguchi, 2007). These observations indicate that the thymus plays a critical role not only in the generation of a functional and diversified repertoire of mature T cells that are capable of recognizing non-self-antigens, but also in preventing the development of autoimmunity.
Primary immunodeficiencies (PIDs) comprise a heterogeneous group of genetic disorders characterized by impaired development and/or function of the immune system (Fischer, 2007; Notarangelo, 2010). In humans, several genetic defects have been identified that result in block at early stages of T cell development: in some of these forms, B and/or NK cell development is also impaired. These disorders are collectively known as severe combined immune deficiency (SCID), whose clinical phenotype is characterized by early onset susceptibility to infections and failure to thrive. SCID is inevitably fatal unless immune reconstitution is achieved by hematopoietic cell transplantation or, in selected cases, by enzyme replacement therapy or gene therapy (Buckley, 2000; Fischer, 2000).
In humans, up to 20–30% of all cases of SCID are caused by defects of V(D)J recombination, a lymphoid-restricted process that allows DNA rearrangements at the immunoglobulin and TCR loci, enabling expression of immunoglobulin and TCR proteins and development of a diversified repertoire of T and B lymphocytes.
Mutations of the recombination-activating gene (RAG) 1 and RAG2 cause SCID by interfering with the initial step of V(D)J recombination, i.e., recognition of recombination signal sequences that flank the Variable (V), Diversity (D), and Joining (J) coding elements and introduction of DNA double strand breaks (DSBs; Schwarz et al., 1996). In contrast, mutations of Artemis, DNA protein kinase catalytic subunit (DNA–PKcs), DNA ligase 4 (LIG4), and Cernunnos/XLF affect the non-homologous end joining (NHEJ) pathway of DNA repair, that is involved at later stages in V(D)J recombination (Riballo et al., 1999; Moshous et al., 2001; Buck et al., 2006a; van der Burg et al., 2009).
While null mutations in genes involved in V(D)J recombination typically cause SCID with virtual absence of T and B lymphocytes (T− B− SCID), hypomorphic mutations in the same genes in humans have been associated with variable degrees of impairment of T and B cell development and frequent occurrence of manifestations of immune dysregulation. In particular, Omenn syndrome (OS) is characterized by oligoclonal expansion of few T cell clonotypes that infiltrate peripheral organs and cause extensive tissue damage (erythroderma, gut villous atrophy, hepatosplenomegaly; Villa et al., 2001). Less severe defects in V(D)J recombination may cause more subtle phenotypes, ranging from leaky SCID (in which residual development of T cells is not associated with generalized skin rash) to delayed-onset combined immunodeficiency with granulomatous manifestations (Schuetz et al., 2008; De Ravin et al., 2010). Furthermore, hypomorphic mutations in genes involved in NHEJ are associated also with extra immune clinical features (microcephaly, short stature, increased occurrence of malignancies), reflecting impairment of DNA repair mechanisms (O’Driscoll et al., 2004; Gennery, 2006; Sobacchi et al., 2006).
We have previously reported severe abnormalities of thymic architecture and impaired expression of Aire and of TSAs in the thymus of patients with OS (Cavadini et al., 2005; Poliani et al., 2009). These observations imply that genetic defects that affect T cell development and prevent generation of a robust and diversified T cell repertoire may also impinge on the differentiation and/or homeostasis of thymic stromal cells, and hence impair deletional and non-deletional mechanisms of central tolerance. In order to address this hypothesis, we have taken advantage of two recently described murine models of leaky SCID: the lig4R/R mouse, homozygous for the hypomorphic R278H mutation in the lig4 gene (Rucci et al., 2010) and the rag1S/S mouse, carrying the homozygous hypomorphic S723C substitution in the rag1 gene (Giblin et al., 2009; Walter et al., 2010).In agreement with the human phenotype, both mutant mice are characterized by severe immunodeficiency with residual development of oligoclonal and functionally impaired T cells. In addition, a minority of rag1S/S mice (but not lig4R/R mice) develop features consistent with OS (Giblin et al., 2009; Walter et al., 2010). Here we show that hypomorphic mutations that affect V(D)J recombination also compromise architecture and homeostasis of thymic stroma, and that the severity of thymic abnormalities correlates with the degree of immune dysregulation that may be observed in these conditions.
Materials and Methods
Mice
Mice harboring the rag1 S723C mutation (rag1S/S; Giblin et al., 2009) and the lig4 R278H mutation (lig4R/R; Rucci et al., 2010) were housed at the Karp Family Research Building under specific pathogen-free conditions. Animal experiments were carried out after approval and in accordance with guidelines from the Subcommittee on Research Animal Care of Children’s Hospital Boston, Harvard Medical School.
Single Cell Suspensions Preparation
Single cell suspensions were prepared from thymuses of lig4R/R, rag1S/S, and wild-type (WT) mice. Tissues were homogenized on 70 μm cell strainers (BD Falcon, Bedford, MA, USA) using FACS buffer: Dulbecco’s Phosphate-Buffered Saline (D-PBS, GIBCO from Invitrogen, Grand Island, NY, USA) containing 2% of heat inactivated and filtered fetal calf serum (FCS, from Gemini Bio-Products, West Sacramento, CA, USA). Red blood cell lysis was performed at room temperature by adding 2 ml of Red Blood Cell Lysing Buffer (Sigma Aldrich Inc., St Louis, MO, USA) for 10 min before proceeding with the specific stainings.
Thymic stromal cells were isolated as previously published (Gray et al., 2002) by digesting thymuses from lig4R/R, rag1S/S, and WT mice with 0.125% (w/v) collagenase D with 0.1% (w/v) DNAse I (both from Roche, Indianapolis, IN, USA) in RPMI 1640 (GIBCO from Invitrogen, Grand Island, NY, USA).
Immunophenotypic Analysis
Thymocytes were incubated with the following antibodies: antigen-presenting cells (APC)-conjugated anti-CD4, biotin-conjugated or PE-conjugated anti-CD8, biotin-conjugated anti-CD4, biotin-conjugated anti-B220, biotin-conjugated anti-CD11b, biotin-conjugated anti-Gr1, FITC-conjugated anti-CD44, PE-conjugated anti-CD25, FITC-conjugated anti-CD69, biotin-conjugated anti-Qa2 (all from BD Biosciences, San Jose, CA, USA). Samples stained with biotin-conjugated antibodies underwent additional incubation with PerCp-conjugated streptavidin. Intranuclear staining for Foxp3 was performed using APC-conjugated anti-mouse/rat Foxp3 staining set (eBioscience, San Diego, CA, USA) following manufacturer’s instructions. At least 20,000 alive cells (defined by physical parameters) were acquired on a FACSCalibur system or FACS Canto (BD Biosciences, San Jose, CA, USA) and analyzed with FLOW-JO software (version 8.3; Treestar Inc.).
Analysis of TECs was performed staining single cell suspensions of stromal cells with PerCp-conjugated anti-CD45, PE-conjugated anti-Ly51, FITC-conjugated anti-MHC-II (BD Biosciences, San Jose, CA, USA). Intranuclear staining for Aire was performed after fixing stromal cells labeled with surface markers, by permeabilization with the BD CytoFix/CytoPerm Fixation/Permeabilization kit (BD Biosciences, San Jose, CA, USA). Anti-Aire antibody (5H12) was a kind gift from Dr. H. Scott (Hubert et al., 2008).
Analysis of thymic DCs was performed staining single cell suspensions of stromal cells with the following antibodies: APC-conjugated anti-CD11c, PE-conjugated anti-CD45RA, biotin-conjugated anti-CD3, anti-Ter119, anti-Gr1, anti-F4/80, anti-CD19, anti-CD11b, anti-CD90 (BD Biosciences, San Jose, CA, USA). Analysis was performed after gating out the biotinylated positive population. At least 100,000 alive cells (defined by physical parameters) were acquired on a FACS Canto (BD Biosciences, San Jose, CA, USA) and analyzed with FLOW-JO software (version 8.3; Treestar Inc.).
Immunohistochemistry and Immunofluorescence
Formalin-fixed paraffin embedded tissue sections were stained with hematoxylin and eosin (H&E) and subjected in parallel to immunohistochemistry. Briefly, sections were deparaffinized, rehydrated, and endogenous peroxidase activity was blocked in 0.3% H2O2/methanol solution for 20 min prior to heat induced antigen retrieval (when necessary) using a thermostatic bath or a microwave-oven in 1.0 mM EDTA (pH 8.00) or 1.0 mM citrate buffer (pH 6.00) respectively. Sections were then washed in TRIS-base buffer at pH 7.4 and incubated for 1 h with the following reagents diluted in TRIS/1% bovine serum albumin (BSA): rabbit anti-CK5 (1:50; Covance, Berkeley, CA, USA), rat anti-CK8 (1:200; clone TROMA-1; kindly provided by Dr. U.H. von Andrian, Harvard Medical School), rabbit anti-claudin 4 (1:100; Zymed Laboratories, San Francisco, CA, USA), rabbit anti-murine Aire (1:3000; kindly provided by Dr. P. Peterson, University of Tartu, Estonia), and biotin-conjugated Ulex europaeus agglutinin-1 (UEA-1; 1:600; Vector Laboratories, Burlingame, CA, USA). After washes, single immunostains were revealed using the ChemMATE Envision Rabbit/Mouse (DAKO Cytomation, Glostrup, Denmark) or NovoLinkTM Polymer Detection System (NovocastraTM Laboratories Ltd, Newcastle Upon Tyne, United Kingdom) followed by diaminobenzidine (DAB) as chromogen and Hematoxylin as counterstain.
The same procedure was applied to double immunofluorescence stainings prior to the incubation with a secondary swine anti-rabbit FITC-conjugated antibody (1:30; DAKO Cytomation, Glostrup, Denmark) for CK5 and a rabbit anti-rat biotinylated antibody (1:200; Vector Laboratories, Burlingame, CA, USA) followed by Streptavidin–Texas Red (1:100; Southern Biotechnology Associates, Birmingham, AL, USA) for CK8. Sections were then counterstained with DAPI.
Images were acquired with an Olympus DP70 digital camera mounted on an Olympus BX60 microscope using CellF imaging software (Soft Imaging System GmbH) and Adobe Photoshop Version 7.0 for the artwork.
RNA Isolation, CDNA Preparation, and Real-Time PCR Analysis
RNA isolation was isolated from thymus single cell suspensions using the mirVana miRNA isolation kit, according to the manufacturer’s protocol (Ambion from Applied Biosystems Inc., Foster City, CA, USA). Reverse transcription was then performed with qScript cDNA SuperMix (Quanta BioSciences, Inc., Gaithersburg, MD, USA) following manufacturer’s instructions.
Real-Time PCR for quantitative expression of Aire and TSAs was performed using TaqMan Gene expression assay with the following assays on demand (all by Applied Biosystems Inc., Foster City, CA, USA): Mm00477461_m1 (Aire); Mm00487224_m1 (Cybp1a2); Mm00433188_m1 (Fabp2); Mm00731595_gH (Ins2); Mm00493214_m1 (EpCAM, used as internal control). Reactions were performed using 2 μl of cDNA obtained from 1 μg of thymic RNA in a final volume of 20 μl using TaqMan PCR Master Mix 2x (Applied Biosystems Inc., Foster City, CA, USA) and specific Gene Expression Assay 20x. Amplification was performed in duplicates in the 7500 Real-Time PCR System (Applied Biosystems Inc., Foster City, CA, USA) and results were analyzed with the 7500 Real-Time PCR Software.
Analysis of T Cell Repertoire
Analysis of T cell repertoire in the thymus of WT, rag1S/S and lig4R/R mice was performed as previously described (Rucci et al., 2010).
Statistics
At least five to six mice per group were analyzed. Results are indicated as mean ± SE or SD. p values were determined by unpaired Student’s t-test (p < 0.05 = *;p < 0.01 = **;p < 0.005 = ***;p < 0.001 = ****).
Results
Lig4R/R and rag1S/S Mice Exhibit an Incomplete Block in T Cell Development
The lig4R/R and rag1S/S mice carry mutations that impair different steps in the V(D)J recombination process. In particular, rag1 mutations interfere with the first step of the process, when DSBs are introduced in the DNA (Fugmann et al., 2000. In contrast, lig4 mutations impair the repair of these breaks (Rooney et al., 2004). Despite these differences, both mutations affect V(D)J recombination and cause an early but incomplete block in T cell development.
Both lig4R/R and rag1S/S mice showed a significant reduction in thymus size and cellularity (Figure 1A), with a significant decrease in the absolute numbers of thymic CD4+ CD8+ DP cells as well as of CD4+ CD8− and CD4− CD8+ SP thymocytes as compared to what observed in WT mice (Figure 1B).
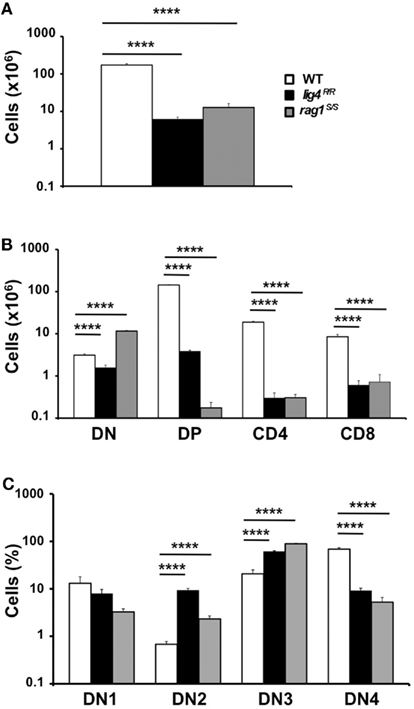
Figure 1. Thymic lymphopenia and impaired T cell development in lig4R/R and rag1S/S mice. (A) Total thymic cellularity from 4 to 5-weeks-old mice shows severe lymphopenia in lig4R/R and rag1S/S mice as compared to WT littermate controls. (B) Thymuses from 4 to 5-weeks-old mice were stained with anti-CD4 and anti-CD8 antibodies and the absolute numbers of live thymocytes at different stages of differentiation are shown in the bar charts (DN, double negative; DP, double positive). (C) Distribution of CD4− CD8− DN thymocytes at various stages of differentiation (DN1–DN4). Mean values ± SE are shown. At least six mice per group were analyzed.
In both mutant models, this block in T cell development was associated with a relative enrichment in the proportion of CD4− CD8− double negative (DN)cells, that was particularly prominent in rag1S/S mice (% DN cells ± SE: WT 1.78 ± 0.13; lig4R/R 25.48 ± 3.5; rag1S/S 90.59 ± 3.27; WT vs. lig4R/R, p < 0.0001; WT vs. rag1S/S, p < 0.0001). Developmental progression of DN thymocytes is characterized by an ordered sequence of expression of CD44 and CD25 markers: CD44+ CD25− (DN1), CD44+ CD25+ (DN2), CD44− CD25+ (DN3), CD44− CD25− (DN4). Analysis of the distribution of DN thymocytes in lig4R/R and rag1S/S mice revealed a severe, but incomplete arrest of thymocyte development at DN3 stage in both models, consistent with a defect in TCRβ rearrangement due to impaired V(D)J recombination (Figure 1C).
Skewed Distribution of SP T Cells and Restricted T Cell Repertoire in the Thymus of lig4R/R and rag1S/S Mice
Newly generated CD4+ and CD8+ SP thymocytes undergo sequential stages of maturation in the medulla. The developmental program of maturing SP thymocytes is associated with progressive down-regulation of CD69 and up-regulation of the Qa2 markers on the cell surface (Lucas et al., 1994; Jin et al., 2008).The earliest stages in SP cell development (SP1–SP2) are characterized by expression of CD69 but lack of Qa2 marker (CD69+ Qa2− cells). The next step of maturation of SP thymocytes (SP3 stage) is marked by lack of expression of either marker (CD69− Qa2− cells). Finally, in the last stage of differentiation (SP4), mature SP thymocytes acquire expression of Qa2, and hence have a CD69− Qa2+ phenotype. Thymocytes that reach this stage are ready to egress from the thymus (Ge and Chen, 1999; Jin et al., 2008). In control mice, the majority of CD4+ SP thymocytes are in the SP1–SP2 stages of differentiation; in contrast, both lig4R/R and rag1S/S mice showed a significant increase in the proportion of CD4+ SP thymocytes with the most mature (SP4) phenotype (Figure 2). A similar pattern was observed for CD8+ SP thymocytes (data not shown).This difference in the distribution of SP thymocytes at various stages of differentiation may reflect several, non-mutually exclusive mechanisms, including accelerated intrathymic T cell maturation, homeostatic T cell proliferation in a lymphopenic environment (Datta and Sarvetnick, 2009) and recirculation of mature T lymphocytes that home back to thymus. In order to distinguish between these possibilities, we have analyzed the distribution of SP thymocytes at late stages (18 ± 2 days) of fetal development, when homeostatic proliferation and recirculation of mature lymphocytes should not prevail. As shown in Figure A1 in Appendix, even at this stage of fetal development, SP thymocytes from lig4R/R and rag1S/S mice were characterized by a more mature (SP3) phenotype than SP thymocytes from age-matched WT mice. Overall, these data indicate that intra thymic maturation of SP thymocytes is accelerated in lig4R/R and rag1S/S mice.
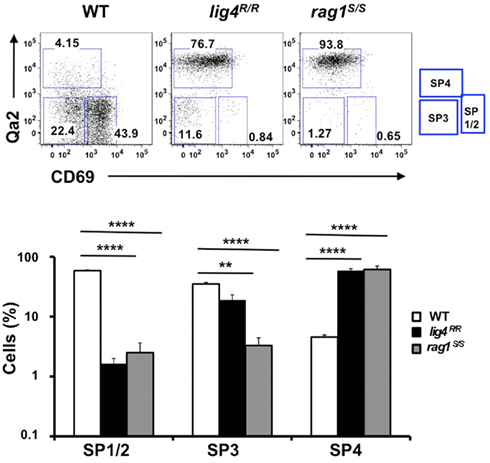
Figure 2. Altered maturation of CD4+ SP medullary thymocytes in the lig4R/R and rag1S/S mice. Upper panels: Representative FACS plots of CD4+ SP medullary thymocytes at various stages of maturation according to the expression of CD69 and Qa2 surface markers. Lower panels: Distribution of the different populations of SP1–SP4 cells. lig4R/R and rag1S/S mice have a significant accumulation of SP4 thymocytes. Mean values ± SE are shown. At least six mice per group were analyzed.
Hypomorphic mutations that affect V(D)J recombination may affect not only the number, but also the TCR repertoire diversity of newly generated thymocytes. We have previously demonstrated that hypomorphic RAG mutations in patients with OS are associated with oligoclonal representation of TCR specificities in the thymus (Signorini et al., 1999). Similarly, a highly restricted T cell repertoire was demonstrated in the thymus of rag1S/S mice (Figure 3). In contrast, homozygosity for the R278H lig4 mutation allowed generation of a broadly polyclonal repertoire of thymocytes (Figure 3). These differences in size and diversity of the thymocyte pool prompted us to investigate thymus morphology in lig4R/R and rag1S/S mice.
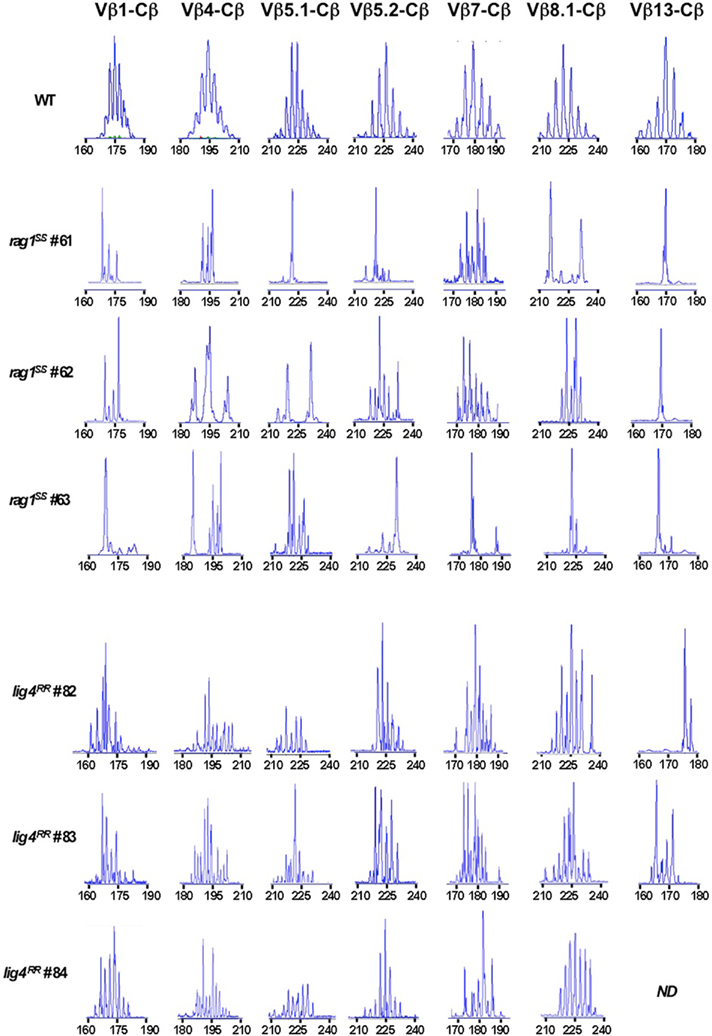
Figure 3. Immunoscope analysis of TCR repertoire in the thymus of rag1S/S and lig4R/R mice. Representative immunoscope profiles of TCRVβ repertoire in the thymus of one WT, three rag1S/S and three lig4R/R mice. Profiles are shown for 7 of the 24 distinct TCR Vβ-Cβ amplification products analyzed. The x axis represents CDR3 length, and arbitrary fluorescence intensity of the run off products is shown on the y axis. ND: not done.
Abnormalities of Thymic Architecture in lig4R/R and rag1S/S Mice
Appropriate interaction between elements of the T cell lineage and stromal cells is crucial to maintain thymic architecture and to support the maturation of both TECs and nascent thymocytes. Severe and early blocks in T cell development are associated with lack of thymic cortico-medullary demarcation (Holländer et al., 1995a). Furthermore, inability to maintain an organized thymic architecture may interfere not only with an orderly maturation of thymocytes, but may also impede establishment of self-tolerance (Holländer et al., 1995b; Derbinski and Kyewski, 2005).
Based on this, we investigated in detail the thymic architecture of lig4R/R and rag1S/S mice. As shown in Figure 4, staining of thymic tissue with H&E revealed severe depletion of cellularity in both lig4R/R and rag1S/S mice. Cortico-medullary demarcation was preserved in lig4R/R mice, whereas only a rudimentary attempt to form a medulla was noticed in rag1S/S mice. Differential expression of cytokeratin 8 (CK8) and cytokeratin 5 (CK5) allows distinction between CK8+ CK5− cTECs and CK8− CK5+ mTECs (Bennett et al., 2002; Takahama, 2006). Analysis of CK5 and CK8 expression by immunohistochemistry and immunofluorescence confirmed significant differences in the degree of thymic architecture abnormalities in lig4R/R and rag1S/S mice. In particular, only few CK5+ cells were detected in rag1S/S mice; furthermore, these cells were largely also CK8+, a pattern observed in immature TEC progenitors (Bennett et al., 2002). In contrast, tiny but well-defined nests of CK5+ CK8− mTECs were appreciated in lig4R/R mice, consistent with what detected by H&E staining.
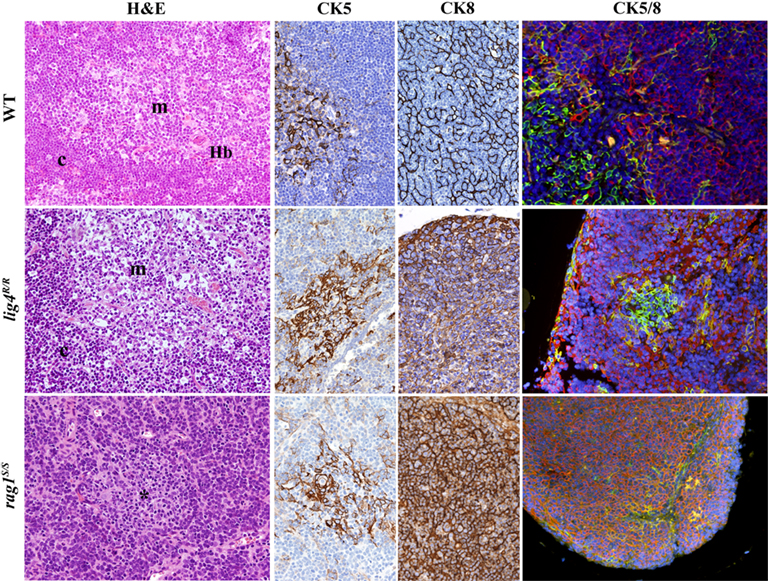
Figure 4. Thymic architecture in wild-type, lig4R/R, and rag1S/S mice. Analysis of thymic architecture and cytokeratin (CK) expression in WT, lig4R/R, and rag1S/S mice. Thymic architecture with identification of cortex (c) and medulla (m) is shown in the first column on the left by hematoxylin and eosin (H&E) staining. The second and third columns show distribution of CK5+ and CK8+ epithelial cells, respectively. Panels in the right column represent dual immunofluorescence (IF) analysis for CK8+ (in red) and CK5+ (in green) cells. Yellow staining identifies cells co-expressing CK5 and CK8. Nuclei are counterstained with DAPI. H&E staining shows normal cortico-medullary demarcation (CMD) in both WT and lig4R/R mutant mice, whereas only focal areas of medullary differentiation (asterisk) are appreciated in rag1S/S mice. A normal distribution of both CK5+ cells, that represent the vast majority of mTECs, and CK8+ cells, that design a fine meshwork of cTECs (upper middle panels, CK5, and CK8 staining), with clear separation between them, is present in WT mice, as shown by IF (upper right panel). Thymuses from lig4R/R mutant mice show CMD with normal distribution of the CK5+ and CK8+ cells, although the CK8+ cTECs show a coarse distribution with a globular morphology (middle panels). A well-defined, but tiny thymic medulla is visualized by IF in lig4R/R mice (right panel). In contrast, thymuses from rag1S/S mice show impaired CMD (H&E, left panel); staining for CKs shows diffuse expression of CK8, and focal expression of CK5 (middle panels). IF shows increased presence of CK5+ CK8+ double positive immature TECs in rag1S/S mice (right panel). All panels are from 20× original magnification. One representative example of 5 mice analyzed per each strain.
Analysis of Maturation of mTECs and of Aire and TSA Expression in the Thymus of lig4R/R and rag1S/S Mice
The thymic medulla plays an essential role in the tolerance to peripheral antigens. Both mature mTECs and thymic DCs have been implicated in mediating central tolerance by presenting nascent thymocytes with a broad repertoire of TSAs whose expression by mTECs is controlled by the transcription factor Aire (Derbinski et al., 2005; Kyewski and Klein, 2006).
Maturation of mTECs is progressively marked by the expression of claudin-4 (Cld4) and the ligand for UEA-1. Furthermore, terminally differentiated mTECs express high levels of MHC-II molecules and a subset of them also express the transcription factor Aire and TSAs (Hamazaki et al., 2007). Residual presence of Cld4+ and of UEA-1+ mature mTECs was detected in the thymus of lig4R/R mice; in contrast, there was no expression of these markers in the thymus from rag1S/S mice, in keeping with our previous observations that they lack a well-defined thymic medulla (Figure 5). Staining with anti-Aire antibody revealed a relative abundance of Aire+ cells in the thymic medulla of WT mice. Aire+ cells were detected also in lig4R/R mice, albeit in low number; in contrast, dramatic depletion of Aire+ cells, that were confined to focal areas of cortico-medullary demarcation, was demonstrated in rag1S/S mice (Figure 5).

Figure 5. Maturation of mTEC cells in wild-type, lig4R/R, and rag1S/S mice. Mature mTECs from WT mice express claudin-4 (Cld4), Ulex europaeus agglutinin 1 (UEA-1) and Aire (upper panels). Insets highlight fully mature mTECs showing immunoreactivity (IR) for Cld4 and the characteristic granular dot-like Aire positivity in the nuclei. Thymuses from lig4R/R mice show residual presence of mTECs that reach full maturation with positivity for UEA-1, Cld4, and Aire expression (middle panels). Loss of CMD with impaired maturation of mTECs was observed in the thymuses from the rag1S/S mice in which only rare UEA-1 IR cells but no mature Cld4+ and Aire+ cells were found (lower panels). IR staining: brown. All panels are from 20× original magnification; insets are from 40× original magnification. One representative example of five mice analyzed per each strain.
Next, we used quantitative real-time polymerase chain reaction (qPCR) to analyze the levels of mRNA specific for Aire and for Aire-dependent TSAs (insulin, cytochrome p450, and fatty acid binding protein) in the thymus of WT, lig4R/R, and rag1S/S mice. A significant reduction in the level of these transcripts was observed in the thymus of both mutant mice; this reduction was particularly pronounced in rag1S/S mice (Figure 6A).
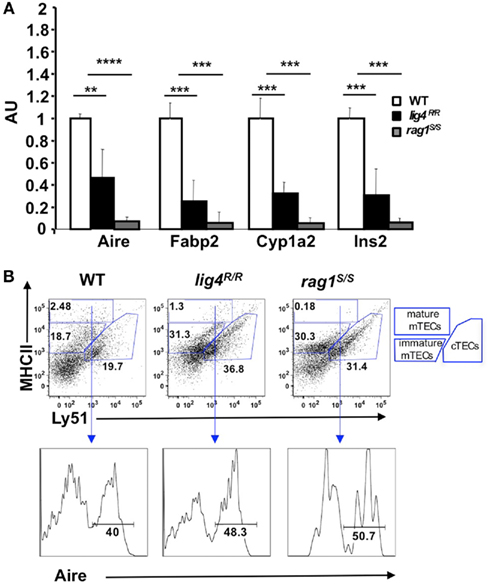
Figure 6. Reduced expression of Aire and of tissue-specific antigens (TSAs) in the thymus of lig4R/R and rag1S/S mice. (A) Reduced expression of Aire and TSAs (fatty acid binding protein, Fabp2; cytochrome p450, Cyp1a2; insulin 2, Ins2) in the thymus of lig4R/R and rag1S/S mice. Real time PCR results were normalized to the pan-epithelial marker EpCAM1. Mean values ± SD are shown. Seven mice per group were analyzed; AU, arbitrary units. (B) Representative example of flow cytometry analysis of cortical and medullary compartments shows that in the thymus of lig4R/R and rag1S/S mice the mature medullary compartment (MHC-IIhi) is poorly represented. The percentage of Aire+ cells among mature mTECs is largely preserved in both mutant mice. At least five mice per group were analyzed.
The observed reduction of Aire and TSA mRNA expression in the thymus of lig4R/R and rag1S/S mice could reflect either impairment of terminal maturation of mTECs or a general depletion of the mTEC compartment. To distinguish between these two possibilities, we used flow cytometry. It has been shown that cTECs and mTECs can be distinguished based on the expression of MHC-II and Ly-51 markers within the CD45− population of thymic stromal cells (Hubert et al., 2008). Both cTECs and mTECs express MHC-II, but only cTECs express Ly51. Furthermore, based on the levels of expression of MHC-II, it is also possible to discriminate between immature (MHC-IIlow Ly-51−)and mature MHC-IIhi Ly-51− mTECs. Upon staining of CD45− thymic stromal cells for Ly-51 and MHC-II, we found that the thymuses of both lig4R/R and rag1S/S mice were significantly depleted of mature mTECs (% MHC-IIhi mTECs ± SE: WT = 2.87 ± 0.8; lig4R/R = 1.12 ± 0.4; rag1S/S = 0.17 ± 0.04; WT vs. lig4R/R p < 0.005; WT vs. rag1S/S, p < 0.005). In contrast, both lig4R/R and rag1S/S mice showed a relative enrichment in thymic cTECs (% cTECs ± SE: WT = 19.49 ± 3.31; lig4R/R = 34.36 ± 2.92; rag1S/S = 33.51 ± 2.23; WT vs. lig4R/R, p < 0.005; WT vs. rag1S/S, p < 0.005; Figure 6B). However, this apparent enrichment in cTECs may also reflect the increased number of immature TECs (as demonstrated by co-expression of CK5 and CK8) and/or an increase in fibroblasts or other stromal CD45− Ly51+ cells, as reported in other murine models of impaired T cell development (Gray et al., 2002; Rodewald, 2008; Alves et al., 2009).
As mentioned above, fully mature MHC-IIhi mTECs are enriched for Aire expressing cells.In spite of the general reduction in the proportion of mature mTECs, we found that the few CD45− MHC-IIhi Ly51low mTECs from lig4R/R and rag1S/S mice retained the ability to express Aire (% Aire+ cells ± SE: WT = 43.05 ± 2.45; lig4R/R = 48.12 ± 5.11; rag1S/S = 47.05 ± 5.4; Figure 6B), indicating that the overall impairment of Aire and TSA expression in these mutant models is due to a reduction of the pool of mature mTECs rather than to intrinsic defects in their developmental and gene expression program.
Analysis of Thymic DCs and Generation of nTreg Cells in lig4R/R and rag1S/S Mice
Thymic DCs are the other population of APCs involved in the negative selection of self-reactive thymocytes. Furthermore, a role for thymic DCs in the induction of nTreg cells has been suggested both in mice and in humans (Proietto et al., 2008; Doan et al., 2009). Thymic DCs can be classified into two major subsets: the CD11c+ CD45RA− conventional DCs (cDCs) and the CD11cint CD45RA+ plasmacytoid DCs (pDCs). The first subgroup is intrathymically generated from early thymic progenitor cells, whereas pDCs arise extrathymically from partially differentiated precursors (Proietto et al., 2009).
In order to analyze the distribution of thymic cDCs and pDCs in WT, lig4R/R, and rag1S/S mice, we stained thymic single cells suspensions with a mixture of monoclonal antibodies against markers specific for T and B lymphocytes, erythroid cells, granulocytes, and macrophages (CD3, CD90, CD19, TER119, Gr-1, CD11b, and F4/80). Upon gating on cells that stained negative for this cocktail of antibodies, we analyzed expression of CD11c and CD45RA to discriminate between cDCs and pDCs. As shown in Figure 7, in WT mice the majority of thymic DCs is composed of cDCs. Although cDCs were more abundant than pDCs also in the thymus of lig4R/R and rag1S/S mice, both mutant strains showed a significant enrichment for pDCs as compared to WT mice (Figure 7).
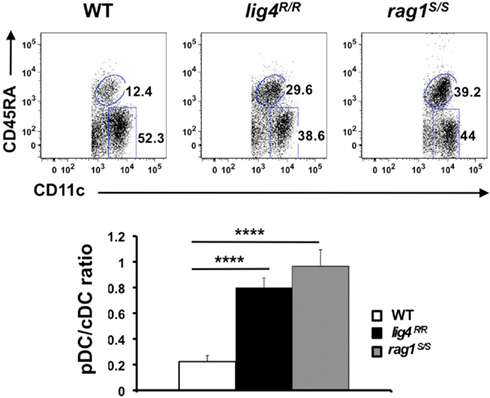
Figure 7. Altered distribution of thymic DCs populations in lig4R/R and rag1S/S thymuses. Top panels: FACS dot plot analysis of the distribution of CD11c+ CD45RA− cDCs and CD11cint CD45RAhi pDCs in the thymus of WT, lig4R/R, and rag1S/S mice after gating on a population of stromal cells positive for CD11c expression but negative for a cocktail of biotinylated markers specific for markers of T and B lymphocytes, erythroid, granulocyte, and macrophage lineages. Lower panels: Proportion of thymic cDCs and pDCs in WT, lig4R/R, and rag1S/S mice. Mean values ± SE are shown. At least six mice per group were analyzed.
To investigate whether the profound abnormalities of thymic stroma observed in lig4R/R and rag1S/S mice could also affect generation of nTreg cells, we analyzed expression of CD25 and Foxp3 within CD4+ SP thymocytes. As shown in Figure 8, both lig4R/R and rag1S/S mice showed preserved ability to express Foxp3 within CD4+ SP thymocytes, and the proportion of Foxp3+ cells was actually increased in lig4R/R mice (% Foxp3+ cells ± SD: WT = 4.72 ± 1.3; lig4R/R = 24.2 ± 3.5; rag1S/S = 6.25 ± 3.3; WT vs. lig4R/R p < 0.0001; WT vs. rag1S/S p < 0.0001; Figure 8). However, it should be noted that both mutant strains display a severe depletion of thymic cellularity, and of CD4+ SP T cells in particular (Figure 1). This has obvious implications also on the absolute number of nTreg cells, in particular in lig4R/R mice. By using immunohistochemistry, we found that a residual number of Foxp3+ cells at the cortico-medullary junction were present in lig4R/R mice, whereas such cells were severely depleted in rag1S/S mice (data not shown).
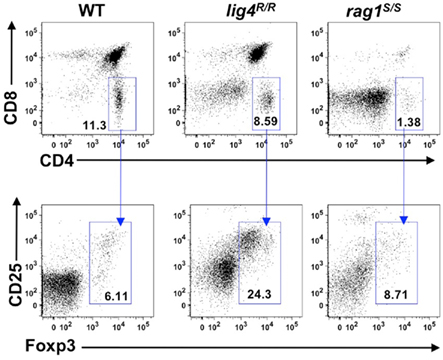
Figure 8. Generation of nTregs in the thymus of lig4R/R and rag1S/S mice. Representative example of flow cytometry analysis of thymocytes stained with anti-CD4, anti-CD8, anti-CD25, and anti-Foxp3 antibodies revealed that generation of nTregs is preserved in the thymus of 4 to 5-weeks-old lig4R/R and rag1S/S mice as compared to what observed in WT age-matched littermates. At least six mice per group were analyzed.
Discussion
There is growing evidence that defects of V(D)J recombination in humans are associated with a variety of clinical and immunological phenotypes. Null mutations in the RAG genes cause T− B− NK+ SCID (Schwarz et al., 1996). In contrast, hypomorphic mutations in RAG have been associated with OS (Villa et al., 1998), atypical/leaky SCID (Villa et al., 2001), combined immunodeficiency with expansion of TCRγδ+ T cells (Ehl et al., 2005), and delayed-onset combined immunodeficiency with granuloma formation (Schuetz et al., 2008; De Ravin et al., 2010). Extreme phenotypic variability has been observed also among patients with LIG4 syndrome, ranging from mild or moderate immunodeficiency to SCID (O’Driscoll et al., 2001; Buck et al., 2006b; Enders et al., 2006; van der Burg et al., 2006). However, only one patient with OS due to LIG4 mutations has been reported (Grunebaum et al., 2008).
The clinical phenotype of patients carrying hypomorphic mutations that affect V(D)J recombination is often characterized by prominent signs of immune dysregulation, as exemplified by infiltration of target organs by activated and oligoclonal T lymphocytes in patients with OS (Signorini et al., 1999) and by the frequent occurrence of autoantibodies in patients with OS or leaky SCID (Walter et al., 2010). Characterization of the molecular and cellular mechanisms that are responsible for the unique association of severe immunodeficiency and autoimmunity has been hampered by lack of adequate animal models.
We have previously shown that lig4R/R and rag1S/S mice represent mouse models of leaky SCID, with profound immunodeficiency and increased risk of autoimmunity (Rucci et al., 2010; Walter et al., 2010). We have also reported that a proportion of rag1S/S, but not of lig4R/R mice, show more prominent features of severe immune dysregulation, resembling OS (Giblin et al., 2009; Rucci et al., 2010; Walter et al., 2010). We now show that hypomorphic mutations in rag1 and lig4 genes in mice affect both normal development of T lymphocytes and organization and maturation of thymic stroma, and compromise key mechanisms involved in central tolerance.
Studies in mice had indicated that signals delivered by thymocytes are crucial to induce maturation of cTECs and mTECs from a common precursor and to support maintenance of thymic architecture (Holländer et al., 1995a; van Ewijk et al., 2000; Akiyama et al., 2008; Hikosaka et al., 2008; Irla et al., 2008). On the other hand, cTECs and mTECs play a critical role in generating and shaping the mature T cell repertoire. In particular, cTECs allow positive selection of thymocytes through a mechanism that involves cTEC-specific expression of thymoproteasome components, allowing expression of a unique repertoire of MHC-bound self-peptides (Murata et al., 2007; Gommeaux et al., 2009). Positively selected thymocytes are then screened for the ability to recognize self-peptide/MHC complexes in the thymic medulla. Expression of Aire by terminally differentiated mTECs allows stochastic expression of TSAs. Newly generated T cells that recognize MHC–self TSAs on the surface of mTECs or of thymic DCs are clonally deleted or diverted to become Foxp3+ nTreg cells (Anderson et al., 2002; Bonasio et al., 2006; Aschenbrenner et al., 2007). Generation of Aire+ mTECs depends on RANK- and CD40-mediated signaling, and is driven by cross-talk of mTEC progenitors with lymphoid tissue inducer cells and CD4+ thymocytes, that express RANK ligand (RANKL) and CD40 ligand (CD40L), respectively (Rossi et al., 2007; Akiyama et al., 2008; Irla et al., 2008). While expression of Aire by mTECs is not strictly dependent on completion of thymocyte development, recent observations indicate that in post-natal life the size of the pool of mature mTECs (and hence the number of Aire+ cells) is regulated by signals delivered by positively selected thymocytes, in particular through activation of the lymphotoxin (LT)α-LTβR axis (White et al., 2010). Consistent with this, Aire+ mTECs are present, but in low number, in Zap70−/− mice (White et al., 2010), in CD40lg−/− mice (Akiyama et al., 2008), and the size of thymic medulla is significantly decreased in IAa−/− mice (Irla et al., 2008), in which lack of expression of MHC-II impairs positive selection of CD4+ thymocytes. Altogether, these data indicate a critical role of positively selected thymocytes, and especially CD4+ SP cells, in supporting maturation of mTECs and hence maintenance of efficient mechanisms of negative selection of self-reactive T cells.
We have previously shown impaired maturation of mTECs and reduced expression of Aire in a variety of human immunodeficiencies that affect T cell development; importantly, similar defects were present also in thymuses from patients carrying hypomorphic mutations that were partially permissive for T cell development. We now confirm that also in mice hypomorphic rag1 and lig4 mutations that cause a severe, but incomplete defect in T cell development, are associated with profound abnormalities of thymic stroma architecture and mTECs maturation. In both models, generation of more mature MHC-IIhi mTECs was severely compromised, without affecting the intrinsic ability of mTECs to express Aire. Abnormalities of thymic architecture, with impaired formation of a well-defined thymic medulla, were more prominent in rag1S/S than in lig4R/R mice, consistent with a more severe block in T cell development in the former, with decreased ability to generate DP thymocytes and a severely restricted thymic TCR repertoire. It is likely that the reduced number of mature mTECs expressing Aire and TSAs may contribute to the increased frequency of manifestations of immune dysregulation in mice and humans with hypomorphic mutations that severely affect T cell development (Cavadini et al., 2005; Marrella et al., 2007; Poliani et al., 2009). We have also shown that SP T cells from rag1S/S and lig4R/R mice are skewed toward a more mature phenotype.
Little is know about the thymic DC compartment in humans and mice with severe defects in T cell development. Mouse studies showed that approximately 27% of thymic cDCs and 35% of thymic pDCs contain IgH gene D–J rearrangements, and express mRNA for CD3 and pre-Tα chains (Corcoran et al., 2003), indicating that a fraction of thymic DCs share early steps of development with the lymphoid lineages. Furthermore, it is known that the earliest thymic progenitors (ETPs) in mice also possess myeloid potential (Bell and Bhandoola, 2008). Hale et al. (2004) reported that the number of CD83+ mature DCs is significantly reduced in the thymus from patients with X-linked SCID, possibly reflecting the failure of a common progenitor for T lymphocytes and DCs, to differentiate in response to γc-dependent signals. We have recently reported that depletion of thymic DCs is not restricted to patients with X-linked SCID, but is common to other genetic conditions with impaired T lymphocyte development (Poliani et al., 2009). In apparent contrast to these human data, we found that both cDCs and pDCs can be detected in the thymus from lig4R/R and rag1S/S. Further studies are needed to define the location and the origin (intrathymic vs. peripheral) of DCs within the thymus of the mutant mice. This is particularly important since peripheral immature DCs that home to the thymic medulla and to the cortico-medullary junction have been shown to mediate self-antigen presentation and intrathymic deletion of autoreactive T cell clones (Bonasio et al., 2006).
Finally, a role for cDCs (in particular for the CD8lo Sirp-α+ fraction) in the induction of murine nTreg cells in the thymic medulla has been demonstrated (Proietto et al., 2008). We have shown that cDCs are present in the thymus of lig4R/R and rag1S/S mice; furthermore, our preliminary data suggest that the proportion of CD8lo Sirp-α+ within thymic cDCs of lig4R/R mice is preserved (data not shown). Consistent with these findings, CD4+ Foxp3+ cells were detected in the thymus of lig4R/R and rag1S/S mice. Somech et al. (2009) have reported a normal proportion of Foxp3+ regulatory T cells in the periphery of patients with OS. However, others have shown that perturbed Treg function may contribute to immune dysregulation in these patients (Cassani et al., 2010). Although our data indicate that generation of nTreg cells is preserved in lig4R/R and rag1S/S mice, the severe defect in T cell development also accounts for the paucity of thymic Foxp3+ T cells in both models.
Conclusion
In summary, we have shown that hypomorphic defects in V(D)J recombination in mice are associated with abnormalities of lymphoid development and thymic architecture. These defects are more prominent and severe in rag1S/S than in lig4R/R mice. This may reflect a different degree of impairment in V(D)J recombination activity associated with rag1 S723C and lig4 R278H mutations, as also suggested by a higher fraction of DP thymocytes in lig4R/R mice. Homozygosity for null mutations in the lig4 gene is associated with embryonic lethality in mice, and patients with LIG4 mutations identified so far carry a hypomorphic mutation on at least one allele. This may explain why clinical manifestations of immune dysregulation have been more frequently reported among patients with RAG than with LIG4 mutations. Alternatively, it is also possible that the different severity of phenotype may reflect the specific role played by RAG and LIG4 genes. The study of new patients and the development of additional animal models with mutations in these genes may help address this issue.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mike Recher, Laura Patrizi, and Divij Matthew for animal care. This work was partially supported by the National Institutes of Health Grant P01 AI076210-01A1 (to Luigi Daniele Notarangelo and Frederick W. Alt), by the Manton Foundation (Luigi Daniele Notarangelo), by Fondazione “Angelo Nocivelli” (Silvia Giliani) and by Fondazione CARIPLO (Pietro Luigi Poliani). Frederick W. Alt is a Howard Hughes Investigator.
References
Akiyama, T., Shimo, Y., Yanai, H., Qin, J., Ohshima, D., Maruyama, Y., Asaumi, Y., Kitazawa, J., Takayanagi, H., Penninger, J. M., Matsumoto, M., Nitta, T., Takahama, Y., and Inoue, J. (2008). The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29, 423–437.
Alves, N. L., Huntington, N. D., Rodewald, H. R., and Di Santo, J. P. (2009). Thymic epithelial cells: the multi-tasking framework of the T cell “cradle.” Trends Immunol. 30, 468–474.
Anderson, G., and Jenkinson, E. J. (2001). Lymphostromal interactions in thymic development and function. Nat. Rev. Immunol. 1, 31–40.
Anderson, M. S., Venanzi, E. S., Klein, L., Chen, Z., Berzins, S. P., Turley, S. J., von Boehmer, H., Bronson, R., Dierich, A., Benoist, C., and Mathis, D. (2002). Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401.
Aschenbrenner, K., D’Cruz, L. M., Vollmann, E. H., Hinterberger, M., Emmerich, J., Swee, L. K., Rolink, A., and Klein, L. (2007). Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 8, 351–358.
Bell, J. J., and Bhandoola, A. (2008). The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature 452, 764–767.
Bennett, A. R., Farley, A., Blair, N. F., Gordon, J., Sharp, L., and Blackburn, C. C. (2002). Identification and characterization of thymic epithelial progenitor cells. Immunity 16, 803–814.
Bonasio, R., Scimone, M. L., Schaerli, P., Grabie, N., Lichtman, A. H., and von Andrian, U. H. (2006). Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat. Immunol. 7, 1092–1100.
Buck, D., Malivert, L., de Chasseval, R., Barraud, A., Fondanèche, M. C., Sanal, O., Plebani, A., Stéphan, J. L., Hufnagel, M., le Deist, F., Fischer, A., Durandy, A., de Villartay, J. P., and Revy, P. (2006a). Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell 124, 287–299.
Buck, D., Moshous, D., de Chasseval, R., Ma, Y., le Deist, F., Cavazzana-Calvo, M., Fischer, A., Casanova, J. L., Lieber, M. R., and de Villartay, J. P. (2006b). Severe combined immunodeficiency and microcephaly in siblings with hypomorphic mutations in DNA ligase IV. Eur. J. Immunol. 36, 224–235.
Buckley, R. H. (2000). Primary immunodeficiency diseases due to defects in lymphocytes. N. Engl. J. Med. 343, 1313–1324.
Cassani, B., Poliani, P. L., Moratto, D., Sobacchi, C., Marrella, V., Imperatori, L., Vairo, D., Plebani, A., Giliani, S., Vezzoni, P., Facchetti, F., Porta, F., Notarangelo, L. D., Villa, A., and Badolato, R. (2010). Defect of regulatory T cells in patients with Omenn syndrome. J. Allergy Clin. Immunol. 125, 209–216.
Cavadini, P., Vermi, W., Facchetti, F., Fontana, S., Nagafuchi, S., Mazzolari, E., Sediva, A., Marrella, V., Villa, A., Fischer, A., Notarangelo, L. D., and Badolato, R. (2005). Aire deficiency in thymus of 2 patients with Omenn syndrome. J. Clin. Invest. 115, 728–732.
Corcoran, L., Ferrero, I., Vremec, D., Lucas, K., Waithman, J., O’Keeffe, M., Wu, L., Wilson, A., and Shortman, K. (2003). The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 170, 4926–4932.
Datta, S., and Sarvetnick, N. (2009). Lymphocyte proliferation in immune-mediated diseases. Trends Immunol. 30, 430–438.
De Ravin, S. S., Cowen, E. W., Zarember, K. A., Whiting-Theobald, N. L., Kuhns, D. B., Sandler, N. G., Douek, D. C., Pittaluga, S., Poliani, P. L., Lee, Y. N., Notarangelo, L. D., Wang, L., Alt, F. W., Kang, E. M., Milner, J. D., Niemela, J. E., Fontana-Penn, M., Sinal, S. H., and Malech, H. L. (2010). Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood 116, 1263–1271.
Derbinski, J., Gäbler, J., Brors, B., Tierling, S., Jonnakuty, S., Hergenhahn, M., Peltonen, L., Walter, J., and Kyewski, B. (2005). Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 202, 33–45.
Derbinski, J., and Kyewski, B. (2005). Linking signalling pathways, thymic stroma integrity and autoimmunity. Trends Immunol. 26, 503–506.
Doan, T., McNally, A., Thomas, R., and Steptoe, R. J. (2009). Steady-state dendritic cells continuously inactivate T cells that escape thymic negative selection. Immunol. Cell Biol. 87, 615–622.
Ehl, S., Schwarz, K., Enders, A., Duffner, U., Pannicke, U., Kühr, J., Mascart, F., Schmitt-Graeff, A., Niemeyer, C., and Fisch, P. (2005). A variant of SCID with specific immune responses and predominance of gamma delta T cells. J. Clin. Invest. 115, 3140–3148.
Enders, A., Fisch, P., Schwarz, K., Duffner, U., Pannicke, U., Nikolopoulos, E., Peters, A., Orlowska-Volk, M., Schindler, D., Friedrich, W., Selle, B., Niemeyer, C., and Ehl, S. (2006). A severe form of human combined immunodeficiency due to mutations in DNA ligase IV. J. Immunol. 176, 5060–5068.
Fugmann, S. D., Lee, A. I., Shockett, P. E., Villey, I. J., and Schatz, D. G. (2000). The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu. Rev. Immunol. 18, 495–527.
Ge, Q., and Chen, W. F. (1999). Phenotypic identification of the subgroups of murine T-cell receptor alphabeta+ CD4+ CD8− thymocytes and its implication in the late stage of thymocyte development. Immunology 97, 665–671.
Gennery, A. R. (2006). Primary immunodeficiency syndromes associated with defective DNA double-strand break repair. Br. Med. Bull. 77–78, 71–85.
Giblin, W., Chatterji, M., Westfield, G., Masud, T., Theisen, B., Cheng, H. L., DeVido, J., Alt, F. W., Ferguson, D. O., Schatz, D. G., and Sekiguchi, J. (2009). Leaky severe combined immunodeficiency and aberrant DNA rearrangements due to a hypomorphic RAG1 mutation. Blood 113, 2965–2975.
Gommeaux, J., Grégoire, C., Nguessan, P., Richelme, M., Malissen, M., Guerder, S., Malissen, B., and Carrier, A. (2009). Thymus-specific serine protease regulates positive selection of a subset of CD4+ thymocytes. Eur. J. Immunol. 39, 956–964.
Gray, D. H., Chidgey, A. P., and Boyd, R. L. (2002). Analysis of thymic stromal cell populations using flow cytometry. J. Immunol. Methods 260, 15–28.
Grunebaum, E., Bates, A., and Roifman, C. M. (2008). Omenn syndrome is associated with mutations in DNA ligase IV. J. Allergy Clin. Immunol. 122, 1219–1220.
Hale, L. P., Buckley, R. H., Puck, J. M., and Patel, D. D. (2004). Abnormal development of thymic dendritic and epithelial cells in human X-linked severe combined immunodeficiency. Clin. Immunol. 110, 63–70.
Hamazaki, Y., Fujita, H., Kobayashi, T., Choi, Y., Scott, H. S., Matsumoto, M., and Minato, N. (2007). Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat. Immunol. 8, 304–311.
Hikosaka, Y., Nitta, T., Ohigashi, I., Yano, K., Ishimaru, N., Hayashi, Y., Matsumoto, M., Matsuo, K., Penninger, J. M., Takayanagi, H., Yokota, Y., Yamada, H., Yoshikai, Y., Inoue, J., Akiyama, T., and Takahama, Y. (2008). The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29, 438–450.
Hogquist, K. A., Baldwin, T. A., and Jameson, S. C. (2005). Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 5, 772–782.
Holländer, G. A., Wang, B., Nichogiannopoulou, A., Platenburg, P. P., van Ewijk, W., Burakoff, S. J., Gutierrez-Ramos, J. C., and Terhorst, C. (1995a). Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature 373, 350–353.
Holländer, G. A., Simpson, S. J., Mizoguchi, E., Nichogiannopoulou, A., She, J., Gutierrez-Ramos, J. C., Bhan, A. K., Burakoff, S. J., Wang, B., and Terhorst, C. (1995b). Severe colitis in mice with aberrant thymic selection. Immunity 3, 27–38.
Hubert, F. X., Kinkel, S. A., Webster, K. E., Cannon, P., Crewther, P. E., Proeitto, A. I., Wu, L., Heath, W. R., and Scott, H. S. (2008). A specific anti-Aire antibody reveals aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J. Immunol. 18, 3824–3832.
Irla, M., Hollander, G., and Reith, W. (2010). Control of central self-tolerance induction by autoreactive CD4+ thymocytes. Trends Immunol. 31, 71–79.
Irla, M., Hugues, S., Gill, J., Nitta, T., Hikosaka, Y., Williams, I. R., Hubert, F. X., Scott, H. S., Takahama, Y., Holländer, G. A., and Reith, W. (2008). Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity 29, 451–463.
Jin, R., Wang, W., Yao, J. Y., Zhou, Y. B., Qian, X. P., Zhang, J., Zhang, Y., and Chen, W. F. (2008). Characterization of the in vivo dynamics of medullary CD4+CD8− thymocyte development. J. Immunol. 180, 2256–2263.
Kyewski, B., and Klein, L. (2006). A central role for central tolerance. Annu. Rev. Immunol. 24, 571–606.
Lucas, B., Vasseur, F., and Penit, C. (1994). Production, selection, and maturation of thymocytes with high surface density of TCR. J. Immunol. 153, 53–62.
Marrella, V., Poliani, P. L., Casati, A., Rucci, F., Frascoli, L., Gougeon, M. L., Lemercier, B., Bosticardo, M., Ravanini, M., Battaglia, M., Roncarolo, M. G., Cavazzana-Calvo, M., Facchetti, F., Notarangelo, L. D., Vezzoni, P., Grassi, F., and Villa, A. (2007). A hypomorphic R229Q Rag2 mouse mutant recapitulates human Omenn syndrome. J. Clin. Invest. 117, 1260–1269.
Miyara, M., and Sakaguchi, S. (2007). Natural regulatory T cells: mechanisms of suppression. Trends. Mol. Med. 13, 108–116.
Moshous, D., Callebaut, I., de Chasseval, R., Corneo, B., Cavazzana-Calvo, M., Le Deist, F., Tezcan, I., Sanal, O., Bertrand, Y., Philippe, N., Fischer, A., and de Villartay, J. P. (2001). Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell 105, 177–186.
Murata, S., Sasaki, K., Kishimoto, T., Niwa, S., Hayashi, H., Takahama, Y., and Tanaka, K. (2007). Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 316, 1349–1353.
O’Driscoll, M., Cerosaletti, K. M., Girard, P. M., Dai, Y., Stumm, M., Kysela, B., Hirsch, B., Gennery, A., Palmer, S. E., Seidel, J., Gatti, R. A., Varon, R., Oettinger, M. A., Neitzel, H., Jeggo, P. A., and Concannon, P. (2001). DNA ligase IV mutations identified in patients exhibiting developmental delay and immunodeficiency. Mol. Cell 8, 1175–1185.
O’Driscoll, M., Gennery, A. R., Seidel, J., Concannon, P., and Jeggo, P. A. (2004). An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome. DNA Repair (Amst.) 3, 1227–1235.
Poliani, P. L., Facchetti, F., Ravanini, M., Gennery, A. R., Villa, A., Roifman, C. M., and Notarangelo, L. D. (2009). Early defects in human T-cell development severely affect distribution and maturation of thymic stromal cells: possible implications for the pathophysiology of Omenn syndrome. Blood 114, 105–108.
Proietto, A. I., van Dommelen, S., and Wu, L. (2009). The impact of circulating dendritic cells on the development and differentiation of thymocytes. Immunol. Cell Biol. 87, 39–45.
Proietto, A. I., van Dommelen, S., Zhou, P., Rizzitelli, A., D’Amico, A., Steptoe, R. J., Naik, S. H., Lahoud, M. H., Liu, Y., Zheng, P., Shortman, K., and Wu, L. (2008). Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. U.S.A. 105, 19869–19874. [Erratum in: Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 1679].
Riballo, E., Critchlow, S. E., Teo, S. H., Doherty, A. J., Priestley, A., Broughton, B., Kysela, B., Beamish, H., Plowman, N., Arlett, C. F., Lehmann, A. R., Jackson, S. P., and Jeggo, P. A. (1999). Identification of a defect in DNA ligase IV in a radiosensitive leukaemia patient. Curr. Biol. 9, 699–702.
Rooney, S., Chaudhuri, J., and Alt, F. W. (2004). The role of the non-homologous end-joining pathway in lymphocyte development. Immunol. Rev. 200, 115–131.
Rossi, S. W., Kim, M. Y., Leibbrandt, A., Parnell, S. M., Jenkinson, W. E., Glanville, S. H., McConnell, F. M., Scott, H. S., Penninger, J. M., Jenkinson, E. J., Lane, P. J., and Anderson, G. (2007). RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J. Exp. Med. 204, 1267–1272.
Rucci, F., Notarangelo, L. D., Fazeli, A., Patrizi, L., Hickernell, T., Paganini, T., Coakley, K. M., Detre, C., Keszei, M., Walter, J. E., Feldman, L., Cheng, H. L., Poliani, P. L., Wang, J. H., Balter, B. B., Recher, M., Andersson, E. M., Zha, S., Giliani, S., Terhorst, C., Alt, F. W., and Yan, C. T. (2010). Homozygous DNA ligase IV R278H mutation in mice leads to leaky SCID and represents a model for human LIG4 syndrome. Proc. Natl. Acad. Sci. U.S.A. 107, 3024–3029.
Schuetz, C., Huck, K., Gudowius, S., Megahed, M., Feyen, O., Hubner, B., Schneider, D. T., Manfras, B., Pannicke, U., Willemze, R., Knüchel, R., Göbel, U., Schulz, A., Borkhardt, A., Friedrich, W., Schwarz, K., and Niehues, T. (2008). An immunodeficiency disease with RAG mutations and granulomas. N. Engl. J. Med. 358, 2030–2038.
Schwarz, K., Gauss, G. H., Ludwig, L., Pannicke, U., Li, Z., Lindner, D., Friedrich, W., Seger, R. A., Hansen-Hagge, T. E., Desiderio, S., Lieber, M. R., and Bartram, C. R. (1996). RAG mutations in human B cell-negative SCID. Science 274, 97–99.
Signorini, S., Imberti, L., Pirovano, S., Villa, A., Facchetti, F., Ungari, M., Bozzi, F., Albertini, A., Ugazio, A. G., Vezzoni, P., and Notarangelo, L. D. (1999). Intrathymic restriction and peripheral expansion of the T-cell repertoire in Omenn syndrome. Blood 94, 3468–4378.
Sobacchi, C., Marrella, V., Rucci, F., Vezzoni, P., and Villa, A. (2006). RAG-dependent primary immunodeficiencies. Hum. Mutat. 27, 1174–1184.
Somech, R., Simon, A. J., Lev, A., Dalal, I., Spirer, Z., Goldstein, I., Nagar, M., Amariglio, N., Rechavi, G., and Roifman, C. M. (2009). Reduced central tolerance in Omenn syndrome leads to immature self-reactive oligoclonal T cells. J. Allergy Clin. Immunol. 124, 793–800.
Takahama, Y. (2006). Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 6, 127–135.
Takahama, Y., Nitta, T., Mat Ripen, A., Nitta, S., Murata, S., and Tanaka, K. (2010). Role of thymic cortex-specific self-peptides in positive selection of T cells. Semin. Immunol. 22, 287–293.
van der Burg, M., Ijspeert, H., Verkaik, N. S., Turul, T., Wiegant, W. W., Morotomi-Yano, K., Mari, P. O., Tezcan, I., Chen, D. J., Zdzienicka, M. Z., van Dongen, J. J., and van Gent, D. C. (2009). A DNA-PKcs mutation in a radiosensitive T-B-SCID patient inhibits Artemis activation and non-homologous end-joining. J. Clin. Invest. 119, 91–98.
van der Burg, M., van Veelen, L. R., Verkaik, N. S., Wiegant, W. W., Hartwig, N. G., Barendregt, B. H., Brugmans, L., Raams, A., Jaspers, N. G., Zdzienicka, M. Z., van Dongen, J. J., and van Gent, D. C. (2006). A new type of radiosensitive T-B-NK+ severe combined immunodeficiency caused by a LIG4 mutation. J. Clin. Invest. 116, 137–145.
van Ewijk, W., Holländer, G., Terhorst, C., and Wang, B. (2000). Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development 127, 1583–1591.
Villa, A., Santagata, S., Bozzi, F., Giliani, S., Frattini, A., Imberti, L., Gatta, L. B., Ochs, H. D., Schwarz, K., Notarangelo, L. D., Vezzoni, P., and Spanopoulou, E. (1998). Partial V(D)J recombination activity leads to Omenn syndrome. Cell 93, 885–896.
Villa, A., Sobacchi, C., Notarangelo, L. D., Bozzi, F., Abinun, M., Abrahamsen, T. G., Arkwright, P. D., Baniyash, M., Brooks, E. G., Conley, M. E., Cortes, P., Duse, M., Fasth, A., Filipovich, A. M., Infante, A. J., Jones, A., Mazzolari, E., Muller, S. M., Pasic, S., Rechavi, G., Sacco, M. G., Santagata, S., Schroeder, M. L., Seger, R., Strina, D., Ugazio, A., Väliaho, J., Vihinen, M., Vogler, L. B., Ochs, H., Vezzoni, P., Friedrich, W., and Schwarz, K. (2001). V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood 97, 81–88.
Walter, J. E., Rucci, F., Patrizi, L., Recher, M., Regenass, S., Paganini, T., Keszei, M., Pessach, I., Lang, P. A., Poliani, P. L., Giliani, S., Al-Herz, W., Cowan, M. J., Puck, J. M., Bleesing, J., Niehues, T., Schuetz, C., Malech, H., DeRavin, S. S., Facchetti, F., Gennery, A. R., Andersson, E., Kamani, N. R., Sekiguchi, J., Alenezi, H. M., Chinen, J., Dbaibo, G., ElGhazali, G., Fontana, A., Pasic, S., Detre, C., Terhorst, C., Alt, F. W., and Notarangelo, L. D. (2010). Expansion of immunoglobulin-secreting cells and defects in B cell tolerance in Rag-dependent immunodeficiency. J. Exp. Med. 207, 1541–1554.
Watanabe, N., Wang, Y. H., Lee, H. K., Ito, T., Wang, Y. H., Cao, W., and Liu, Y. J. (2005). Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436, 1181–1185.
White, A. J., Nakamura, K., Jenkinson, W. E., Saini, M., Sinclair, C., Seddon, B., Narendran, P., Pfeffer, K., Nitta, T., Takahama, Y., Caamano, J. H., Lane, P. J., Jenkinson, E. J., and Anderson, G. (2010). Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. J. Immunol. 185, 4769–4776.
Keywords: severe combined immunodeficiency, recombination-activating gene 1, DNA ligase 4, thymic epithelial cells, thymus, dendritic cells, Aire, regulatory T cells
Citation: Rucci F, Poliani PL, Caraffi S, Paganini T, Fontana E, Giliani S, Alt FW and Notarangelo LD (2011) Abnormalities of thymic stroma may contribute to immune dysregulation in murine models of leaky severe combined immunodeficiency. Front. Immun. 2:15. doi: 10.3389/fimmu.2011.00015
Received: 14 February 2011;
Accepted: 01 May 2011;
Published online: 11 May 2011.
Edited by:
Menno C. Van Zelm, Erasmus MC, University Medical Center, NetherlandsReviewed by:
Mirjam Van der Burg, Erasmus MC, NetherlandsPärt Peterson, University of Tartu, Estonia
Copyright: © 2011 Rucci, Poliani, Caraffi, Paganini, Fontana, Giliani, Alt and Notarangelo. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Luigi Daniele Notarangelo, Division of Immunology and The Manton Center for Orphan Disease Research, Karp Research Building, Room 9210, 1 Blackfan Circle, Boston, MA 02115, USA. e-mail: luigi.notarangelo@childrens.harvard.edu
