- 1Institut de Recherche en Cancérologie de Montpellier (IRCM), INSERM U1194, Montpellier, France
- 2Université de Montpellier, Montpellier, France
- 3Institut Régional du Cancer de Montpellier (ICM), Montpellier, France
While adjuvant treatments of early breast cancers (BCs) had significantly improved patients’ overall survival, some of them will still develop locoregional relapses and/or severe late radio-induced toxicities. Here, we propose to review how to personalize locoregional treatment by identifying patients at high and low risk of locoregional relapse, patients at risk of late radio-induced side effects. We will, therefore, discuss how to enhance BC radiosensitivity. Finally, we will address how personalized radiotherapy could be implemented in prospective clinical trials.
Introduction
In France, incidence of early breast cancer (BC) is 54,062 in 2015 (data from FRANCIM, French network of cancer registries). Adjuvant treatments of early stage BC had significantly improved patients’ overall survival (OS) over time. In patients younger than 75 years, the French 5-years OS is 91–93% in 2005–2010 period versus 83–88% in 1994–1998 period. Similar observations were noticed worldwide, and this improvement could be attributable to mammography and advances in treatment. Indeed, in the last two decades, biological tumor analysis permitted to classify BC prognostic outcomes according to molecular classification: luminal (positive estrogen and/or progesterone receptors—ER/PgR), triple negative (negative ER/PgR/negative Her2), and Her2 overexpression phenotypes (positive Her2) (1, 2). Since the introduction of BC molecular classification, systemic therapies are tailored according to BC heterogeneity (biomarkers as ER/PgR, Her2, Ki67) and aggressiveness (histological subtypes, histological grade, and tumor stage). Different methods have been developed to help clinicians in BC management as prognostic tools (Adjuvant! Online, Predict, the Nottingham Prognostic Index); as commercialized mRNA based gene classifiers [OncotypeDx®/Genomic Health; MammaPrint®/Agendia; MapQuantDxTM (Genomic Grade Index—GGI)/Ipsogen/QIAGEN; ProSigna®/nanoString; EndoPredict®/Sividon/Myriad Genetics].
Nevertheless, personalizing treatments in the field of locoregional treatment is less developed. Here, we propose to review which BC population would be at high risk of locoregional relapse, the trends of radiosensitization in BC, and how to identify patients at risk of late radio-induced side effects.
Predictive Biomarkers for BC Radiotherapy
Current Prognostic Factors of Locoregional Recurrences
Adjuvant BC radiotherapy reduced both recurrence and BC mortality after mastectomy and axillary lymph nodes dissection (3) and after breast conserving surgery (BCS) (4). Usual prognostic factors of locoregional relapses (LRR) reported in the literature were young age, axillary lymph nodes involvement, tumor size, involved margins, histological grade, negative estrogen receptor, and presence of extensive ductal in situ and lymphovascular invasion (5). A recent meta-analysis showed that patients with luminal-A subtype BC displayed lesser LRR than triple-negative (TN) or Her2-positive BC (6). Those results are consistent with others reported in the literature such as Braunstein and colleagues’ study (7): among 2,233 BC patients treated from 1998 to 2007, a total of 69 local relapse were observed with a median follow-up of 106 months. They observed that non luminal-A BCs were significantly at higher risk of local relapses with a hazard ratio (HR) at 2.64 for luminal-B, at 5.42 for Her2-enriched, and at 4.32 for triple negative BCs, respectively (p < 0.001 for each).
Luminal-A versus Non Luminal-A BCs: Is Molecular Subtype Predictive of Radiotherapy Efficacy?
Luminal-A BC and Patients at Low Risk of Local Relapse
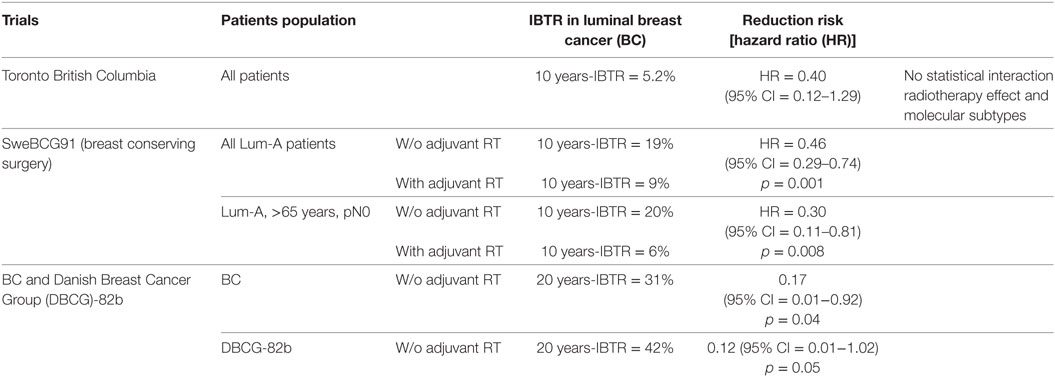
Patients’ population enrolled in the Toronto–British Columbia trial was luminal-A BC phenotype and was considered at low risk of local relapse (older than 50 years, T1-2 and node-negative BC) (Table 1). They were randomly assigned to tamoxifen or tamoxifen and breast RT (8). A recent molecular phenotypes analysis through tissue micro-arrays (TMA) was performed in order to determine whether an intrinsic subtyping would be a predictive biomarker of adjuvant BC radiotherapy benefit (9). Luminal A subtype was defined as followed: positive ER and/or PgR (immunostaining seen in >1% of tumor nuclei), negative Her2 and Ki67 < 14% (10, 11). A total of 501 formalin-fixed paraffin-embedded blocks were retrieved among the 769 patients enrolled. The median follow-up was 10 years, and the 10y-OS was 84% in both arms. Usual clinical and pathological factors were studied (age, tumor size, tumor grade) as well as BC phenotypes (luminal-A, luminal-B, basal-like, and Her2-enriched BC). Luminal-A BC patients significantly displayed a lower risk of local relapse compared to other phenotypes with a 10y-ipsilateral breast tumor recurrence (IBTR) at 5.2%. While luminal-A and -B BCs seemed to have a lesser benefit from adjuvant BC radiotherapy (luminal-A, HR = 0.40; 95% confidence interval = 0.12–1.29; and luminal-B, HR = 0.51; 95% CI = 0.19–1.36), no significant interaction was observed between molecular phenotype and treatments.
Molecular phenotype in such patients population is a significant prognostic factor of IBTR, but not predictive. The recent report of a meta-analysis showed similar findings (6).
Patients Treated With BCS and LUMINAL-A BC
The Swedish Breast Cancer Group 91 Radiotherapy Randomized Clinical Trial (SweBCG91-RT trial) assigned patients to undergo BCS alone versus BCS followed by adjuvant radiotherapy in node-negative stage I-II BC patients (12). Systemic treatments, chemo or hormone therapy, were mostly omitted (92% of patients). A total of 958 TMA was performed among the 1,003 patients enrolled (95% of patients population) and tumor subtypes were defined according to the St. Gallen International Breast Cancer Conference (2013) Expert Panel (13). BC subtypes were prognostic for time to IBTR with a shorter interval time of relapse for TN and Her2-enriched BC (mostly in the first 5 years of follow-up).
After a median follow-up of 15 years, adjuvant radiotherapy significantly decreased the risk of 10y-IBTR as first event in luminal-A (from 19 to 9%; HR = 0.46; 95% CI = 0.29–0.74; p = 0.001) and luminal-B (non Her2 enriched; from 24 to 8%; HR = 0.33; 95% CI = 0.16–0.65; p = 0.001) phenotypes but not for Her2-enriched (from 15 to 19%; HR = 1.29; 95% CI = 0.38–4.40; p = 0.6), and triple negative BC patients (from 21 to 6%; HR = 0.25; 95% CI = 0.05–1.12; p = 0.08). A subpopulation of patients were defined at low risk (age over than 65 years, N0 and luminal A molecular subtype). Among those patients, the risk of IBTR was not as low as expected with a cumulative incidence of IBRT as first event at 10 years at 20% in absence of radiotherapy and at 6% in presence of radiotherapy (HR = 0.30; 95% CI = 0.11–0.81; p = 0.008). However, BC subtype was not predictive of response to radiotherapy (p = 0.21 for interaction tests).
Patients Treated With Radical Mastectomy and Luminal-A BC
A recent study included The British Columbia randomized radiation trial and the Danish Breast Cancer Group (DBCG) protocol 82b assessed whether intrinsic subtypes would be predictive on postmastectomy radiotherapy (PMRT) efficacy regarding LRR in young premenopausal and lymph node-positive BC patients (14). After 20 years of follow-up, the cumulative incidence of LRR in the entire cohort and in absence of PMRT was 36 and 42% in British Columbia and DBCG-82b trials, respectively. During time, PMRT significantly decreased LRR risk in both clinical trials with a HR at 0.35 (95% CI = 0.17−0.72; p = 0.004) in the British Columbia and HR at 0.30 (95% CI = 0.11−0.83; p = 0.02) in the DBCG-82b trials, respectively. As luminal-A BCs are considered at low risk of LRR, the authors studied their cumulative incidence in absence of PMRT: 31 and 42% of LRR at 20 years in British Columbia and DBCG-82b trials, respectively. Authors tested whether PMRT was useful in luminal-A patients’ cohort and they observed that PMRT significantly decreased LRR risk in such patients with a HR at 0.17 (95% CI = 0.01−0.92; p = 0.04) and at 0.12 (95% CI = 0.01−1.02; p = 0.05) in British Columbia and DBCG-82b trials, respectively. The low numbers of patients in each subgroup is one of the major limitations of this study, and therefore, no robust conclusions could be stated.
In conclusion, to date, BC subtypes are not predictive of adjuvant BC radiotherapy regardless the type of surgery, while molecular subgroups are associated with LRR risk. Therefore, in daily practice, no predictive tool could be used to personalized adjuvant radiotherapy in BC settings.
Perspectives in Personalization of Adjuvant BC Radiotherapy
Torres-Rocca has developed a 10 genes-signature, namely RadioSensitive Index (RSI), which estimates BC radiosensitivity (low RSI = radiosensitive profile/RSI-S; high RSI = radioresistant profile/RSI-R; intermediate RSI/RSI-I) (15). In patients treated by surgery and adjuvant radiotherapy, RSI was related to patients’ outcome: radiosensitive patients displayed a significant higher relapse-free survival and distant metastases-free survival at 5 years (5 years RFS = 95%; 5 years DMFS = 77%) than radioresistant patients (5 years RFS = 75%; 5 years DMFS = 64%) [5 years RFS: HR = 7.47 (95% CI = 1–56.01), p = 0.02; 5 years DMFS: HR = 1.74 (95% CI = 1.02–2.99), p = 0.049]. In absence of adjuvant RT, the RSI signature was not able to distinguish patients at risk to develop relapses regardless the type of surgery (lumpectomy or mastectomy) (15). When RSI was combined to molecular subtypes defined according to Eroles and colleagues (16), the authors observed that local relapse was significantly increased in triple negative and RSI-R BC with a HR at 0.37 (95% CI = 0.15–0.92; p = 0.02) (17). Interestingly, when triple negative BC had a sensitive or intermediate RSI, patients’ outcome reached luminal BC outcome. Even though no impact of RSI was observed among the entire cohort of luminal BCs in terms of patients’ outcome, a dose effect was noticed in those latter patients population. Hence, increasing total dose by a boost of 16 Gy to tumor bed significantly improved patients’ outcome with RSI-R luminal BC with a 5 years-local relapse survival at 91 versus 78% (no boost).
As triple negative BCs displayed a higher risk of LRR, some preclinical studies assessed whether the addition of a radiosensitizer would increase response to ionizing radiation in those BCs. For instance, Yes-associated protein 1 (YAP1), which is the terminal effector of the Hippo pathway is involved in TNBC radioresistance. The use of verteporfin, a YAP1 inhibitor, radiosensitized TNBC cell lines by inhibiting EGFR/PI3K/AKT signaling pathway and by increasing DNA damages (18). Other preclinical approaches have been developed as the use of niclosamide, a potent inhibitor of Wnt/β-catenin signaling (19). Yin and colleagues observed that niclosamide radiosensitized TNBC cell lines in vitro by increasing radio-induced apoptosis and induced tumor growth delay in vivo.
In addition to TNBC, Her2-enriched BC also displayed a poor prognosis and a high LRR risk. More than one decade ago, Pietras and colleagues showed that Her2 inhibition radiosensitized BC cell lines by increasing radio-induced DNA damages and apoptosis (20). When BC cells displayed positive Her2 phenotype associated to BC stem cells phenotype (i.e., CD44+CD24low), those cells showed an aggressiveness and radioresistant phenotype (21). When those cells were in presence to trastuzumab, a specific Her2 antibody, their aggressiveness, and radioresistance significantly decreased. More recently, Hou and colleagues showed that Her2 was involved in BC radioresistance by promoting focal adhesion kinase (Fak) phosphorylation and thereby by promoting epithelial-to-mesenchymal transition (22). Therefore, targeting Fak would be a potential target for Her2 BC radiosensitization.
Radio-Induced Toxicity Biomarkers ERA
Toxicities after adjuvant BC radiotherapy, such as a poor cosmetic outcome, can have a negative impact on quality of life and a marked effect on subsequent psychological outcome. Severe toxicities will still occur in 5–10% of patients treated by 3D-conformal radiotherapy, such as radio-induced fibrosis (RIF), even though in the respect of dose–volume constraints to organs-at-risk (23, 24). Furthermore, owing to considerable progress in cancer management in recent decades, the number of long-term survivors significantly increased in BC patients’ population. In that context, many normal tissue radiosensitivity assays were developed to predict the risk of late toxicities occurrence.
Fibroblast-Based Assays
Clinical onset of severe radio-induced toxicities in children with ataxia telangiectasia (AT) was the starting point to develop assays to predict intrinsic radiosensitivity (19, 25). AT is an autosomal recessive disorder related to ATM gene mutations, which is involved in DNA damage repair. Several fibroblast-based assays were developed to predict late radio-induced toxicities occurrence as well as clonogenic assays, residual double strand breaks, micronuclei formation, and comet assays (19, 23–25). The rational was that irradiation-induced DNA damage leads to cell death and, therefore, was the mirror of normal tissue response to ionizing radiation exposure. Even though those tests showed promising results, none of them has been validated in a large clinical trial (26–28). More recently, a new ATM protein-based assay has been developed from patients’ skin biopsy in patients who had developed severe radio-induced toxicities (29). Those latter patients displayed a delay in nucleoshuttling of the ATM protein in response to ionizing radiation of normal tissue. To date, no prospective study showed that one of those assays was able to predict severe late toxicities occurrence (26, 27).
Lymphocytes-Based Assays
Since 1995, a rapid (72 h) radiosensitivity assay was developed and based on flow cytometric assessment of radiation-induced CD8 T-lymphocyte apoptosis (RILA) (28). RILA was first compared between AT and healthy children. A low RILA value was significantly associated with AT syndrome (29, 30). Based on this exploratory study, RILA value was retrospectively assessed in patients with DNA repair-related diseases (i.e., Nijmegen breakage and AT syndromes). Those patients with individual radiosensitivity also displayed a low RILA rate. Therefore, a prospective study included miscellaneous cancers was conducted to determine whether RILA assay would be helpful to identify patients at high risk of severe radio-induced toxicities. This study concluded that patients with severe toxicities displayed a compromised apoptotic response (31) as well as other studies which drew similar conclusions (32–35). The predictive value of RILA in RIF occurrence was validated within the PHRC2005 (NCT00893035), a prospective multicenter French study published in December 2015 (36). In this study, a significant relationship between RILA and toxicities occurrence was observed: an increased RIF development was significantly related to RILA.
Based on the data from the PHRC2005 (NCT00893035), a multiparametric nomogram was developed. The nomogram incorporates both the RILA value, as a continuous variable, and other independent parameters related to the patient’s environment and treatment. This virtual biomarker has shown to significantly improve the performance of the RILA assay alone (p < 0.0013). The prediction of RIF, based on the RILA assay, should now include the nomogram analysis.
In the meanwhile, other assays were developed since many years such as residual γH2AX foci, G2 metaphase, G0 micronuclei assays or GWAS, SNPs studies. Most of these assays-related studies are retrospective or in case of prospective studies, results were non-statistically significant [reviewed in Ref (37, 38)]. For instance, the last published study compared four assays in a recent small cohort (n = 12). Even though this study was very small, RILA still performed best (39).
Validity of Predictive Assays According to REMARK Guidelines
According to REMARK guidelines (40, 41), RILA get the highest level of evidence (level I) while level of evidence is quite low for others assays (level III or IV).
Clinical Implementation
As stated by Azria and colleagues (42), personalized radiotherapy could be address in prospective clinical trials according to tumor control (LRRhigh or LRRlow) and normal tissue complications (RIFhigh or RIFlow) probability (Table 2).
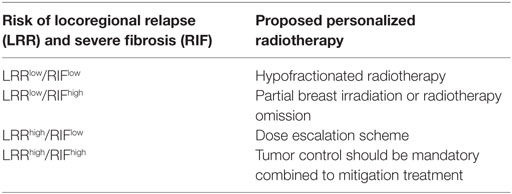
Table 2. Proposed prospective clinical trials according to tumor control (LRRhigh or LRRlow) and normal tissue complications (RIFhigh or RIFlow) probability.
For instance, BC patients at LRRlow/RIFlow risk, hypofractionated radiotherapy schedule could be proposed. In case of patients at LRRlow/RIFhigh risk, the authors suggested either a more conformal radiotherapy, as accelerated partial breast irradiation, or an alternative approach by using surgery to avoid radiotherapy.
For patients at LRRhigh/RIFlow risk, dose escalation, such as a greater boost dose or irradiation field extensions could be considered to improve local control without severe toxicities, providing enhanced clinical benefit. For patients at LRRhigh/RIFhigh risk, tumor control should be mandatory. In those latter patients, mitigation treatment could be proposed when late and severe toxicities will occur such as the use of pentoxifyllin–tocopherol combination (43, 44) or the use of statins (45).
Author Contributions
All authors participated in conception of the review, drafting and revising the article, and providing approval for publication of the content.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature (2000) 406:747–52. doi:10.1038/35021093
2. van ’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature (2002) 415:530–6. doi:10.1038/415530a
3. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet (2014) 383:2127–35. doi:10.1016/S0140-6736(14)60488-8
4. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet (2011) 378:1707–16. doi:10.1016/S0140-6736(11)61629-2
5. van der Leij F, Elkhuizen PH, Bartelink H, van de Vijver MJ. Predictive factors for local recurrence in breast cancer. Semin Radiat Oncol (2012) 22:100–7. doi:10.1016/j.semradonc.2011.12.001
6. Pan XB, Chen RJ, Huang ST, Jiang YM, Zhu XD. Systematic review and meta-analysis of the efficacy of breast conservation therapy followed by radiotherapy in four breast cancer subtypes. Oncotarget (2017) 8:57414–20. doi:10.18632/oncotarget.18205
7. Braunstein LZ, Taghian AG, Niemierko A, Salama L, Capuco A, Bellon JR, et al. Breast-cancer subtype, age, and lymph node status as predictors of local recurrence following breast-conserving therapy. Breast Cancer Res Treat (2017) 161:173–9. doi:10.1007/s10549-016-4031-5
8. Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med (2004) 351:963–70. doi:10.1056/NEJMoa040595
9. Liu FF, Shi W, Done SJ, Miller N, Pintilie M, Voduc D, et al. Identification of a low-risk luminal a breast cancer cohort that may not benefit from breast radiotherapy. J Clin Oncol (2015) 33:2035–40. doi:10.1200/JCO.2014.57.7999
10. Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res (2008) 14:1368–76. doi:10.1158/1078-0432.CCR-07-1658
11. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol (2010) 28:1684–91. doi:10.1200/JCO.2009.24.9284
12. Sjöström M, Lundstedt D, Hartman L, Holmberg E, Killander F, Kovács A, et al. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the swedish breast cancer group 91 radiotherapy randomized clinical trial. J Clin Oncol (2017) 35:3222–9. doi:10.1200/JCO.2017.72.7263
13. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus On The Primary Therapy Of Early Breast Cancer 2013. Ann Oncol (2013) 24:2206–23. doi:10.1093/annonc/mdt303
14. Laurberg T, Tramm T, Nielsen T, Alsner J, Nord S, Myhre S, et al. Intrinsic subtypes and benefit from postmastectomy radiotherapy in node-positive premenopausal breast cancer patients who received adjuvant chemotherapy – results from two independent randomized trials. Acta Oncol (2018) 57(1):38–43. doi:10.1080/0284186X.2017.1401735
15. Eschrich SA, Fulp WJ, Pawitan Y, Foekens JA, Smid M, Martens JW, et al. Validation of a radiosensitivity molecular signature in breast cancer. Clin Cancer Res (2012) 18:5134–43. doi:10.1158/1078-0432.CCR-12-0891
16. Eroles P, Bosch A, Perez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev (2012) 38:698–707. doi:10.1016/j.ctrv.2011.11.005
17. Torres-Roca JF, Fulp WJ, Caudell JJ, Servant N, Bollet MA, van de Vijver M, et al. Integration of a radiosensitivity molecular signature into the assessment of local recurrence risk in breast cancer. Int J Radiat Oncol Biol Phys (2015) 93:631–8. doi:10.1016/j.ijrobp.2015.06.021
18. Andrade D, Mehta M, Griffith J, Panneerselvam J, Srivastava A, Kim TD, et al. YAP1 inhibition radiosensitizes triple negative breast cancer cells by targeting the DNA damage response and cell survival pathways. Oncotarget (2017) 8:98495–508. doi:10.18632/oncotarget.21913
19. Yin L, Gao Y, Zhang X, Wang J, Ding D, Zhang Y, et al. Niclosamide sensitizes triple-negative breast cancer cells to ionizing radiation in association with the inhibition of Wnt/beta-catenin signaling. Oncotarget (2016) 7:42126–38. doi:10.18632/oncotarget.9704
20. Pietras RJ, Poen JC, Gallardo D, Wongvipat PN, Lee HJ, Slamon DJ. Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res (1999) 59:1347–55.
21. Duru N, Fan M, Candas D, Menaa C, Liu HC, Nantajit D, et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res (2012) 18:6634–47. doi:10.1158/1078-0432.CCR-12-1436
22. Hou J, Zhou Z, Chen X, Zhao R, Yang Z, Wei N, et al. HER2 reduces breast cancer radiosensitivity by activating focal adhesion kinase in vitro and in vivo. Oncotarget (2016) 7:45186–98. doi:10.18632/oncotarget.9870
23. Taghian NR, Miller CL, Jammallo LS, O’Toole J, Skolny MN. Lymphedema following breast cancer treatment and impact on quality of life: a review. Crit Rev Oncol Hematol (2014) 92:227–34. doi:10.1016/j.critrevonc.2014.06.004
24. Hau E, Browne L, Capp A, Delaney GP, Fox C, Kearsley JH, et al. The impact of breast cosmetic and functional outcomes on quality of life: long-term results from the St. George and Wollongong randomized breast boost trial. Breast Cancer Res Treat (2013) 139:115–23. doi:10.1007/s10549-013-2508-z
25. Morgan JL, Holcomb TM, Morrissey RW. Radiation reaction in ataxia telangiectasia. Am J Dis Child (1968) 116:557–8.
26. Peacock J, Ashton A, Bliss J, Bush C, Eady J, Jackson C, et al. Cellular radiosensitivity and complication risk after curative radiotherapy. Radiother Oncol (2000) 55:173–8. doi:10.1016/S0167-8140(00)00173-0
27. Somaiah N, Chua ML, Bourne S, Daley F, A’ Hern R, Nuta O, et al. Correlation between DNA damage responses of skin to a test dose of radiation and late adverse effects of earlier breast radiotherapy. Radiother Oncol (2016) 119:244–9. doi:10.1016/j.radonc.2016.04.012
28. Ozsahin M, Ozsahin H, Shi Y, Larsson B, Würgler FE, Crompton NE. Rapid assay of intrinsic radiosensitivity based on apoptosis in human CD4 and CD8 T-lymphocytes. Int J Radiat Oncol Biol Phys (1997) 38:429–40. doi:10.1016/S0360-3016(97)00038-2
29. Crompton NE, Miralbell R, Rutz HP, Ersoy F, Sanal O, Wellmann D, et al. Altered apoptotic profiles in irradiated patients with increased toxicity. Int J Radiat Oncol Biol Phys (1999) 45:707–14. doi:10.1016/S0360-3016(99)00256-4
30. Crompton NE, Shi YQ, Emery GC, Wisser L, Blattmann H, Maier A, et al. Sources of variation in patient response to radiation treatment. Int J Radiat Oncol Biol Phys (2001) 49:547–54. doi:10.1016/S0360-3016(00)01477-2
31. Ozsahin M, Crompton NE, Gourgou S, Kramar A, Li L, Shi Y, et al. CD4 and CD8 T-lymphocyte apoptosis can predict radiation-induced late toxicity: a prospective study in 399 patients. Clin Cancer Res (2005) 11:7426–33. doi:10.1158/1078-0432.CCR-04-2634
32. Bordón E, Henríquez Hernández LA, Lara PC, Pinar B, Fontes F, Rodríguez Gallego C, et al. Prediction of clinical toxicity in localized cervical carcinoma by radio-induced apoptosis study in peripheral blood lymphocytes (PBLs). Radiat Oncol (2009) 4:58. doi:10.1186/1748-717X-4-58
33. Schnarr K, Boreham D, Sathya J, Julian J, Dayes IS. Radiation-induced lymphocyte apoptosis to predict radiation therapy late toxicity in prostate cancer patients. Int J Radiat Oncol Biol Phys (2009) 74:1424–30. doi:10.1016/j.ijrobp.2008.10.039
34. Pinkawa M, Brzozowska K, Kriehuber R, Eble MJ, Schmitz S. Prediction of radiation-induced toxicity by in vitro radiosensitivity of lymphocytes in prostate cancer patients. Future Oncol (2016) 12:617–24. doi:10.2217/fon.15.334
35. Fuentes-Raspall MJ, Caragol I, Alonso C, Ramón y Cajal T, Fisas D, Seoane A, et al. Apoptosis for prediction of radiotherapy late toxicity: lymphocyte subset sensitivity and potential effect of TP53 Arg72Pro polymorphism. Apoptosis (2015) 20:371–82. doi:10.1007/s10495-014-1056-2
36. Azria D, Riou O, Castan F, Nguyen TD, Peignaux K, Lemanski C, et al. Radiation-induced CD8 T-lymphocyte apoptosis as a predictor of breast fibrosis after radiotherapy: results of the prospective multicenter french trial. EBioMedicine (2015) 2:1965–73. doi:10.1016/j.ebiom.2015.10.024
37. Brengues M, Lapierre A, Bourgier C, Pèlegrin A, Özsahin M, Azria D. T lymphocytes to predict radiation-induced late effects in normal tissues. Expert Rev Mol Diagn (2017) 17:119–27. doi:10.1080/14737159.2017.1271715
38. Bourgier C, Lacombe J, Solassol J, Mange A, Pèlegrin A, Ozsahin M, et al. Late side-effects after curative intent radiotherapy: identification of hypersensitive patients for personalized strategy. Crit Rev Oncol Hematol (2015) 93:312–9. doi:10.1016/j.critrevonc.2014.11.004
39. Vandevoorde C, Depuydt J, Veldeman L, De Neve W, Sebastià N, Wieme G, et al. In vitro cellular radiosensitivity in relationship to late normal tissue reactions in breast cancer patients: a multi-endpoint case-control study. Int J Radiat Biol (2016) 92:823–36. doi:10.1080/09553002.2016.1230238
40. Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst (2009) 101:1446–52. doi:10.1093/jnci/djp335
41. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat (2006) 100:229–35. doi:10.1007/s10549-006-9242-8
42. Azria D, Lapierre A, Gourgou S, Ruysscher D, Colinge J, Lambin P, et al. Data-based radiation oncology: design of clinical trials in the toxicity biomarkers era. Front Oncol (2017) 7:83. doi:10.3389/fonc.2017.00083
43. Delanian S, Porcher R, Balla-Mekias S, Lefaix JL. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol (2003) 21:2545–50. doi:10.1200/JCO.2003.06.064
44. Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol (2005) 23:8570–9. doi:10.1200/JCO.2005.02.4729
45. Bourgier C, Rivera S, Vozenin MC, Boisselier P, Azria D, Lassau N, et al. Pravastatin reverses established radiation-induced cutaneous and subcutaneous fibrosis in head and neck cancer patients: results of a biology-driven clinical trial, pravacur phase 2. Int J Radiat Oncol Biol Phys (2017) 99:S74. doi:10.1016/j.ijrobp.2017.06.180
Keywords: radiosensitization, normal tissue complications, predictive factors, biomarkers, breast cancer
Citation: Azria D, Brengues M, Gourgou S and Bourgier C (2018) Personalizing Breast Cancer Irradiation Using Biology: From Bench to the Accelerator. Front. Oncol. 8:83. doi: 10.3389/fonc.2018.00083
Received: 30 January 2018; Accepted: 12 March 2018;
Published: 05 April 2018
Edited by:
Pelagia G. Tsoutsou, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Pavankumar Tandra, University of Nebraska Medical Center, United StatesAbraham Kuten, Rambam Health Care Campus, Israel
Copyright: © 2018 Azria, Brengues, Gourgou and Bourgier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Azria, david.azria@icm.unicancer.fr
 David Azria
David Azria Muriel Brengues
Muriel Brengues Sophie Gourgou
Sophie Gourgou Celine Bourgier
Celine Bourgier