Synthesis of Zr2WP2O12/ZrO2 Composites with Adjustable Thermal Expansion
- 1Department of Electrical and Mechanical Engineering, Guangling College, Yangzhou University, Yangzhou, China
- 2School of Physiccal Science and Technology, Yangzhou University, Yangzhou, China
Zr2WP2O12/ZrO2 composites were fabricated by solid state reaction with the goal of tailoring the thermal expansion coefficient. XRD, SEM and TMA were used to investigate the composition, microstructure, and thermal expansion behavior of Zr2WP2O12/ZrO2 composites with different mass ratio. Relative densities of all the resulting Zr2WP2O12/ZrO2 samples were also tested by Archimedes' methods. The obtained Zr2WP2O12/ZrO2 composites were comprised of orthorhombic Zr2WP2O12 and monoclinic ZrO2. As the increase of the Zr2WP2O12, the relative densities of Zr2WP2O12/ZrO2 ceramic composites increased gradually. The coefficient of thermal expansion of the Zr2WP2O12/ZrO2 composites can be tailored from 4.1 × 10−6 K−1 to −3.3 × 10−6 K−1 by changing the content of Zr2WP2O12. The 2:1 Zr2WP2O12/ZrO2 specimen shows close to zero thermal expansion from 25 to 700°C with an average linear thermal expansion coefficient of −0.09 × 10−6 K−1. These adjustable and near zero expansion ceramic composites will have great potential application in many fields.
Introduction
Lots of materials known to show positive thermal expansion as temperature increase. In contrast, some materials show completely different thermal expansion properties and contract upon heating. This negative thermal expansion (NTE) phenomena has been found in some A2(MO4)3 compounds, where the A cation can be a trivalent main group metal, transition metal, or rare earth element ranging from Lu to Ho, while M corresponds to W or Mo (Sumithra and Umarji, 2004, 2006; Liu H. F. et al., 2012; Liu Q. Q. et al., 2012; Liu et al., 2015). In addition, compounds with aliovalent cations on the A and M site have been reported. For example, Zr2WP2O12 has been reported to exhibit strong and stable NTE over a wide temperature range. Zr2WP2O12 adopts the orthorhombic Sc2W3O12 structure, which consists of ZrO6 octahedra that share corners with two WO4 tetrahedra and four PO4 tetrahedra. Zr-O-W (P) linkages in this structure will lead to the volume contraction due to transverse vibration of bridging oxygen atoms as temperature increase (Isobe et al., 2008, 2009; Cetinkol and Wilkinson, 2009; Tani et al., 2010).
Thermal expansion is an important property of materials, and mismatch in thermal expansion often induces unstable performance or failure of devices in the field of microelectronics, optics and micromachines. To avoid the above problems, control of thermal expansion of materials can be necessary. An easy approach is to mix the NTE material with the positive thermal expansion material in the right proportion.
Most studies describing attempts to synthesize controllable thermal expansion composites mainly focus on ZrW2O8 based composites, such as ZrW2O8/ZrO2 (De Buysser et al., 2004; Lommens et al., 2005; Yang et al., 2007; Khazeni et al., 2011; Romao et al., 2015), ZrW2O8/Cu and ZrW2O8/polyimide (Yilmaz, 2002; Sullivan and Lukehart, 2005; Yang et al., 2010; Hu et al., 2014). The coefficient of thermal expansion (CTE) of the composites drops with the increase of the ZrW2O8 filler. However, the cubic NTE phase of ZrW2O8 is metastable at room temperature, and has to be prepared by rapid quenching after sintering at 1,200°C. Cubic ZrW2O8 show a isotropic NTE over a wide temperature range, but a phase transition from α -ZrW2O8 to β -ZrW2O8 occurs around 160°C, which leads to the decrease of CTE. This change in thermal expansion may be disadvantageous for composite design. Moreover, when heated to 740°C, ZrW2O8 decomposes into ZrO2 and WO3 (Mary et al., 1996; Banek et al., 2010; Gao et al., 2016). In addition, cubic ZrW2O8 undergoes a pressure induced phase transition to an orthorhombic phase with a positive CTE. This transformation has been observed in composites during thermal cycling, and leads to irreproducible thermal expansion behavior (Perottoni and Jornada, 1998; Miao et al., 2004; Varga et al., 2007; Liu et al., 2014).
Zr2WP2O12 is a new NTE material for use as a filler to adjust the CTE of ceramics, glasses, metals, and polymers. It exhibits a strong NTE over the broadest temperature range (room temperature to its sublimation point of about 1,600°C). Moreover, it does not suffer from the same limitations as ZrW2O8, as it is thermodynamically stable and does not undergo any phase transformations.
The synthesis and NTE behavior of Zr2WP2O12 ceramics have been reported previously (Isobe et al., 2008, 2009; Cetinkol and Wilkinson, 2009; Tani et al., 2010). Zr2WP2O12 ceramics show stable NTE with an average linear CET of about −5 × 10−6 K−1. In addition, the Zr2WP2O12 ceramics display excellent mechanical properties (Isobe et al., 2008, 2009; Cetinkol and Wilkinson, 2009). ZrO2 ceramics and fibers has been widely used in optics, electronics and high temperature fields (Lommens et al., 2005; Yang et al., 2007). In some special occasions, ZrO2 need to keep precision dimensional stability with the change in temperature, because a mismatch in size among different precision devices can cause some problems. The average linear CTE of ZrO2 is about 10 × 10−6 K−1 from room temperature to 1,000°C. The absolute values of the CTE of ZrO2 and Zr2WP2O12 are thus similar but have opposite signs, suggesting that these materials are good candidates for the preparation of ceramic composites with tunable CTEs. It is beneficial that ZrO2 does not react with Zr2WP2O12 at high temperatures, as it is a starting material in the solid state synthesis of Zr2WP2O12.
A new series of Zr2WP2O12/ZrO2 ceramic composites that are expected to show an adjustable CTE were synthesized by a solid state reaction method. This work is devoted to exploring the effects of mass ratio of Zr2WP2O12 and ZrO2 on the microstructure, density, and CTE values of the Zr2WP2O12/ZrO2 ceramic composites.
Experimental Details
All Zr2WP2O12, ZrO2, and Zr2WP2O12/ZrO2 ceramics (mass ratios: 1:1, 2:1, 3:1, 4:1) were synthesized through a conventional solid state route. The raw materials were ZrO2 (Aladdin, purity ≥99.95%), WO3 (Aladdin, purity ≥99.95%), and NH4H2PO4 powders (Aladdin, purity ≥99.5%). A summary of samples prepared can be found in Table 1. Reactant mixtures were milled for 6 h to form a homogeneous powder and dried at 80°C, followed by heating at 500°C for 3 h. After this pre-sintering step, the mixtures were uni-axially cold pressed into pellets of 7 mm in diameter and about 2 mm in thickness. Pellets were calcined at 1,200°C in air for 6 h and cooled down in the furnace.
Powder X-ray diffraction experiments were performed on a Shimadzu XRD 7000 using CuKα radiation. Data were collected at 40 kV and 30 mA over the 10° to 60° 2θ range with a scanning speed of 5°/min. The fractured surface morphologies of the samples were observed using a TESCAN VEGA3 scanning electron microscope (SEM). The relative densities of the resulting samples were measured using Archimedes' method. The CTEs of the samples were measured with a Seiko 6300 TMA/SS thermal mechanical analyzer at a heating rate of 5°C/min in air between 25 and 700°C.
Results and Discussion
XRD Analysis
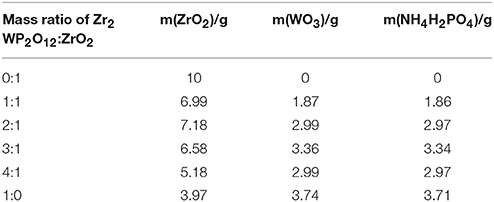
Figure 1 shows typical room temperature XRD patterns of Zr2WP2O12/ZrO2 composites with different mass ratios synthesized at 1,200°C for 6 h. The XRD patterns of pure ZrO2 and pure Zr2WP2O12 ceramics are also displayed for reference. For pure ZrO2 ceramics (Figure 1A), all observed reflections could be well indexed and attributed to monoclinic ZrO2 in agreement with JCPDS card number 65–1,023. For pure Zr2WP2O12 ceramics (Figure 1F), all diffraction peaks matched those expected for orthorhombic Zr2WP2O12 (JCPDS 43-0258). No impurity phases were detected. XRD patterns of Zr2WP2O12/ZrO2 composites with mass ratios of 1:1, 2:1, 3:1, and 4:1 (Figures 1B–E) displayed diffraction peaks belonging to both monoclinic ZrO2 and orthorhombic Zr2WP2O12. As no intermediate phase exists between ZrO2 and Zr2WP2O12, no reaction can occur between excess ZrO2 and Zr2WP2O12. As expected, the diffraction peaks of Zr2WP2O12 became more intense with increasing mass ratio of Zr2WP2O12.
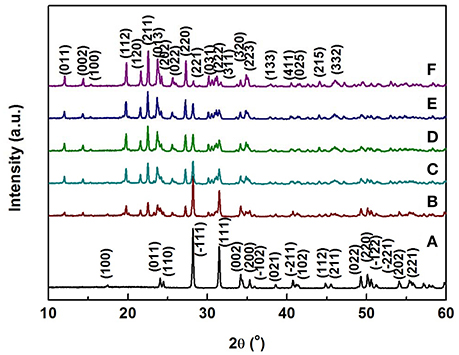
Figure 1. XRD patterns of ZrO2, Zr2WP2O12, and Zr2WP2O12-ZrO2 composites with different mass ratios sintered at 1,200°C for 6 h. (A) ZrO2, (B) 1:1 Zr2WP2O12:ZrO2, (C) 2:1 Zr2WP2O12:ZrO2, (D) 3:1 Zr2WP2O12:ZrO2, (E) 4:1 Zr2WP2O12-ZrO2, (F) Zr2WP2O12.
SEM and Density Analysis
SEM micrographs of different weight ratio Zr2WP2O12/ZrO2 ceramic composites, ZrO2 and Zr2WP2O12 ceramics after sintering at 1,200°C for 6 h are shown in Figure 2. The SEM image of the ZrO2 ceramics (Figure 2a revealed significant porosity, which is likely due to insufficient sintering. It is known that the sintering temperature required to fabricate dense and tough ZrO2 ceramics is higher than 1,400°C (Varga et al., 2007). Figures 2b–e show SEM images of sintered Zr2WP2O12/ZrO2 ceramic composites as a function of different mass ratios. With increasing amount of Zr2WP2O12, Zr2WP2O12/ZrO2 ceramic composites sintered for the same time at the same temperature became denser and displayed larger grain sizes and less porosity. The average grain size of 1:1 Zr2WP2O12/ZrO2 composites was about 2–3 μm, but increased to about 6–8 μm when the mass ratio of Zr2WP2O12/ZrO2 was increased to 4:1. Pure Zr2WP2O12 (Figure 2f) showed a wide size distribution of spherical grains with some residual porosity, which is in agreement with results reported earlier (Isobe et al., 2008, 2009; Cetinkol and Wilkinson, 2009). Figure 3 shows the composition maps analysis of the 2:1 Zr2WP2O12:ZrO2 composite. Homogeneous spatial distributions of Zr, P, W, and O elements were observed. These results indicates that Zr2WP2O12 and ZrO2 phase uniformly distributed as expected.
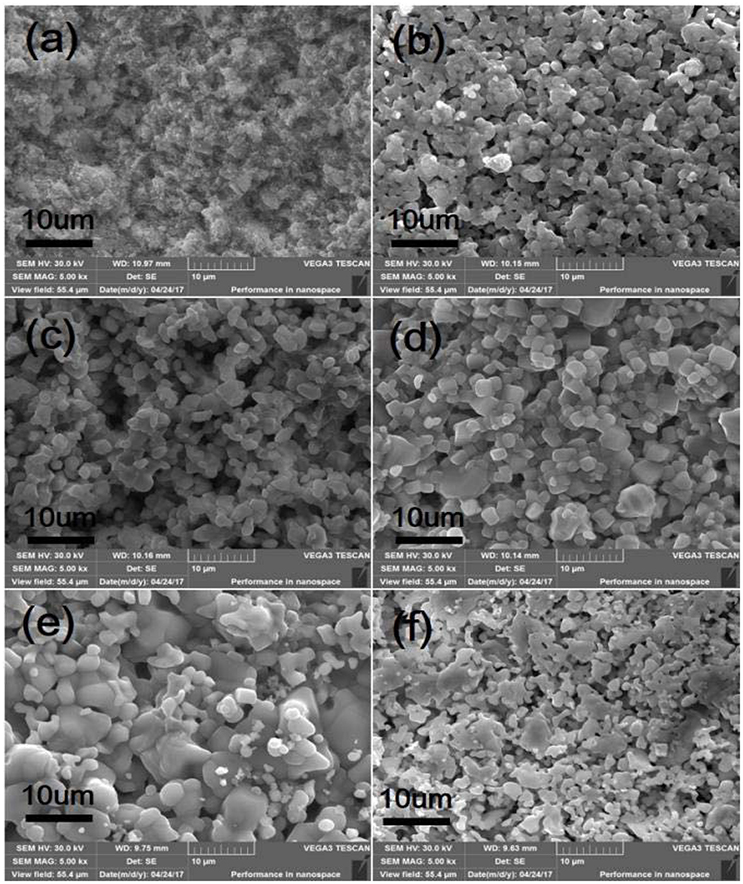
Figure 2. SEM images of ZrO2, Zr2WP2O12, and Zr2WP2O12-ZrO2 composites with different mass ratios sintered at 1,200°C for 6 h, (a) ZrO2, (b) 1:1 Zr2WP2O12:ZrO2, (c) 2:1 Zr2WP2O12:ZrO2, (d) 3:1 Zr2WP2O12:ZrO2, (e) 4:1 Zr2WP2O12-ZrO2, (f) Zr2WP2O12.
Figure 3. EDX composition maps (a) Zr, P, W and O, (b) Zr, (c) P, (d) O, and (e) W analysis for 2:1 Zr2WP2O12:ZrO2 composite.
In this work, the densities of the resulting Zr2WP2O12, ZrO2, and Zr2WP2O12/ZrO2 (mass ratio: 1:1, 2:1, 3:1, 4:1) ceramics were also measured using Archimedes' technique. The relative densities were calculated from theoretical values for Zr2WP2O12 (3.63 g/cm3) and ZrO2 (5.817 g/cm3). As shown in Table 2, the results are consistent with the SEM analysis above. The relative densities of pure Zr2WP2O12 and ZrO2 were low, however, the densities of Zr2WP2O12/ZrO2 (mass ratio: 1:1, 2:1, 3:1, 4:1) ceramics increased with increasing content of Zr2WP2O12. For a 4:1 mass ratio Zr2WP2O12/ZrO2 composite, the relative density of the sample reached 91.5% of the theoretical density values. The sintering temperature of Zr2WP2O12 is lower than that of ZrO2, which results in a decreased sintering temperature and better densification of Zr2WP2O12/ZrO2 ceramics with increasing content of Zr2WP2O12.

Table 2. Relative densities of ZrO2, Zr2WP2O12, and Zr2WP2O12-ZrO2 composites with different mass ratios.
Thermal Expansion Analysis
Figure 4 gives the information about the thermal expansion of all the Zr2WP2O12/ZrO2 ceramic composites synthesized at 1,200°C for 6 h. For purposes of comparison, the thermal expansion curves of pure ZrO2 and pure Zr2WP2O12 ceramics are also given in Figure 4. Average linear CTEs of the obtained ZrO2, Zr2WP2O12, and Zr2WP2O12/ZrO2 ceramics with different mass ratios are summarized in Table 3. Pure ZrO2 ceramics (Figure 4A) showed positive thermal expansion between 25 and 700°C, and the average linear CTE was measured to be 4.1 × 10−6 K−1, which is lower than the value reported in the literature (Lommens et al., 2005; Yang et al., 2007). This is likely due to insufficient sintering of the ZrO2 ceramics, as some of the expansion can be absorbed by the empty pore space. Pure Zr2WP2O12 ceramics (Figure 4F) showed NTE in the testing temperature range. The average linear CTE of the Zr2WP2O12 ceramics was measured to be −3.3 × 10−6 K−1 in the temperature range of 25–700°C, which is consistent with literature reports (Cetinkol and Wilkinson, 2009; Isobe et al., 2009). As can be expected, the CTEs of the Zr2WP2O12/ZrO2 composites decreased from 4.1 × 10−6 K−1 to −3.3 × 10−6 K−1 as the weight fraction of Zr2WP2O12 was increased. As shown in Figure 4C, the 2:1 Zr2WP2O12/ZrO2 specimen showed close to zero thermal expansion with an average linear CTE of −0.09 × 10−6 K−1 in the temperature range of 25–700°C. This near zero expansion ceramic composite will have a number of potential applications in many fields. These results suggest that the CTE of the Zr2WP2O12-ZrO2 composites can be modified in the range from 4.1 × 10−6 K−1 to −3.3 × 10−6 K−1, and that it is even possible to achieve zero thermal expansion by adjusting the mass ratios of Zr2WP2O12 and ZrO2.
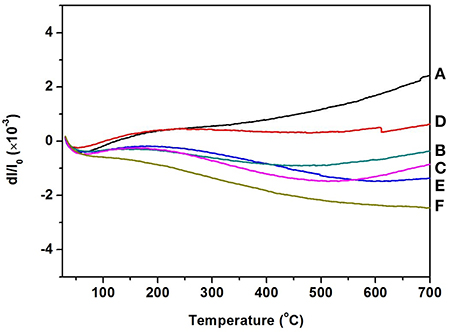
Figure 4. Thermal expansion curves of ZrO2, Zr2WP2O12, and Zr2WP2O12-ZrO2 composites. (A) ZrO2, (B) 1:1 Zr2WP2O12:ZrO2, (C) 2:1 Zr2WP2O12:ZrO2, (D) 3:1 Zr2WP2O12:ZrO2, (E) 4:1 Zr2WP2O12-ZrO2, (F) Zr2WP2O12.
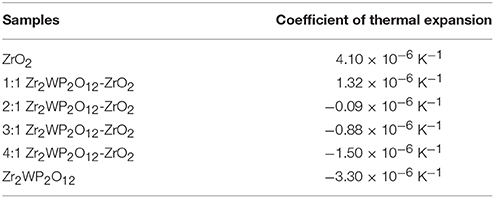
Table 3. Average linear thermal expansion coefficients of ZrO2, Zr2WP2O12, and Zr2WP2O12-ZrO2 composites in corresponding testing temperature range from 25 to 700°C.
Conclusions
Zr2WP2O12/ZrO2 ceramic composites with adjustable thermal expansion coefficients were successfully fabricated by a solid state reaction method. The composites consisted of orthorhombic Zr2WP2O12 and monoclinic ZrO2 with no intermediate phases observed. With increasing amount of Zr2WP2O12, the relative densities of the Zr2WP2O12/ZrO2 ceramic composites increased gradually. The CTE of the Zr2WP2O12/ZrO2 composites can be tailored from 4.1 × 10−6 K−1 to −3.3 × 10−6 K−1 by changing the weight fraction of Zr2WP2O12. For a mass ratio of Zr2WP2O12/ZrO2 of 2:1, the Zr2WP2O12/ZrO2 ceramic composite showed close to zero thermal expansion with an average linear CTE of −0.09 × 10−6 K−1 between 25 and 700°C.
Author Contributions
HL, XC, and ZZ designed experiments; WS and GX carried out experiments; HL, ZZ, and XZ analyzed experimental results and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the National Natural Science Foundation of China (No.51602280 and No.51102207). Qing Lan Project of Jiangsu Province. Guang ling College of Yangzhou University Natural Science Research Foundation (No. ZKZD17001). The authors are grateful to Dr. Cora Lind-Kovacs for revising the paper.
References
Banek, N. A., Baiz, H. I., Latigo, A., and Lind, C. (2010). Autohydration of nanosized cubic zirconium tungstate. J. Am. Chem. Soc. 132, 8278–8279. doi: 10.1021/ja101475f
Cetinkol, M., and Wilkinson, A. P. (2009). Pressure dependence of negative thermal expansion in Zr2(WO4)(PO4)2. Solid State Commun. 149, 421–424. doi: 10.1016/j.ssc.2009.01.002
De Buysser, K., Lommens, P., De Meyer, C., Bruneel, E., Hoste, S., and Driessche, I. V. (2004). ZrO2-ZrW2O8 composites with tailor-made thermal expansion. Ceram. Silikaty 48, 139–144.
Gao, X., Coleman, M. R., and Lind, C. (2016). Surface modification of ZrW2O8 and ZrW2O7(OH)2·2H2O filler particles for controlled thermal expansion polycarbonate composites. Pol. Comp. 37, 1359–1368. doi: 10.1002/pc.23304
Hu, L., Chen, J., Fan, L. L., Ren, Y., Rong, Y. C., Pan, Z., et al. (2014). Zero thermal expansion and ferromagnetism in cubic Sc1−xMxF3 (M = Ga, Fe) over a wide temperature range. J. Am. Chem. Soc. 136, 13566–13569. doi: 10.1021/ja5077487
Isobe, T., Kato, Y., Mizutani, M., Ota, T., and Daimon, K. (2008). Pressureless sintering of negative thermal expansion ZrW2O8/Zr2WP2O12 composites. Mater. Lett. 62, 3913–3915. doi: 10.1016/j.matlet.2008.05.046
Isobe, T., Umezome, T., Kameshima, Y., Nakajima, A., and Okada, K. (2009). Preparation and properties of negative thermal expansion Zr2WP2O12 ceramics. Mater. Res. Bull. 44, 2045–2049. doi: 10.1016/j.materresbull.2009.07.020
Khazeni, N., Mavis, B., Gunduz, G., and Colak, U. (2011). Synthesis of zirconium tungstate-zirconia core-shell composite particles. Mater. Res. Bull. 46, 2025–2031. doi: 10.1016/j.materresbull.2011.07.006
Liu, H. F., Pan, K. M., Jin, Q., Zhang, Z. P., Wang, G., and Zeng, X. H. (2014). Negative thermal expansion and shift in phase transition temperature in Mo-substituted ZrW2O8 thin films prepared by pulsed laser deposition. Ceram. Int. 40, 3873–3878. doi: 10.1016/j.ceramint.2013.08.028
Liu, H. F., Zhang, W., Zhang, Z. P., and Chen, X. B. (2012). Synthesis and negative thermal expansion properties of solid solutions Yb2−xLaxW3O12 (0 ≤ x ≤ 2). Ceram. Int. 38, 2951–2956. doi: 10.1016/j.ceramint.2011.11.072
Liu, H. F., Zhang, Z. P., Ma, J., Zhu, J., and Zeng, X. H. (2015). Effect of isovalent substitution on phase transition and negative thermal expansion of In2−xScxW3O12 ceramics. Ceram. Int. 41, 9873–9877. doi: 10.1016/j.ceramint.2015.04.062
Liu, Q. Q., Yang, J., Cheng, X. N., Liang, G. S., and Sun, X. J. (2012). Preparation and characterization of negative thermal expansion Sc2W3O12/Cu core–shell composite. Ceram. Int. 38, 541–545. doi: 10.1016/j.ceramint.2011.07.041
Lommens, P., Meyer, C. D., Bruneel, E., Buysser, K. D., Driessche, I. V., and Hoste, S. (2005). Synthesis and thermal expansion of ZrO2/ZrW2O8 composites. J. Eur. Ceram. Soc. 25, 3605–3610. doi: 10.1016/j.jeurceramsoc.2004.09.015
Mary, T. A., Evans, J. S. O., Vogt, T., and Sleight, A. W. (1996). Negative thermal expansion from 0.3 K to 1050 K in ZrW2O8. Science 272, 90–92. doi: 10.1126/science.272.5258.90
Miao, X. G., Sun, D., Hoo, P. W., Liu, J. L., Hu, Y. F., and Chen, Y. M. (2004). Effect of titania addition on yttria-stabilised tetragonal zirconia ceramics sintered at high temperatures. Ceram. Int. 30, 1041–1047. doi: 10.1016/j.ceramint.2003.10.025
Perottoni, C. A., and Jornada, J. A. H. (1998). Pressure-induced amorphization and negative thermal expansion in ZrW2O8. Science 280, 886–888. doi: 10.1126/science.280.5365.886
Romao, C. P., Marinkovic, B. A., Werner-Zwanzige, U., and White, M. A. (2015). Thermal expansion reduction in alumina-toughened zirconia by incorporation of zirconium tungstate and aluminum tungstate. J. Am. Chem. Soc. 98, 2858–2865. doi: 10.1111/jace.13675
Sullivan, L. M., and Lukehart, C. M. (2005). Zirconium tungstate (ZrW2O8)/polyimide nanocomposites exhibiting reduced coefficient of thermal expansion. Chem. Mater. 17, 2136–2141. doi: 10.1021/cm0482737
Sumithra, S., and Umarji, A. M. (2004). Role of crystal structure on the thermal expansion of Ln2W3O12 (Ln = La, Nd, Dy, Y., Er and Yb). Solid State Sci. 6, 1313–1319. doi: 10.1016/j.solidstatesciences.2004.07.023
Sumithra, S., and Umarji, A. M. (2006). Negative thermal expansion in rare earth molybdates. Solid State Sci. 8, 1453–1458. doi: 10.1016/j.solidstatesciences.2006.03.010
Tani, J., Takahashi, M., and Kido, H. (2010). Fabrication and thermal expansion properties of ZrW2O8/Zr2WP2O12 composites. J. Eur. Ceram. Soc. 30, 1483–1488. doi: 10.1016/j.jeurceramsoc.2009.11.010
Varga, T., Lind, C., Wilkinson, A. P., Xu, H., Lesher, C. E., and Navrotsky, A. (2007). Heats of formation for several crystalline polymorphs and pressure-induced amorphous forms of AMo2O8 (A = Zr, Hf) and ZrW2O8. Chem. Mater. 19, 468–476. doi: 10.1021/cm0617743
Yang, J., Yang, Y. S., Liu, Q. Q., Xu, G. F., and Cheng, X. N. (2010). Preparation of negative thermal expansion ZrW2O8 powders and its application in polyimide/ZrW2O8 composites. J. Mater. Sci. Technol. 26, 665–668. doi: 10.1016/S1005-0302(10)60103-X
Yang, X. B., Cheng, X. N., Yan, X. H., Yang, J., Fu, T. B., and Qiu, J. (2007). Synthesis of ZrO2/ZrW2O8 composites with low thermal expansion. Compos. Sci. Technol. 67, 1167–1171. doi: 10.1016/j.compscitech.2006.05.012
Keywords: Zr2WP2O12, ZrO2, composites, thermal expansion, ceramics
Citation: Zhang Z, Sun W, Liu H, Xie G, Chen X and Zeng X (2017) Synthesis of Zr2WP2O12/ZrO2 Composites with Adjustable Thermal Expansion. Front. Chem. 5:105. doi: 10.3389/fchem.2017.00105
Received: 27 September 2017; Accepted: 03 November 2017;
Published: 21 November 2017.
Edited by:
Jun Chen, University of Science and Technology Beijing, ChinaReviewed by:
Peng Tong, Institute of Solid State Physics, Hefei Institutes of Physical Science (CAS), ChinaRongjin Huang, Technical Institute of Physics and Chemistry (CAS), China
Copyright © 2017 Zhang, Sun, Liu, Xie, Chen and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongfei Liu, liuhf@yzu.edu.cn
 Zhiping Zhang1,2
Zhiping Zhang1,2  Hongfei Liu
Hongfei Liu