- 1Centre for Health Economics and Medicines Evaluation, Bangor Institute for Health and Medical Research, Bangor University, Bangor, United Kingdom
- 2UCL Institute of Child Health, University College London, London, United Kingdom
This article is based on a study first reported in Health Technology Assessment: Harron K, Mok Q, Dwan K, Ridyard CH, Moitt T, Millar M, et al. CATheter Infections in CHildren (CATCH): a randomised controlled trial and economic evaluation comparing impregnated and standard central venous catheters in children. Health Technol Assess 2016; 20(18), doi: 10.3310/hta20180
Background: Antibiotic-impregnated central venous catheters (CVCs) reduce the risk of bloodstream infections (BSIs) in patients treated in pediatric intensive care units (PICUs). However, it is unclear if they are cost-effective from the perspective of the National Health Service (NHS) in the UK.
Methods: Economic evaluation alongside the CATCH trial (ISRCTN34884569) to estimate the incremental cost effectiveness ratio (ICER) of antibiotic-impregnated (rifampicin and minocycline), heparin-bonded and standard polyurethane CVCs. The 6-month costs of CVCs and hospital admissions and visits were determined from administrative hospital data and case report forms.
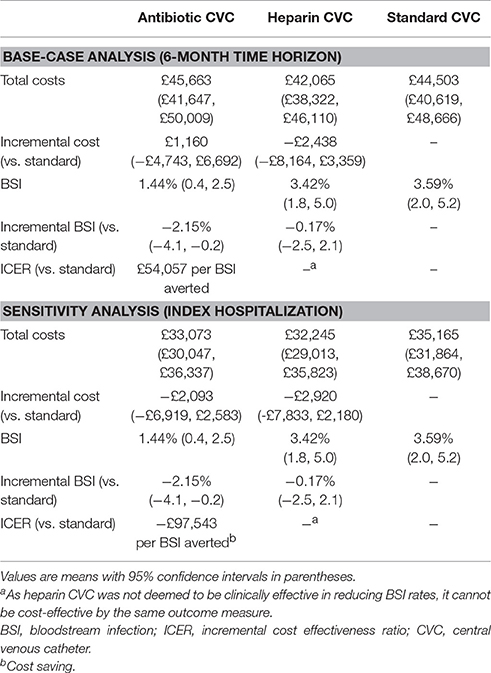
Results: BSIs were detected in 3.59% (18/502) of patients randomized to standard, 1.44% (7/486) to antibiotic and 3.42% (17/497) to heparin CVCs. Lengths of hospital stay did not differ between intervention groups. Total mean costs (95% confidence interval) were: £45,663 (£41,647–£50,009) for antibiotic, £42,065 (£38,322–£46,110) for heparin, and £44,503 (£40,619–£48,666) for standard CVCs. As heparin CVCs were not clinically effective at reducing BSI rate compared to standard CVCs, they were considered not to be cost-effective. The ICER for antibiotic vs. standard CVCs, of £54,057 per BSI avoided, was sensitive to the analytical time horizon.
Conclusions: Substituting standard CVCs for antibiotic CVCs in PICUs will result in reduced occurrence of BSI but there is uncertainty as to whether this would be a cost-effective strategy for the NHS.
Introduction
Central venous catheters (CVCs) are a large yet potentially avoidable cause of health-care associated infections in hospitals. In pediatric intensive care units (PICUs), catheter-related bloodstream infections (BSIs) occur in 3–8% of all CVC insertions (Hockenhull et al., 2008). BSIs are associated with increased morbidity, mortality, lengths of hospital stay, and healthcare costs (Abou Elella et al., 2010; Nowak et al., 2010). Since between 40 and 60% (Harron et al., 2014) of the 16,000 annual admissions to English PICUs (PICANet, 2014) require CVCs, BSIs represent a major burden to patients and the National Health Service (NHS) (Elward et al., 2005; Abou Elella et al., 2010).
The incidence of BSI in adults may be reduced with CVCs impregnated with antibiotics, antibacterial agents or heparin. These are recommended for use in adults at highest risk of BSI (Department of Health, 2007), but evidence in children is lacking (Balain et al., 2015). CVC use in children presents a greater theoretical risk of BSI owing to the narrower lumens within which blood may thrombose more readily. The CATheter Infections in Children (CATCH) trial (NCT01029717) was a pragmatic, three-arm randomized controlled trial aimed to determine the clinical and cost-effectiveness of antibiotic or heparin CVCs compared with standard CVCs in children requiring intensive care. Both heparin-bonded and antibiotic-impregnated CVCs prevent biofilm formation which prevents bacterial colonization. Heparin inhibits thrombus formation and heparin-bonded CVCs use benzalkonium chloride as an anti-infective bonding agent. The primary analyses of CATCH, however, showed no effect of impregnated compared with standard CVCs (Gilbert et al., 2016; Harron et al., 2016a); but secondary analyses revealed antibiotic CVCs to be superior to heparin CVCs with a hazard ratio (HR) for time to first BSI of 0.42 (95% CI, 0.19–0.93), and to standard polyurethane CVCs (HR 0.43; 95% CI 0.20–0.96). Heparin CVCs were no different from standard (HR 1.04; 95% CI, 0.53–2.03).
As impregnated CVCs are more expensive than standard, decisions on their broader use within the NHS requires evidence of their cost-effectiveness. Existing economic analyses are limited in their applicability to the PICU setting in the UK as they relate to adult populations and, with one exception (Hockenhull et al., 2008), apply to different healthcare systems [Australia (Halton et al., 2009), Germany (Frank et al., 2003), and the USA (Veenstra et al., 1999; Marciante et al., 2003; Shorr et al., 2003)]. These studies indicate, however, that antibiotic-impregnated CVCs are associated with improved health outcomes and are cost saving.
Previous economic evaluations are reliant on modeled costs and consequences of BSI using data from a range sources, often observational studies. As such, they rely on assumed attribution of hospital lengths of stay (the main cost driver) and mortality to BSI. The economic evaluation which adopted an NHS cost perspective assumed catheter-related BSIs increase the length of hospital stay by 6 additional days in intensive care units (ICU) and 5 additional days in a general medical ward (Hockenhull et al., 2008). A US cohort study of 1,339 pediatric cases of catheter-related BSI matched to controls by propensity-score, identified a higher mean attributable length of stay of 19 days (Goudie et al., 2014). While this is comparable with the 21 days excess length of stay estimated for BSI in pediatric hematology/oncology patients (Wilson et al., 2014), studies of this nature are based on retrospective observational data and are prone to bias. Patients who are more ill are more likely to develop BSI, making it difficult to separate the contribution of BSI to excess length of stay from the underlying condition.
The aim of the present study was to assess the cost-effectiveness of antibiotic and heparin CVCs relative to commonly used standard polyurethane CVCs in a UK PICU setting using data collected as part of the CATCH randomized controlled trial.
Methods
Design and Results of CATCH
CATCH recruited 1,485 children <16 years who were admitted to any of 14 PICUs in England and who were expected to require a CVC for ≥3 days. Children were randomized equally to receive heparin-bonded, antibiotic-impregnated (rifampicin and minocycline), or standard polyurethane CVCs. The intervention was blinded to everyone except the clinicians responsible for inserting the catheter. The primary outcome was the time to first BSI occurring between 48 h after randomization and 48 h after CVC removal. This occurred in 3.59% (18/502) children randomized to standard CVC, 1.44% (7/486) to antibiotic, and 3.42% (17/497) to heparin CVCs. In the primary analysis, impregnated CVCs (antibiotic and heparin) were no more effective than standard CVCs (HR 0.71; 95% CI 0.37–1.34). Antibiotic CVCs were superior to standard CVCs in secondary analysis (HR 0.43; 0.20–0.96) but heparin CVCs were not (HR 1.04; 0.53–2.03). There were no differences between intervention groups in other outcomes, including time to thrombosis, 30-day mortality, or antibiotic resistance (minocycline or rifampicin). Trial results are presented in full elsewhere (Gilbert et al., 2016; Harron et al., 2016a).
The CATCH trial is registered with the ClinicalTrials.gov (Trial registration: NCT01029717 Registered 9 December 2009), and was conducted in accordance with the recommendations of the Research Ethics Committee for South West England, with prospective or deferred written informed consent obtained from all subjects in accordance with the Declaration of Helsinki. The protocol was approved by the Research Ethics Committee for South West England (reference number 09/H0206/69), and is available at www.nets.nihr.ac.uk/projects/hta/081347.
Economic Evaluation
We conducted a cost-effectiveness analysis as it is not possible to estimate health utilities in children in a PICU setting (Thorrington and Eames, 2015). While this precluded any evidence on allocative efficiency, it allowed for an assessment of technical efficiency for selecting the most cost-effective CVC for reducing the occurrence of BSIs.
Resource Use
The economic analysis adopted the perspective of the NHS in England, with resource use measurement focused on the principal cost drivers, which were PICU, High Dependency Unit (HDU) and ward stays (including readmissions), outpatient clinic visits, Accident and Emergency (A&E) admissions and the costs of the CVCs. The 6-month time horizon of the base-case analysis was chosen to include the costs associated with managing BSIs and associated complications. Shorter time horizons were explored in sensitivity analyses.
Patients' use of hospital services were obtained from trial case report forms (CRF), Hospital Episode Statistics (HES), the Paediatric Intensive Care Audit Network (PICANet), and hospital Patient Administration Systems (PAS). CRFs were accessed for data on dates of hospital discharge, transfer to other hospitals and CVC removal. HES data on Healthcare Resource Groups (HRGs) corresponding to the type of care patients receive at a ward-level, outpatient visits and A&E admissions, were accessed from NHS Digital1. We accessed the PICANet dataset2 for the National Schedule of Reference Cost HRGs for HDU and ICU stays3, and for verifying the dates of hospital admission, transfer and discharge. The finance offices of each participating hospital provided data from Patient Administration Systems (PAS) on patients' lengths of stay in ICUs and wards, and on relevant HRGs. These were used to supplement data that were otherwise missing from other sources.
Costs Analysis
HRGs reflect NHS hospital payments for patients' use of hospital services. Unit costs from the 2012 to 2013 National Schedule of Reference Costs were applied to all HRG codes; the most significant being those associated with PICU, Neonatal Intensive Care Unit (NICU) and HDU (Table 1). Basic HDU (XB07Z) or ICU (XB05Z) codes were applied in the 10% of cases where HRG codes were missing.
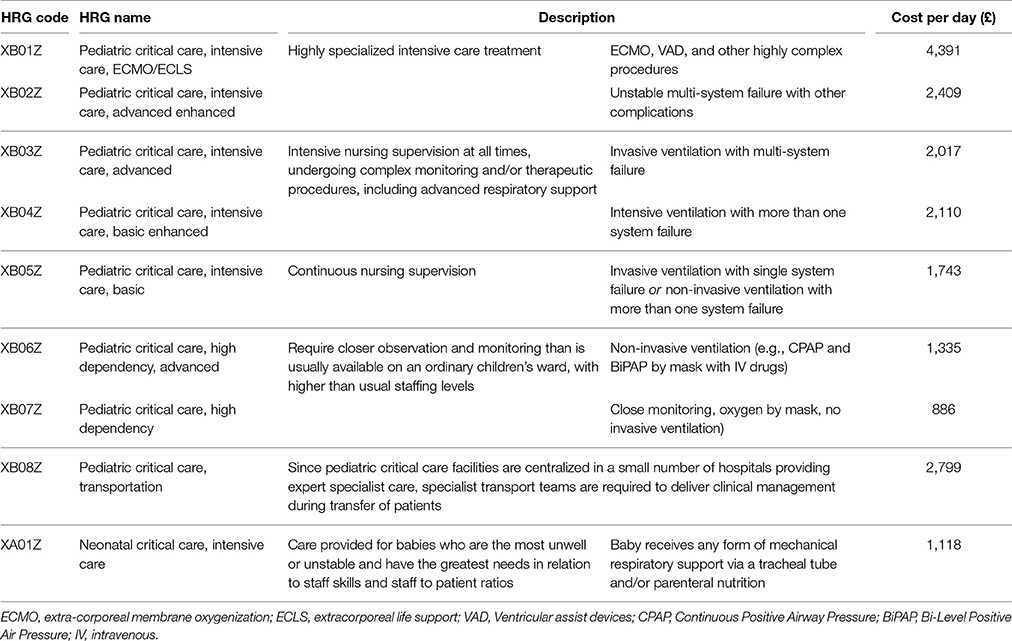
Table 1. Unit cost for intensive care and high dependency care, based on HRGs from the National Schedule of Reference Costs (2012–13).
Unit costs of ward, outpatient and A&E attendances are presented in the Supplementary Appendix Tables 1–3. Any missing HRGs from HES or PAS data were replaced with ward costs based on bed-day rates provided by hospital finance offices (Supplementary Appendix Table 4). Bed-day rates were also applied to unassignable HRG codes appearing in the HES and PAS data, but overall, bed-day rates were used to cost <1% of admissions.
Catheter list prices were provided by the supplier (Cook Medical, Bloomington, IN, USA).
The costs of care for the 6-months prior to randomization were calculated from HES and PICANet data. Given that HRGs relate to episodes of care, we calculated patient costs for the 6-months following randomization according to:
Where n and N are the number of days patients were hospitalized prior to, and following randomization, respectively.
Patients' use of healthcare resources and total costs were calculated for the intention to treat population, and summary statistics were generated by intervention group.
Outcomes
The health outcome for the cost-effectiveness analysis was the presence of a first BSI. These were defined in CATCH by a positive blood culture from a sample that was clinically indicated and taken more than 48 h after CVC insertion and up to 48 h after CVC removal (Gilbert et al., 2016).
Incremental Analysis
Each CVC was ranked in order of decreasing effectiveness and dominated interventions (those which are less effective or ineffective) or extendedly dominated interventions eliminated. The incremental cost effectiveness ratio (ICER) was calculated for remaining CVCs as the difference in the means of total costs divided by the difference in the proportion of bloodstream infections.
Uncertainty Analysis
Bias-adjusted 95% central ranges for differences in costs and BSI were calculated from 10,000 replicate bootstrap analyses. The joint uncertainty in costs and BSI was depicted in a cost-effectiveness acceptability curve (CEAC) which presented the probability of CVCs being cost effective for different threshold willingness to pay for each BSI averted (Fenwick et al., 2001).
Uncertainty in total costs was further assessed by adjusting for the contribution of baseline factors to overall variability (Mihaylova et al., 2011).
Sensitivity Analysis
Given the dependency of costs and therefore the ICER on the analytic time horizon, a sensitivity analysis was performed in which costs were limited to those incurred during the index hospitalization (that is, excluding any re-admissions that may have occurred over the 6-month period).
Regression Analysis
Regression analyses were performed to control for possible baseline imbalances between intervention groups (Mihaylova et al., 2011) and, by including a variable to representing the presence of a BSI, to estimate the value of healthcare resources associated with the management of BSI. The following pre-specified variables were tested for their independent associations with total costs: Age, body weight, 6-month pre-randomization costs (all log-transformed), gender, pre-existing CVC 72 h prior to randomization, health status before PICU admission, reason for admission (cardiovascular, endocrine or metabolic, infection, neurological, oncology, respiratory, trauma, other), suspected infection at randomization, immune compromised, positive blood culture within 72 h prior to randomization, numbers of devices in situ, and admission type (elective or emergency). Where data were missing, we assumed: patients to be healthy (n = 1), not immunocompromised (n = 19), and no positive blood culture (n = 5). Missing data for weight (n = 2) were imputed with the mean (11.95 kg).
Variables that were significant at the 5% level were included using a stepwise approach in multivariable generalized linear models (GLMs) that were specified using a combination of families (e.g., gamma and poisson) and links (e.g., log, square root and identity). Modified Park's test and Akaike Information Criterion were used to assess GLM goodness of fit but were inconclusive. The identity link function performed best according to the Pearson Correlation, Pregibon Link, and the Modified Hosmer and Lemeshow tests. We therefore specified an ordinary least squares regression based on the comparatively large sample size which guaranteed near-normality of sample means (Glick et al., 2007).
All analyses were performed using STATA Version 10, and the economic analysis was reported according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement (Husereau et al., 2013).
Results
Resource Use and Costs
Cost data were available for all patients. Hospital ICU/HDU length of stay and total costs were comparable between intervention groups during the 6-months prior to randomization.
In the 6-months following randomization, patients randomized to antibiotic-impregnated CVCs were in PICU for a mean of 10.8 days (95% CI, 9.3–12.4), compared with 9.9 days (95% CI, 8.6–11.4) for those randomized to heparin-bonded CVC and 10.5 days (95% CI, 9.2–11.9) for standard CVCs (Table 2). Mean durations of hospitalization were 34.8 days (95% CI, 31.2–38.5) for antibiotic-impregnated CVC, 31.4 days (95% CI, 28.2–34.7) for heparin-bonded CVC and 31.7 (95% CI, 28.8–34.7) for the standard CVC group. Six HRGs (from a total of 349) relating to congenital or other cardiac surgery and lower respiratory tract disorders, accounted for 50% of ward costs.
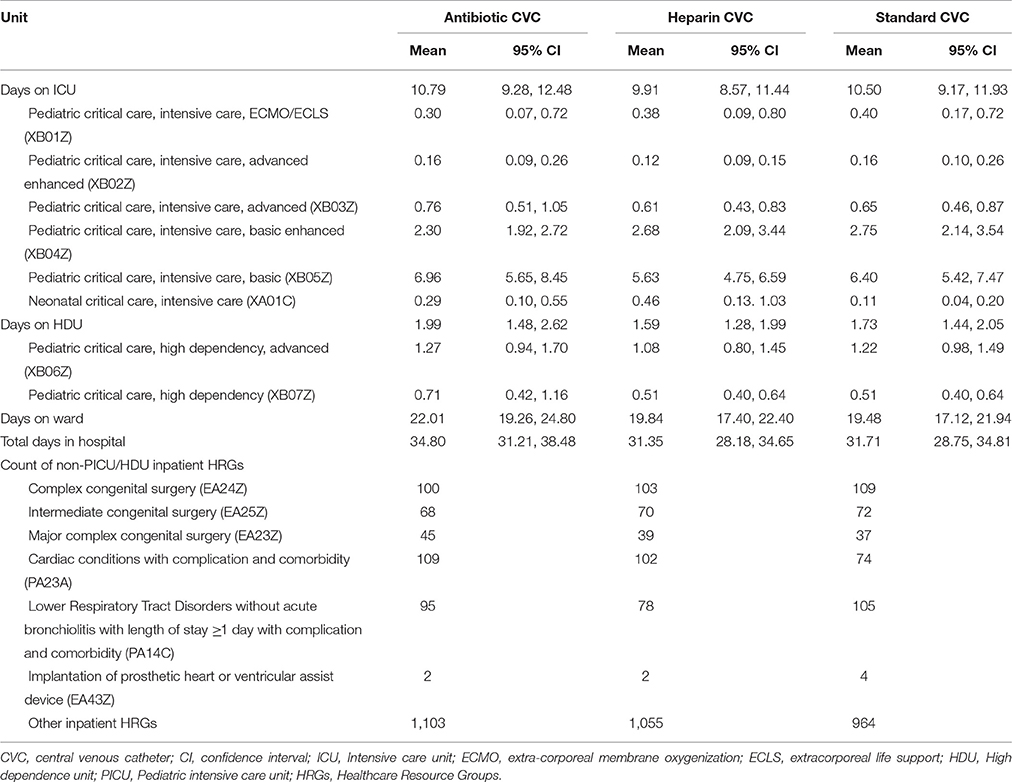
Table 2. Patients' lengths of stay from randomization to 6-months (including readmissions), according to place and intensity of care and by intervention group.
Mean 6-month costs were £44,503 (median £28,952; range £1,786–£360,983; 95% CI, £40,619–£48,666) for standard CVC, £45,663 (median £29,793; range £2,189–£442,365; 95% CI, £41,647–£50,009) for antibiotic-impregnated CVC, £42,065 (median £27,621; range £2,638–£382,431; 95% CI, £38,322–£46,110) for heparin-bonded CVC (Table 3). Costs were not significantly different by CVC group over the 6-month timeframe.
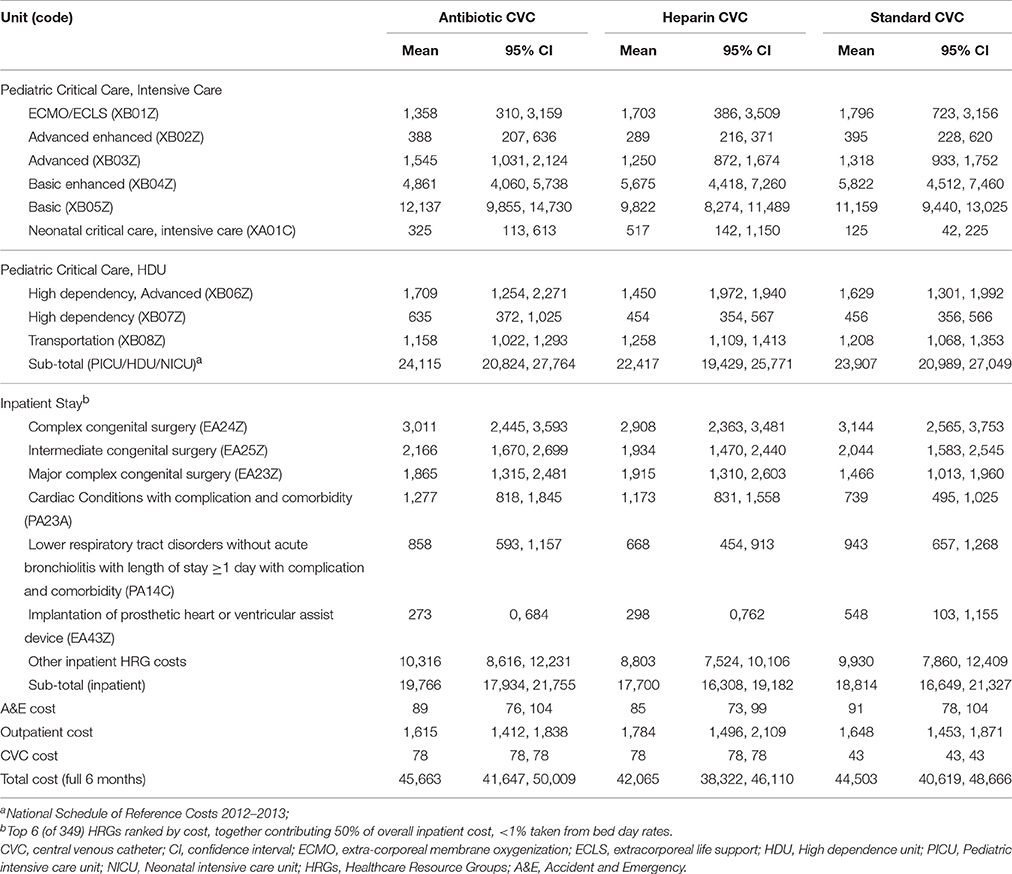
Table 3. Disaggregated and total costs (£) by intervention group from randomization to end of the 6-month timeframe.
Variables tested for the cost regression were evenly balanced between intervention groups (Balain et al., 2015). The residual variability in total cost could be explained, in part, by the following significant explanatory variables: age (in days), 6-month pre-randomization costs (both log-transformed), health status at randomization, reason for admission, immune status, and admission type (elective or emergency). The adjusted incremental costs associated with antibiotic CVCs, in relation to standard CVCs, were £1,220 (95% CI, −£4,332 to £6,773), and with heparin CVCs, −£2,399 (95% CI, to −£7,914 to £3,120).
Outcomes
Seven patients from 486 randomized to antibiotic CVCs experienced a BSI, compared with 17/497 in the heparin CVC group and 18/502 in the standard CVC group. A statistically significant absolute risk differences was found only for antibiotic vs. standard CVCs (−2.15%; 95% CI, −4.09 to −0.20). Heparin CVCs were not clinically effective with a risk difference of −0.17% (95% CI, −2.45 to 2.12) vs. standard CVC.
Value of Healthcare Resources Associated with BSI
Patients who had a BSI (n = 42) experienced 6.5 more days (95% CI, 1.4–11.6) in PICU than those with no BSI (n = 1,443), and 15.1 additional total days (95% CI, 4.0–26.2) of hospitalization. The mean 6-month costs for patients with a BSI was £60,481 (95% CI, £47,873–£73,809) compared to £43,578 (95% CI, £41,185–£45,970) for those without; a difference of £17,263 (95% CI, −£3,076 to £31,450). The adjusted difference in mean costs was £10,975 (95% CI, −£2,801 to £24,751).
Incremental and Uncertainty Analysis
Heparin CVCs were not clinically effective when compared to standard CVC, and are more expensive, and so cannot be cost-effective by the same measure of BSI. The ICER for antibiotic-impregnated vs. standard CVCs was £54,057 per BSI averted (Table 4).
The probabilities of antibiotic CVCs being cost-effective at thresholds of £10,000, £50,000, and £100,000 per BSI averted, were 0.38, 0.49, and 0.62, respectively (Figure 1). There is a probability of 0.650 for standard CVCs dominating antibiotic CVCs.
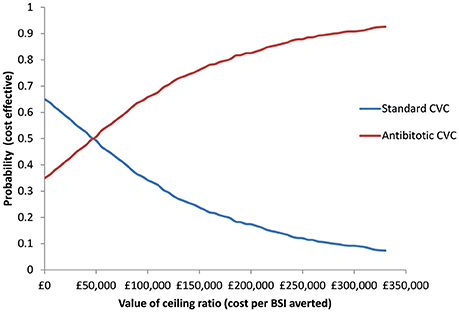
Figure 1. Cost-effectiveness acceptability curve presenting the probability of antibiotic and standard CVCs being cost-effective for a given values of ceiling ratio expressed as cost per bloodstream infection (BSI) averted.
Sensitivity Analysis
Considering only the index hospitalization, total costs in the antibiotic CVC group were £33,073 (95% CI, £30,047–£36,337) compared to £32,245 (95% CI, £29,013–£35,823) in the heparin CVC group and £35,165 (95% CI, £31,864–£38,670) in the standard CVC group. Antibiotic CVCs therefore dominated standard CVC with a difference of 2.15% in the risk of BSI, and a saving of £97,543 per BSI averted.
Discussion
The results of the base-case analysis indicate that heparin-bonded CVCs are not cost-effective while the incremental cost-effectiveness ratio of antibiotic-impregnated CVCs vs. standard CVCs is £54,057 per BSI averted. However, there is considerable uncertainty in this estimate. Restricting costs to the index hospital stay resulted in an ICER of £97,543 saved per BSI averted for antibiotic compared to standard CVCs. Antibiotic CVCs are highly cost-effective when considering costs accruing over comparable periods to events.
The economic analysis benefits from having been designed and executed as an integral part of a pragmatic clinical trial that provided an unbiased comparison of CVCs in the context of current practice in 14 UK PICUs. The cost-effectiveness analysis was conducted to accepted methodological standards of trial-based economic evaluations (Ramsey et al., 2015). Patient-level HES data were used to reflect NHS payments to hospitals for their services, and we exploited different sources to ensure a complete dataset.
However, there are limitations to the analysis. First, the CATCH trial was not powered to demonstrate statistically significant differences in effectiveness or costs among each of the three types of CVCs. However, differences in the rates of BSI were pre-specified in a secondary analysis, and a lack of a difference in costs between intervention groups is less relevant in the context of net benefits (Claxton, 1999). The joint uncertainty in costs and BSI is considered in the CEAC which indicated antibiotic CVCs as having a probability of 0.35 of dominating standard CVCs. Despite not being effective at reducing BSI rates, the mean costs associated with heparin CVCs were lower than for either antibiotic or standard CVCs. This is likely to be explained by BSI being a rare event, with associated costs diluted in the overall costs of managing patients in intensive care.
A second limitation was in our choice of economic outcome. The quality-adjusted life-year (QALY), which is the preferred measure of health outcome for cost-utility analyses (National Institute for Health and Care Excellence, 2013), could not be estimated in the study population (Thorrington and Eames, 2015). The majority of trial participants (58%) were aged <1 year, and even if utilities were measured by proxy, these would be unreliable, especially in the context of intensive care. Using BSI averted as the denominator of the ICER calculation also fails to fully capture other possible consequences of BSI, including long term neurological defects, mortality, antibiotic resistance (Falagas et al., 2007) and other adverse events (Tsai et al., 2016). While neurological outcomes were not monitored in CATCH, there were no differences in 30-day mortality for antibiotic vs. standard (HR 0.96; 95% CI, 0.61–1.51) or for heparin vs. standard CVC (HR 0.65; 95% CI, 0.40–1.07). There were also no differences between intervention groups in microbial resistance to minocycline or rifampicin, or in adverse event rates (Gilbert et al., 2016; Harron et al., 2016a).
In contrast to QALYs, where an explicit threshold range has been defined (£20,000–£30,000 per QALY gained for most health technologies in the UK), there is no threshold for BSIs averted. Interpretation may therefore be dependent on previous economic evaluations, such as Shorr et al. (2003) who considered US$9600 to be cost-effective, or assumptions concerning the impact of BSI on health. For instance, if BSIs are assumed to impair patients' quality of life by a year, (i.e., 1 QALY decrement on average), then antibiotic CVCs may not be cost-effective.
The choice of analytical time horizon represents a further limitation. Six months was selected to capture the costs of subsequent hospital readmissions and transfers to other hospitals. However, as the cost-effectiveness calculation considered only the first BSI, costs accrued over time with no corresponding change to the number of BSI (these all occurred within 30 days). Consequently, the ICER continued to increase over time.
Our estimates of the costs associated with the management of BSI are broadly in line with other economic evaluations (Hockenhull et al., 2008); however there are appreciable differences in our estimate of the ICER. Previous economic analyses indicated the dominance of antibiotic CVCs over standard CVCs. Possible explanations for this discrepancy are that model-based analyses are based on a synthesis of data from disparate sources, require strong assumptions on the attribution of hospital lengths of stay and mortality to BSI and assume independence of the cost of managing BSIs and CVC type.
In conclusion, the results of the economic evaluation indicate that replacing standard polyurethane CVCs with antibiotic-impregnated CVCs in PICUs will result in reduced rates of BSI. Given the low background rate of BSI, the variation in costs between CVCs and the sensitivity of the ICER to the time-horizon of analysis, it remains uncertain if antibiotic-impregnated CVCs are cost-effective from a UK NHS perspective. Given the focus of the evaluation, there is limited generalizability outside the UK to other payers, healthcare systems or jurisdictions; however, our economic findings from CATCH add to evidence on the generalizability of trial participants in the UK, and on the cost implications of using antibiotic-impregnated CVCs to the NHS (Harron et al., 2016b).
Ethics Statement
The Research Ethics Committee for South West England approved the study protocol (reference number 09/H0206/69). Prospective written parental consent during preoperative assessment was sought for children admitted to pediatric intensive care units after elective surgery. For children who needed a central venous catheter as an emergency, we sought written parental consent after randomization and stabilization (deferred consent) to avoid delaying treatment. Parents consented to the use of their child's data for the trial, to follow-up using routinely recorded clinical data, and to an additional 0.5 mL of blood being collected for PCR testing whenever a blood culture was clinically needed.
CATCH Trial Management Group
Ruth Gilbert (chair and chief investigator), Carrol Gamble, Kerry Dwan, Tracy Moitt, Rachel Breen, Colin Ridyard, Angie Wade, Dyfrig Hughes, Quen Mok, Liz Draper, Shane Tibby, Mike Millar, Oliver Bagshaw and Padmanabhan Ramnarayan, Julia Harris and Darren Hewett. Other contributors were Michaela Blundell (quality assurance checks), Susan Howlin and Lynsey Finnetty (data management), and Ivana Pribramska (administrative support).
Author Contributions
DH and RG conceptualized the study; CR, RG, and DH made substantial contribution to the study design and acquisition of data; CR, CP, RG, and DH made substantial contribution to the analysis and interpretation of data, revised the paper critically for important intellectual content and approved the final manuscript.
Funding
This study was funded by the National Institute of Health Research Health Technology Assessment programme (project number 08/13/47). DH is recipient of a Health and Care Research Wales Senior Researcher Award. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS or the Department of Health.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This article is a republication of the economic evaluation of CATCH, reported in: Harron K, Mok Q, Dwan K, CR, Moitt T, Millar M, Ramnarayan P, Tibby SM, Muller-Pebody B, DH, Gamble C, RG. CATheter Infections in CHildren (CATCH): a randomized controlled trial and economic evaluation comparing impregnated and standard CVCs in children. Health Technol Assess. 2016 Mar;20(18):vii-xxviii, 1–219. doi: 10.3310/hta20180. The authors gratefully acknowledge the contributions of Katie Harron of the Institute of Child Health, University College London; and Carrol Gamble, Kerry Dwan, Tracy Ball, Sue Howlin, and Andrew McKay from the Medicines for Children Clinical Trials Unit for their support and for collating data used for this study. We thank the children and families who participated in the CATCH trial and the principal investigators and research nurses at each study site (in order of number of patients recruited): GOSH (Quen Mok, Twin Yen Lee, Samantha Riordan), Southampton General Hospital (Iain Macintosh, Jenni McCorkell, Katie Stearn, Rosie Mitchell), Evelina Children's Hospital (Shane Tibby, Julia Harris, Paul Wellman), Birmingham Children's Hospital (Oliver Bagshaw, Jenna Spry, Simon Laker, Nikki Holdback), Leeds General Infirmary (John Roche, Sian Cooper, Darren Hewett), Alder Hey Children's Hospital (Steve Kerr, Felicity Haigh), Bristol Royal Hospital for Children (Michelle White, Margrid Schindler, Clare Traub, Nina Worrin), Glenfield Hospital (Raghu Ramaiah, Rekha Patel), Royal Brompton Hospital (Duncan Macrae, Sarah Bacon), St Mary's Hospital, London (Mehrengise Cooper, Amina Abdulla, Amy Brewer), Royal Victoria Infirmary (Rachel Agbeko, Christine Mackerness), Queens Medical Centre (Patrick Davies, Daniel Walsh, Lindsay Crate), Freeman Hospital (Rachel Agbeko, Clare Simmister), Leicester Royal Infirmary (Raghu Ramaiah, Rekha Patel). We thank the Local Research Networks (LRNs) in England for supporting the trial implementation; the Trial Steering Committee [Robert Tasker (chair) and Stephen Playfor (chair), Andy Vail, Derek Roebuck, and Jim Gray] and the Independent Data Safety and Monitoring Committee [Paul Ewings (chair), Mike Sharland, Neena Modi] for their oversight of the study.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphar.2017.00644/full#supplementary-material
Footnotes
1. ^Health and Social Care Information Centre Data Linkage and Extract Service. Available online at: http://www.hscic.gov.uk/dles (Accessed August 10, 2017).
2. ^Paediatric Intensive Care Audit Network. Available online at: http://www.picanet.org.uk/ (Accessed August 10, 2017).
3. ^National Schedule of Reference Costs 2012-13. Available online at: https://www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013 (Accessed August 10, 2017).
References
Abou Elella, R., Najm, H., Balkhy, H., Bullard, L., and Kabbani, M. (2010). Impact of bloodstream infection on the outcome of children undergoing cardiac surgery. Pediatr. Cardiol. 31, 483–489. doi: 10.1007/s00246-009-9624-x
Balain, M., Oddie, S. J., and McGuire, W. (2015). Antimicrobial-impregnated central venous catheters for prevention of catheter-related bloodstream infection in newborn infants. Cochrane Database Syst. Rev. 9:CD011078. doi: 10.1002/14651858.CD011078.pub2
Claxton, K. (1999). The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J. Health Econ. 18, 341–364. doi: 10.1016/S0167-6296(98)00039-3
Department of Health (2007). Saving Lives: Reducing Infection, Delivering Clean and Safe Care. London: Department of Health.
Elward, A. M., Hollenbeak, C. S., Warren, D. K., and Fraser, V. J. (2005). Attributable cost of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics 115, 868–872. doi: 10.1542/peds.2004-0256
Falagas, M. E., Fragoulis, K., Bliziotis, I. A., and Chatzinikolaou, I. (2007). Rifampicin-impregnated central venous catheters: a meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 59, 359–369. doi: 10.1093/jac/dkl522
Fenwick, E., Claxton, K., and Sculpher, M. (2001). Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 10, 779–787. doi: 10.1002/hec.635
Frank, U., Chojnacki, T., Dettenkofer, M., and Daschner, F. D. (2003). Cost-effectiveness of an antiseptic impregnated central venous catheter in the ICU. Intensive Care Med. 29:139. doi: 10.1007/s00134-002-1559-0
Gilbert, R. E., Mok, Q., Dwan, K., Harron, K., Moitt, T., Millar, M., et al. (2016). Impregnated central venous catheters for prevention of bloodstream infection in children (the CATCH trial): a randomised controlled trial. Lancet 387, 1732–1742. doi: 10.1016/S0140-6736(16)00340-8
Glick, H. A., Doshi, J. A., Sonnad, A. A., and Polsky, D. (2007). Economic Evaluation in Clinical Trials. Oxford: Oxford University Press.
Goudie, A., Dynan, L., Brady, P. W., and Rettiganti, M. (2014). Attributable cost and length of stay for central line-associated bloodstream infections. Pediatrics 133, e1525–e1532. doi: 10.1542/peds.2013-3795
Halton, K. A., Cook, D., Whitby, M., Paterson, D. L., and Graves, N. (2009). Cost effectiveness of antimicrobial catheters in the intensive care unit: addressing uncertainty in the decision. Crit. Care 13:R35. doi: 10.1186/cc7744
Harron, K., Mok, Q., Dwan, K., Ridyard, C. H., Moitt, T., Millar, M., et al. (2016a). CATheter Infections in CHildren (CATCH): a randomised controlled trial and economic evaluation comparing impregnated and standard central venous catheters in children. Health Technol. Assess. 20, vii–xxviii, 1–219. doi: 10.3310/hta20180
Harron, K., Mok, Q., Hughes, D., Muller-Pebody, B., Parslow, R., Ramnarayan, P., et al. (2016b). Generalisability and cost-impact of antibiotic-impregnated central venous catheters for reducing risk of bloodstream infection in paediatric intensive care units in england. PLoS ONE 11:e0151348. doi: 10.1371/journal.pone.0151348
Harron, K., Mok, Q., Parslow, R., Muller-Pebody, B., Gilbert, R., and Ramnarayan, P. (2014). Risk of bloodstream infection in children admitted to paediatric intensive care units in England and Wales following emergency inter-hospital transfer. Intensive Care Med. 40, 1916–1923. doi: 10.1007/s00134-014-3516-0
Hockenhull, J., Dwan, K., Boland, A., Smith, G., Bagust, A., Dündar, Y., et al. (2008). The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technol. Assess. xi–xii, 1–154. doi: 10.3310/hta12120
Husereau, D., Drummond, M., Petrou, S., Carswell, C., Greenberg, D., Augustovski, F., et al. (2013). Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 346:f1049. doi: 10.1136/bmj.f1049
Marciante, K. D., Veenstra, D. L., Lipsky, B. A., and Saint, S. (2003). Which antimicrobial impregnated central venous catheter should we use: modeling the costs and outcomes of antimicrobial catheter use. Am. J. Infect. Control 31, 1–8. doi: 10.1067/mic.2003.35
Mihaylova, B., Briggs, A., O'Hagan, A., and Thompson, S. G. (2011). Review of statistical methods for analysing healthcare resources and costs. Health Econ. 20, 897–916. doi: 10.1002/hec.1653
National Institute for Health and Care Excellence (2013). National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013. Available onine at: https://www.nice.org.uk/process/pmg9 (Accessed August 10, 2017).
Nowak, J. E., Brilli, R. J., Lake, M. R., Sparling, K. W., Butcher, J., Schulte, M., et al. (2010). Reducing catheter-associated bloodstream infections in the pediatric intensive care unit: business case for quality improvement. Pediatr. Crit. Care Med. 11, 579–587. doi: 10.1097/PCC.0b013e3181d90569
PICANet (2014). Paediatric Intensive Care Audit Network: A Decade of Data. Universities of Leeds and Leicester. Available online at: http://www.picanet.org.uk/Audit/Annual-Reporting/PICANet_A_Decade_of_Data_2014_Annual_Report_Summary.pdf (Accessed August 10, 2017).
Ramsey, S. D., Willke, R. J., Glick, H., Reed, S. D., Augustovski, F., Jonsson, B., et al. (2015). Cost-effectiveness analysis alongside clinical trials II-An ISPOR good research practices task force report. Value Health 18, 161–172. doi: 10.1016/j.jval.2015.02.001
Shorr, A. F., Humphreys, C. W., and Helman, D. L. (2003). New choices for central venous catheters: potential financial implications. Chest 124, 275–284. doi: 10.1016/S0012-3692(15)36021-9
Thorrington, D., and Eames, K. (2015). Measuring health utilities in children and adolescents: a systematic review of the literature. PLoS ONE 10:e0135672. doi: 10.1371/journal.pone.0135672
Tsai, M. H., Lee, C. W., Chu, S. M., Lee, I. T., Lien, R., Huang, H. R., et al. (2016). Infectious complications and morbidities after neonatal bloodstream infections: an observational cohort study. Medicine 95:e3078. doi: 10.1097/MD.0000000000003078
Veenstra, D., Saint, S., and Sullivan, S. (1999). Cost-effectiveness of antiseptic-impregnated central venous catheters for the prevention of catheter-related blood stream infection. JAMA 282, 554–560. doi: 10.1001/jama.282.6.554
Keywords: cost-effectiveness analysis, bloodstream infection, central venous catheter, pediatric intensive care, antibiotic, heparin
Citation: Ridyard CH, Plumpton CO, Gilbert RE and Hughes DA (2017) Cost-Effectiveness of Pediatric Central Venous Catheters in the UK: A Secondary Publication from the CATCH Clinical Trial. Front. Pharmacol. 8:644. doi: 10.3389/fphar.2017.00644
Received: 25 June 2017; Accepted: 30 August 2017;
Published: 19 September 2017.
Edited by:
Dominique J. Dubois, Free University of Brussels, BelgiumReviewed by:
Zoltan Kalo, Eötvös Loránd University, HungaryMihajlo Jakovljevic, University of Kragujevac, Serbia
Copyright © 2017 Ridyard, Plumpton, Gilbert and Hughes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dyfrig A. Hughes, d.a.hughes@bangor.ac.uk
†Names given in the CATCH Trial Management Group section.
 Colin H. Ridyard
Colin H. Ridyard Catrin O. Plumpton
Catrin O. Plumpton Ruth E. Gilbert2
Ruth E. Gilbert2 Dyfrig A. Hughes
Dyfrig A. Hughes